Трополон и его производные: структура, синтез, применение в медицине (обзор)
Автор: Ходакова Д.В., Гончарова А.С., Галина А.В., Гурова С.В., Головинов И.В., Шульга А.А., Камлык Д.В.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 5 т.24, 2025 года.
Бесплатный доступ
Актуальность. Трополоны и его производные принадлежат к семейству природных продуктов, содержащих трополоноидный мотив (уникальный циклогепта-2,4,6-триеноновый фрагмент), который представляет собой семичленное небензоидное ароматическое кольцо. С развитием органической химии было открыто и синтезировано большое количество новых соединений трополонового ряда. За последние 10 лет было опубликовано свыше 350 исследований, в которых использовались данные вещества. Цель исследования – обобщение опубликованных данных о структуре, методах синтеза и применении в разных областях медицины соединений трополонового ряда. Материал и методы. Проведен поиск литературы с использованием баз данных PubMed, eLibrary и Google Scholar по ключевым словам «tropolones», «chemical structure of tropolones», «synthesis of tropolones», «biological activity of tropolones». Из отобранных для изучения 535 источников литературы подробно было проанализировано 39 публикаций. В представленном обзоре рассматриваются результаты исследований за период с 1936 по 2024 г. Результаты. Рассматриваются структура широко распространенных трополонов и вопрос о химическом синтезе трополонов различными методами из шестии семичленных углеродных колец посредством реакций циклизации и циклоприсоединения, а также получение новых соединений при помощи конденсации трополона с другими функциональными группами. Результаты современных исследований, выполненных на культурах опухолевых клеток, различных видах бактерий и лабораторных животных, демонстрируют, что представители соединений трополонового ряда обладают широким спектром биологических эффектов, что делает их перспективными ведущими молекулами для разработки различных лекарственных средств. Трополоны проявляют свои свойства преимущественно посредством координации катионов в активных центрах металлоферментов. Авторы в своих работах предлагают различные пути и мишени их действия. Заключение. Трополоны обладают широким спектром биологической активности, включая противовирусную, противомикробную и противоопухолевую. Исследование зависимости «структура – активность» и накопление информации о химико-физических свойствах полученных веществ привели к разработке более эффективных методов синтеза и получению соединений трополонового ряда с улучшенной эффективностью и селективностью, что подтверждается в исследованиях как in vitro, так и in vivo. Однако точные механизм(ы) действия трополонов остаются неизвестными, поэтому требуются дальнейшие исследования в этом направлении.
Трополоны, химическая структура трополонов, синтез трополонов, биологическая активность, опухолевая культура клеток, модели in vivo
Короткий адрес: https://sciup.org/140312772
IDR: 140312772 | УДК: 616-006:547.572:615.31]-092.7 | DOI: 10.21294/1814-4861-2025-24-5-163-170
Текст научной статьи Трополон и его производные: структура, синтез, применение в медицине (обзор)
Tropolone and its derivatives are classified as alkaloids, defined as nitrogen-containing organic compounds of natural origin that can exhibit diverse chemical formulas and structures, physiological activities, and properties. The study of these compounds has made a significant contribution to the development of both theoretical organic chemistry and medical practice. Despite the wealth of knowledge amassed through previous research, the field continues to garner significant interest from the scientific community [1].
Tropolones contain a distinctive cyclohepta-2,4,6-trienone fragment in their structure, which consists of a seven-membered non-benzenoid aromatic ring and various side groups. The nomenclature of this group of substances was first introduced in 1945 by the Indian chemist M.D.S. Dewar [2]. His seminal research, which involved the chemical characterization of tropolone, made a substantial contribution to the advancement of the theoretical underpinnings of aromaticity in organic chemistry. This research also led to sustained interest among scientists in the study of biosynthetic pathways [3].
Tropolone-based structures have been identified in more than 200 natural compounds, ranging from simple tropolone derivatives to complex multicyclic systems such as pycnidione and pyrerubrin A. These structures are primarily synthesized by plants and fungi, and they play an antibacterial defense role in these organisms [4]. Extensive research has revealed that tropolones possess a multitude of biological activities, including antiviral, antioxidant, antiinflammatory, insecticidal, and antitumor properties, thus sustaining their scientific interest [1, 5].
This review delineates the chemical structure, synthesis, and biological activity of tropolone compounds, while concurrently presenting the latest advances in their use in oncology.
Results
The chemical nature of tropolones
Structure of the tropolone molecule
Tropolone (2-hydroxytropone, 2-oxy-cyclohepta-trien-2,4,6-one-1) is an aromatic compound of the non-benzenoid type. It consists of a simple structure consisting of a seven-membered carbon ring in which the pi electrons are delocalized (Fig. 1). The distance between the carbon atoms is 1.39 Å (comparable to that of benzene). The bond lengths between the atoms are 0.14 nm (C–C), 0.125 nm (C=O), and 0.134 nm (C–O), indicating the molecule’s asymmetry. The gross formula (molecular formula) of the substance isC7H6O2. A ketone group is attached to the first carbon atom, and a hydroxyl group is attached to the second. This configuration endows the compound with amphoteric properties. The compound exhibits the ability to accept a proton from acids and to form anions in the presