Tissue specific responses alter the biomass accumulation in wheat under gradual and sudden salt stress
Автор: Yumurtaci Aysen, Uncuoglu A.A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.8, 2012 года.
Бесплатный доступ
Salinity is one the major limiting environmental factors which has negative side effects on crop production. The purpose of this study was to investigate the differences between the gradual and sudden salt stress effects on biomass accumulation associated with whole plant development in three different tissues of two wheat species (Triticum aestivum and Triticum durum) under hydroponic conditions in the long term. Considering the effects of sudden and gradual stress for biomass accumulation, while importance of salinity x genotype interaction for fresh weights was 5%, association for salinity x tissue type was found as 1% important. Interestingly, root branching and development of lateral roots were much more negatively affected by gradual stress rather than sudden salt application. Our results demonstrated that root and leaf were both critical tissues to test the salt tolerance by physiologically but sheath tissue might be used as an alternative source of variation for solving the interactions between root and leaves in wheat.
Biomass, hydroponics, physiology, salinity, wheat
Короткий адрес: https://sciup.org/14323681
IDR: 14323681
Текст научной статьи Tissue specific responses alter the biomass accumulation in wheat under gradual and sudden salt stress
Due to the devastating global effects, salinity is a problem which is expecting to pay attention in our ongoing agricultural efforts for sustainable nutrition of humanity in the near future (Boyer 1982; Wang et al. 2001, 2003). Bread wheat ( Triticum aestivum L.) is commonly regarded as moderately tolerant to the salt stress while durum wheat ( T. turgidum L.) is more susceptible (Francois et al. 1986). One of the results showed that soil salinity reduces the ability of plants to take up water which quickly causes reductions in growth rate along with metabolic changes identical to those caused by water stress (Munns and Tester
-
2008 ). Beside this, a sudden increase in soil salinity causes leaf cells to lose water, but this loss of cell volume and turgor is transient. Within hours, cells regain their original volume and turgor owing to osmotic adjustment, but despite this, cell elongation rates are reduced (Cramer 2002; Fricke and Peters 2002; Passioura and Munns 2000; Yeo et al. 1991). Comparing the response of cultivars varied in salt tolerance and using different tissues provided a convenient and useful tool for unveiling the basic mechanisms involved in salt tolerance (Ashraf et al. 1999). Also, differences in salt tolerance existed not only among different genera
and species, but also within the different organs of the same species (Flowers and Hajibagheri 2001; Ismail 2003; Sadat-Noori and McNeilly 2000). Large numbers of bread and durum wheat genotypes have been screened for salt tolerance in greenhouse, the criteria being biomass production at high salinity (up to 250 mM NaCl) relative to biomass in control conditions (Kingsbury and Epstein, 1984) and Sayed (1985) screened of 5000 wheat lines under solution culture, based on survival of high salinity. In the recent experiment of Rahnama et al. (2011), salt gradient was applied for documenting of genotypic differences in the salinity response in seminal root elongation and branch root elongation.
Importantly, plant biomass reduction is one of the reported physiological “markers” for plant screening for salt tolerance (Munns and James 2003). Also, Na+ specific damage is associated with the accumulation of Na+ in the leaf tissues and results in necrosis of older leaves, starting at the tips and margins and working back through the leaf. Growth and yield reduction occur as a result of shortening of the life-time of individual leaves, thus reducing the productivity and crop yield (Munns 1993, 2002). The primary explanation for the slow inadequate progress towards the understanding of salinity tolerance has always been the complexity of this trait. Although intracellular phenomena of salt stress response are well described, much less is known about the organismal response coordination in different organs in changing environments (Hasegawa et al. 2000).
The ion-specific effects of Na+ accumulation on growth and leaf senescence begin to appear only after several weeks of salt treatment and show substantial genotypic variation (Davenport et al. 2005). Curiously, shoot growth is more sensitive than root growth and root growth is usually less affected than leaf growth (Munns 2002). The growth reduction regulated by hormonal signals coming from roots to sheath tissue which carries the signals from the root to the leaf tissue might be maintain much more exact results for comparing the salt stress effects (Davenport 2005). In this study, the differences between the gradual and sudden salt stress effects on biomass accumulation, which have positive correlation with plant yield in a direct way, associated with whole plant development in three different tissues have been synchronously investigated in salt sensitive and tolerant wheat genotypes in the long term by using 150mM NaCl concentration to mimic salinity observed in field conditions.
MATERIALS AND METHODS
Plant material
The following plant material, two bread wheat genotypes ( Triticum aestivum L. cvs.) Dagdas (salt-tolerant) and Yildiz (salt-sensitive) and two durum wheat ( Triticum durum L. cvs.) Meram (salt-tolerant) and Selcuklu (salt-sensitive) were used. Seeds were gently provided from University of Sabanci, Turkey.
Experimental set up and salt stress application
Seeds were surface sterilized in 5% NaHClO 3 solution for 30 min. After extensive rinses with running and distilled water, seeds were germinated in rolls of moistened neutral pH “germ-test” paper. Four weeks-old seedlings of each genotype, selected for uniform size, were transferred into the holes of 42 liter pots which was filled with half strength of Hoagland (Hoagland and Arnon 1950) nutrient solution (pH 6.0) circulated by air pumps in a growth chamber at 22oC, with a photoperiod of 16h light/8h dark for three weeks to investigate salt stress effects on different tissues of wheat plants.
For gradual stress imposition, salt concentration was daily increased by adding 50mM NaCl to nutrient solution until reaching the application concentration of 150mM NaCl. On the other hand, 150mM NaCl was added directly to the solution for sudden stress treatment and these two different salinity treatments were performed at the same time with control groups for comparative evaluation more accurately at physiological level. The level of Ca2+ in the external solution needed to be a ratio of 10:1 for maximal growth in saline conditions as reported by Hawkins and Lewis (1993) for wheat. So, supplemental CaCI 2 was added together with NaCI to maintain a Na+:Ca2+ concentration ratio of 10:1 on a molar basis. Nutrient solutions were replenished after every 5 days and pH 6.0 adjusted with H 2 SO 4 was daily maintained and loss of water was made regularly. Root, leaf and sheath tissues of all genotypes without NaCl treatment were synchronously harvested as control at 21st day of stress application with the treatment groups. This phase of growth is chosen due to the fact that at this developmental stage, physiological results of salt could be distinguished much more clearly as compared to untreated plants at the whole plant level.
Quantitative and qualitative measurements
Fresh and dry weights of the different tissues (roots, leaf and sheath) were measured immediately after harvesting the plant material grown under the discrete salt regimes at 21st day. Initially, all harvested tissues of each plant were separately rinsed with distilled water for 5 minutes and blotted with tissue paper before fresh weighting. Dry biomass calculations of control and treated groups of each genotype were performed after drying each part of plants in an aerated oven at +70ºC for three days. Also, total and green leaf numbers were categorized for both treated and control groups of bread and durum wheat genotypes to calculate the percentage of dead leaves after the exposure of 150mM NaCI stress treatment both gradually and sudden type.
Statistical Analyses
Experiments were performed as completely randomized design and measurements were recorded for the groups of gradual and sudden salinity treated samples individually as a result of two replications and standard deviations were calculated. Data were subjected to analysis of variance for testing significant differences between means.
RESULTS
Effects of gradual and sudden salt stress on fresh weights
In the current research, variations between plant growth characteristics (fresh and dry weights of leaf, root and sheath tissue, decrease percentage of dead leaves and root morphology) and their alterations in the respect of stress treatment were tested to investigate the long term effects of two different type of salt stress in hydroponically grown bread and durum wheat genotypes. It was generally observed that average values of fresh weights were lower than that of control groups for both gradual and sudden salt stress treatments within all genotypes (Fig. 1) and also the same negative effects of salinity on dry biomass accumulation was clearly observed for root, leaf and sheath tissues at various levels (Fig. 2). Moreover, detailed calculations about the separate plant parts initially showed that the level of decline for the average percentage values of root fresh weights had a lower pattern for sudden salt stressed groups (for Dagdas;
56.19%, Yildiz; 63.31%, Meram; 34.28% and Selcuklu; 46.18%) than the gradual ones (for Dagdas; 68.45%, Yildiz; 71.6%, Meram; 66.7% and Selcuklu; 79.41%) for both bread and durum wheat genotypes as compared to their control groups (Fig. 1A). Briefly, fresh weights of root tissues were markedly affected by gradual stress (70% decreases) than sudden salinity (50% decreases) application for whole genotypes.
As the average decrease percentage for fresh weights of sheath tissue was calculated for bread wheat genotypes (Dagdas and Yildiz), it was 80.08% for sudden salt stress treatment, while the reduction in the gradual stress was 83.9%. Additionally, an opposite pattern was exhibited for durum wheat genotypes (Meram and Selcuklu) under sudden (79.53% decline) and gradual (78.76% decline) salinity conditions compared to control, even if the difference between reduction rates of sudden and gradual salinity applications was not too evident and while this decrease was still stayed behind the values of bread wheat genotypes (Fig. 1B). In the case of sheath tissue, sudden salt stress generally caused much more significant negative effects on fresh weights of all genotypes (almost 80% reduction) while fresh weights of root tissue reduced almost 50% under the same salt stress conditions.
As shown in Figure1C, the reduction in leaf fresh weights of bread wheat (Dagdas and Yildiz) genotypes reached the average value of 80.5% for gradual stress treatment and the same dramatic effect was found as nearly as 80.78% for sudden salt stress compared to control genotypes. However, there was a considerable variation in the mean values of decrease percentage for leaf fresh weights which was detected as 6.57% difference between sudden (77.81%) and gradual (84.38%) salt stress treated groups of durum wheat (Meram and Selcuklu) genotypes.
Effects of gradual and sudden salt stress on dry weights
Data obtained from dry weights of all genotypes displayed that there was a higher reduction in the rate of root tissues after gradual stress application as compared to sudden salinity results for both bread (Dagdas, 66.75% and 34.58%; Yildiz, 65.62% and 6.05%) and durum (Meram, 58.4% and 28.45%; Selcuklu, 42.05% and 33.69%) wheat genotypes respectively (Fig. 2A). Additionally, when treatment group of sheath tissue compared to its control group, it was reduced 57.93% and 69.64% for Meram and Selcuklu genotypes respectively, while it was 47.90% for Yildiz and 46.68% for Dagdas under sudden salt stress conditions (Fig. 2B). However, gradual stress resulted an opposite pattern for the bread (Dagdas 58.71%; Yildiz 65.35%) and durum (Meram 54.67%: Selcuklu 41.61%) changed as wheat genotype’s sheath tissues represented in Fig. 2B. Concisely, there was a parallel correlation between stress and genotype tolerance under sudden salt stress conditions but gradual stress did not reflected the same effect and caused a different pattern for all genotypes.
On the other hand, for dry weights of leaf tissues, there was only 2.51% difference in reduction values between bread (average decline percentage 49.83%) and durum (average decline percentage 47.32%) wheat after gradual stress imposition. However, this difference was striking as 13.49% between bread (average decline 37.15%) and durum (average decline 50.64%) wheat genotypes after sudden salt stress (Fig. 2C). Response of leaf dry weights demonstrated much more visible alterations than gradual one.
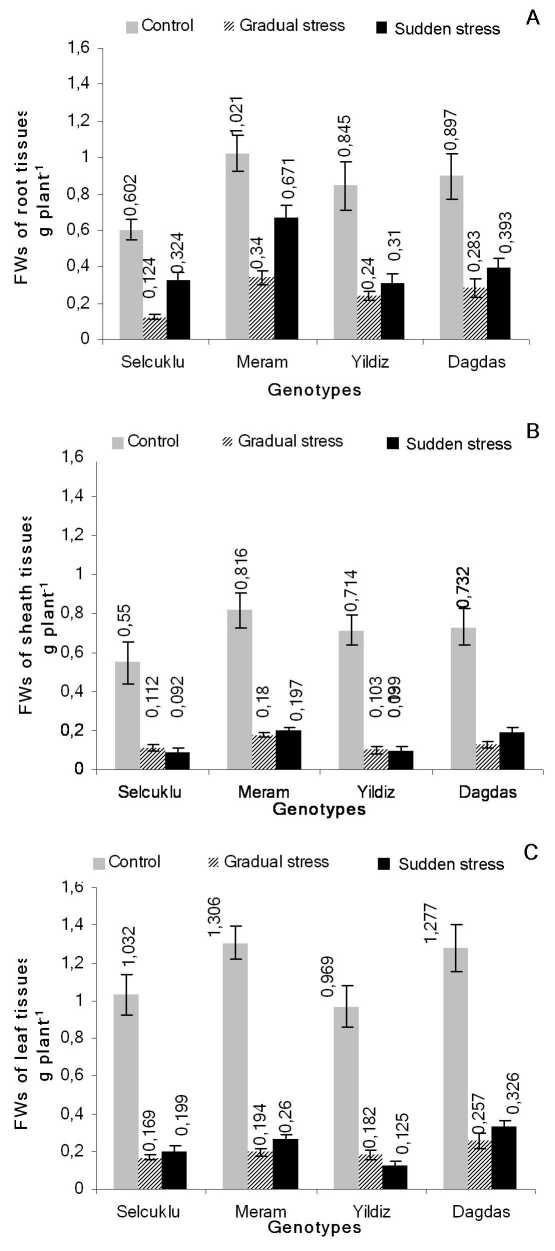
Figure 1 Effects of gradual and sudden salt stress treatment on fresh weights (FW) among bread and durum wheat genotypes after three weeks. A- root tissue, B- sheath tissue, C- leaf tissue. Vertical bars show ±SE (n=10).
Selcuklu Meram Yildiz Dagdas
Genotypes
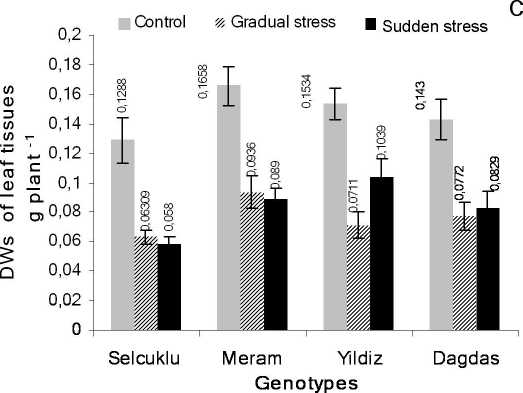
Figure 2 Effects of gradual and sudden salt stress treatment on dry weights (DW) among bread and durum wheat genotypes after three weeks of application. A- root tissue, B- sheath tissue, C- leaf tissue. Vertical bars show ±SE (n=10).
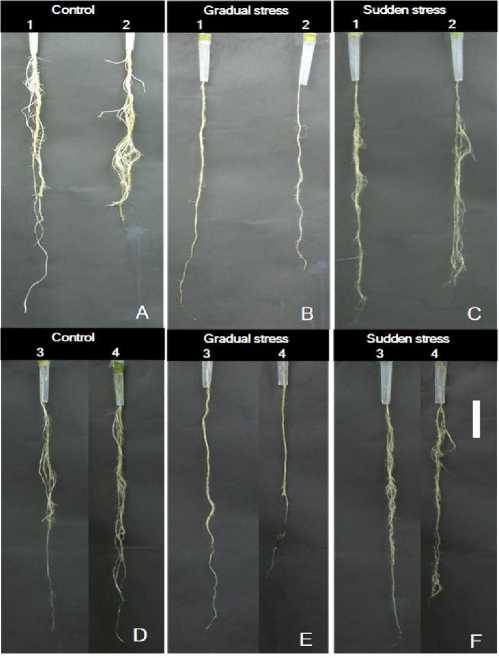
Figure 3 Effects of salinity on root morphology and representative plants root of bead and durum wheat genotypes which were exposed to gradual and sudden salinity treatment (150mM NaCl). White bar indicates the length of 10cm and genotypes displayed as in number order; 1-Meram, 2-Selcuklu, 3- Dagdas, 4-Yildiz.
Table1: Percentage means of dead leaves at 21st day of gradual and sudden salt stress application
|
Genotypes |
Control |
Gradual Salt Stress |
Sudden Salt Stress |
|
Selcuklu |
12% |
95% |
67% |
|
Meram |
11.5% |
87% |
64% |
|
Yildiz |
9% |
75% |
57% |
|
Dagdas |
5.5% |
58% |
51% |
Table2: Evaluations for effects of salt stress on fresh and dry matter yields and their interactions with genotype, tissue type according to the analysis of variance.
Effects of gradual and sudden salt stress on leaf number and root morphology
Salt stress altered the amount of leaves and its effect was much more pronounced in gradual stress for all genotypes, especially for durum wheat (Meram and Selcuklu), as displayed in Table1. Interestingly, values indicated a highly significant difference between gradual and sudden salinity treatment for the decrease percentage means of dead leaves except for Dagdas as a salt tolerant genotype which was lost almost 51% of its total leaves under sudden salt stress and followed its growth during the treatment time. Also, Figure 3 represented that the negative effects of gradual salt stress on the root morphology were much more observable and dominated while sudden salt application did not differed any significant morphological variation among the treatment and control groups after 21 days of stress under the hydroponic conditions. When branching of lateral roots were examined, it was observed that root branching was almost eliminated in gradually salt stressed plants and roots belong to the gradual stressed group (Fig. 3B-E) were thinner than sudden salt applied ones (Fig. 3C-F) as compared to the control plants (Fig. 3A-D). However, lateral roots of sudden salt applied plants displayed much more tolerance rather than gradual one, their development was stayed behind the control group.
Statistical mining
It was evident with the results as summarized in Table2, only fresh weights did not affected by genotype variation but on the other hand, tissue type had an important role for both fresh and dry weight accumulation at P<0.01 significance level. Salinity x tissue type interactions produced much more attractable values ( P<0.01 ) rather than variation of salinity x genotype interactions ( P<0.05 )
for fresh weights. However, associations between genotype x salinity and tissue x salinity indicated prominent results for dry weights at P<0.01 significance level.
DISCUSSION
In this study, according to fresh weight comparisons, root was the least affected tissue under gradual and sudden salt stress (Fig. 1A) whilst the effects of both type of salinity were much more observable for sheath (Fig. 1B) and leaf (Fig. 1C) tissues. The reason for the reduction of whole tissues under salt stress might be due to the imbalanced distribution of plant nutrients in addition to the toxic effects of salt and can be attributed to consequences of the change in osmotic potential around the roots as well as specific ion toxicity. This is because root growth, in contrast to leaf growth, recovers remarkably well from the addition of salt or other osmotica (Hsiao and Xu 2000). It has been shown that salt stress caused serious reductions for both dry biomass of sheath and leaf tissues however this decline was not too favorable for the roots. With these results, our biomass data which were obtained after 21 days of stress application were in agreement with Tuna et al. (2008) and indicated that the effect of salinity might be varied based on tissue type. Previously, Jeannette et al. (2002) observed that total fresh weight of root and shoot of cultivated accessions was reduced with increased salt stress. Also, plant salinity tolerance is usually measured at the end of the experimental period by comparing the weight or yield of control plants against those grown in salt-stressed conditions (Genc et al. 2007; Munns and Rawson 1999). In recent work, gradual and sudden salt stress application time was extended to weekly time period of 21 days. In this respect, remarkable results were obtained after comparisons of the root morphology between the control and treatment groups in addition to the biomass variations under salinity.
Plants exhibit substantial capacity for salt tolerance provided that stress imposition is gradual (Hasegawa et al. 1994). Obviously, the rate of the effectiveness of salinity varied considerably with species and is markedly influenced by the explant of genotype so that some cultivars responded only at tissue level. Some reports documented that each plant tissues perceived to allow a more rapid and successful overall response to salinity stress like halophytes (Flowers et al. 1977; Glenn et al. 1999; Yeo 1998). Rajendran et at. (2009) reported that tissue tolerance might be used as an additional component to expand our understanding about salt tolerance in cereals helping with the results of sodium exclusion and osmotic tolerance. In the current research, plant salt tolerance strictly showed variations not only for the tissue level, but also type of salinity affected the responses of each cultivar after 21st day of stress. According to the results, type of salinity exhibited an important role on biomass accumulation and also it was changed by the tolerance level of genotype (P<0.05) and tissue response at significant P<0.01. The most observable effects of salt stress reflected with the results of decrease percentage of sheath tissue for both fresh and dry weights under the both saline regimes defined as gradual and sudden stress treatment. The response of sheath tissue to salinity both at gradual and sudden treatment level was always showed a closed pattern as in the leaf for limiting the biomass accumulation. The ability to sequester Na+ before it enters leaves prolongs leaf productivity under salt stress and leaf sheath of salt tolerant durum wheat had the capacity to extract and sequester Na+ as it entered leaves (Davenport et al. 2005). In the present study, this was proved by the corresponding changes in fresh weights of sheath and leaf tissues after 21st days of gradual stress. Namely, the decline under gradual salinity condition was lower than sudden salt stress for sheath fresh weights of durum wheat genotypes. This was resulted as a reverse pattern for the leaf fresh weights of durum wheat. This might be also because of response of the sheath tissue due to the course of time and type of stress. Hence, sheath tissue may maintain a bridge between both parts of plant root and leaf tissue.
The detrimental effects of salt on plants are a consequence of both a water deficit resulting in osmotic stress and the effects of excess salt ions on critical biochemical processes (Wyn-Jones 1981). These findings were approved by Termatt and Munns (1986) and Munns (1993), the reduction in growth is a consequence of several physiological responses, including water status, modification of ion balance, carbon allocation and utilization and toxic ions. However, one of the earlier studies showed that a highly significant reduction in fresh and dry weights was observed after 180 mM NaCl for bread wheat cultivar Banysoif 1 (Tammam et al. 2008), in this study 150 mM NaCl exhibited the same impact on all genotypes as well.
El-Hendawy et al. (2005) concluded that an increase in tiller number per plant and spikelet number per spike will improve the salt tolerance of wheat genotypes in breeding programs. Salt stress also resulted in considerable decline in fresh and dry weights of leaves, stems, tillers, fertile tillers and roots (AliDenar et al. 1999; Chartzoulakis and Klapaki 2000; Shahzad 2007). All cultivars exposed to salinity exhibited significantly lower fresh and dry weights than measured plants under control conditions. Salinity declined dry weight per plant significantly at all growth stages. The reduction in total biomass in the sensitive genotypes was probably due to the extra energy utilization for osmotic accumulation, which is much more ATP consuming for osmotic adjustment (Wyn-Jones and Gorham 1993).
From the results obtained from fresh weights, gradual stress had much more negative effects rather than sudden salt stress for all genotypes. The decline in shoot and root yield of all cultivars with the addition of salt could be due to the reduction of physiological availability of water with the increase in solute suction from saline media or accumulation of toxic ions in plants (Munns et al. 1995). The reduction in dry weights was closely related to leaf number and leaf area (Hu et al. 1997). In the saltsensitive genotypes, in which salt is not effectively excluded from the transpiration stream, salt will build up to toxic levels in the leaves, resulting in death of old leaves and new leaves becoming injured and succulent (Munns and James, 2003). Consequently, the number of green and healthy leaves will ultimately decline. Munns (1993) found the same growth reduction in the growth of two wheat cultivars under 150mM salt stress after four weeks, according to our results, there were significant differences between all cultivars both dry and fresh weight level for several tissues of bread and durum wheat cultivars under 150 mM NaCl application and three weeks later (Table1). This study also leads to the conclusion that there is a large variation in root, leaf and sheath tissues and their response characteristics within hexaploid and tetraploid level as well as salinity type in the frame of the time dependent manner, which enable our attentions to physiological effects of salt stress to the specific tissues such as sheath for future experiments. Although, both type of salinity made the most significant impact on sheath tissues for whole genotypes according to their controls, these results confirmed with the findings of the differences in biomass accumulation between treated and untreated groups.
Briefly, physiological responses of different tissues could be expected to make positive contribution to the phenotypic screening studies in addition to the molecular ones which was performed to understand the behavior of plant and also to measure overall growth in wheat. It is assumed that studies established under different salt regimes could be used to discriminate the realistic responses of wheat.
ACKNOWLEDGEMENTS
The present study is an output of a project funded by The Scientific and Technological Research Council of Turkey and numbered as TBAG-104T464. Authors thank to Yelda Borekci and Ebru Ozkan for their technical help about data measuring and collecting the plant material.
Список литературы Tissue specific responses alter the biomass accumulation in wheat under gradual and sudden salt stress
- AliDenar, H.M., Ebert, G., and Ludders, P. (1999) Growth, chlorophyll content, photosynthesis and water relations in guava (Psidium guajava L.) under salinity and different nitrogen supply. Gartenbauwissenschaft 64, 54-59.
- Ashraf, M.Y., Waheed, R.A., Bhatti, A.S., Baig, A., and Aslam, Z., (1999) Salt tolerance potential in different Brassica species. Growth studies. In: Hamdy AH, Leith M, Todorovic and Moscheuko M. eds., Halophyte Uses in Different Climates II. Backhuys Pubs. Leiden, The Netherlands pp: 119-125.
- Boyer, J.S. (1982) Plant productivity and environment. Science 218, 443-448.
- Cramer, G.R. (2002) Response of abscisic acid mutants of Arabidopsis to salinity. Functional Plant Biology 29,561-567.
- Chartzoulakis, K. and Klapaki, G. (2000) Response of two green house pepper hybrids to NaCl salinity during different growth stages. Sci. Horticulture 86, 247-260.
- Davenport, R., James, R.A., Zakrisson-Plogander, A., Tester, M. and Munns, R. (2005) Control of sodium transport in durum wheat. Plant Physiology 137, 807-818.
- El-Hendawy, S.E., Hu, Y., Yakout, G.M., Awad, A.M., Hafiz, S.E. and Schmidhalter, U. (2005) Evaluating salt tolerance of wheat genotypes using multiple parameters. European Journal of Agronomy 22, 243-253.
- Flowers, T.J. and Hajiabagheri, M.A. (2001) Salinity tolerance in Hordeum vulgare: ion concentration in root cells to cultivars differing in salt tolerance. Plant Soil 231,1-9.
- Flowers, T.J., Troke, P.F. and Yeo, A.R. (1977) The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology 28, 89-121.
- Francois, L.E., Maas, E.V., Donovan, T.J. and Youngs, V.L. (1986) Effect of salinity on grain yield and quality, vegetative growth and germination of semi-dwarf and durum wheat. Agronomy Journal 78, 1053-1058.
- Fricke, W. and Peters, W.S. (2002) The biophysics of leaf growth in salt-stressed barley.A study at the cell level. Plant Physiology 129, 374-388.
- Genc, Y., McDonald, G.K. and Tester, M. (2007) Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant, Cell and Environment 30, 1486-1498.
- Glenn, E.P., Brown, J.J. and Blumwald, E. (1999) Salt tolerance and crop potential of halophytes. Critical Review of Plant Science 18, 227-255.
- Hasegawa, P.M., Bressan, R.A., Nelsen, D.E., Samaras, Y., Rhodes, D. (1994) Tissue culture in the improvement of salt tolerance in plants. In Soil Mineral Stresses. Approaches to Crop Improvement Monogr. Yeo AR, Flowers TJ eds. Berlin:Springer-Verlag pp 83-125.
- Hasegawa, P.M., Bressan, R.A., Zhu, J.K. and Bohnert, H.J. (2000) Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology Plant Molecular Biology 51, 463-499.
- Hawkins, H.J. and Lewis, O.A.M. (1993) Effect of NaCl salinity, nitrogen form, calcium and potassium concentration on nitrogen uptake and kinetics in Triticum aestivum L. cv. gamtoos New Phytologist 124, 171-177.
- Hsiao, T.C. and Xu, L.K. (2000) Sensitivity of growth of roots versus leaves to water stress: biophysical analysis and relation to water transport. Journal of Experimental Botany 51, 1595-1616.
- Hoagland, D.R. and Arnon, D.I. (1950) The Water Culture Method for Growing Plants With out Soil. Circular, College of Agriculture University of California, Berkeley pp 347.
- Hu, Y., Oertli, J.J. and Schmidhalter, U. (1997) Interactive effects of salinity and macronutrient level on wheat: I. Growth. Journal Plant Nutrition 20,1155-1167.
- Ismail, A.M. (2003) Effect of salinity on physiological responses of selected lines/variety of wheat. Acta Agronomica 51, 1-9.
- Jeannette, S., Craig, R. and Lynch, J.P. (2002) Salinity tolerance of phaseolus species during germination and early seedling growth. Crop Science 42, 1584-1594.
- Kingsbury, R.W. and Epstein, E. (1984) Selection for salt-resistant spring wheat. Crop Science 24, 310-314.
- Munns, R. (1993) Physiological processes limiting plant growth in saline soil: some dogmas and hypotheses. Plant Cell Environment 16, 15-24.
- Munns, R. (2002) Comparative physiology of salt and water stress. Plant, Cell and Environment 25, 239-250.
- Munns, R. and Rawson, H.M. (1999) Effect of salinity on salt accumulation and reproductive development in the apical meristem of wheat and barley. Functional Plant Biology 26, 459-464.
- Munns, R. and James, R.A. (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253, 201-18.
- Munns, R. and Tester, M. (2008) Mechanisms of Salinity Tolerance. Annual Review of Plant Biology 59, 651-681.
- Munns, R. and Schachtman, D.P. and Condon, A.G. (1995) The significance of two-phase growth response to salinity in wheat and barley. Australian Journal of Plant Physiology 22, 561-569.
- Passioura, J.B. and Munns, R. (2000) Rapid environmental changes that affect leaf water status induce transient surges or pauses in leaf expansion rate. Australian Journal of Plant Physiology 27, 941-948.
- Rahnama, A., Munns, R., Poustini, K. and Watt, M.A. (2011) Screening method to identify genetic variation in root growth response to a salinity gradient. Journal of Experimental Botany 62, 69-77.
- Rajendran, K., Tester, M. and Roy, S.J. (2009) Quantifying the three main components of salinity tolerance in cereals. Plant, Cell and Environment 32, 237-249.
- Sadat-Noori, S.A. and McNeilly, T. (2000) Assessment of variability in salt tolerance based on seedling growth in Triticum durum Desf. Genetic Resources Crop Evolution 47, 285-291.
- Sayed, H.I. (1985) Diversity of salt tolerance in a germplasm collection of wheat (Triticum spp.) Theoretical Applied Genetics 69, 651-657.
- Shahzad, A. (2007) Biochemical markers for screening wheat for salt tolerance. Doctor of Philosophy in Soil Science, Institute of Soil and Environmental Sciences, University of Agriculture Faisalabad
- Tammam, A.A., Alhamd, M.F.A. and Hemeda, M.M. (2008) Study of salt tolerance in wheat (Triticum aestium L.) cultivar Banysoif 1. Australian Journal of Crop Science 1, 115-125.
- Termaat, A, and Munns, R. (1986) Use of concentrated macronutrient solutions to separate osmotic from NaCl-specific effects on plant growth. Australian Journal of Plant Physiology 13, 509-22.
- Tuna, A.L., Kaya, C., Higgs, D., Murillo-Amador, B., Aydemir, S. and Girgin, A.R. (2008) Silicon improves salinity tolerance in wheat plants. Environmental and Experimental Botany 62, 10-16.
- Wang, W.X., Vinocur, B., Shoseyov, O. and Altman, A. (2001) Biotechnology of plant osmotic stress tolerance: physiological and molecular considerations. Acta Horticulture 560, 285-292.
- Wang, W., Vinocur, B. and Altman, A. (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1-14.
- Wyn-Jones, R.G. (1981) Physiological processes limiting plant productivity, In: Johnson CB eds. Butterworths, London pp 271-292.
- Yeo, A.R. (1998) Molecular biology of salt tolerance in the context of whole-plant physiology. Journal of Experimental Botany 49, 915-929.
- Yeo, A.R., Lee, K.S., Izard, P., Boursier, P.J. and Flowers, T.J. (1991) Short and long-term effects of salinity on leaf growth in rice (Oryza sativa L.). Journal of Experimental Botany 42, 881-889.


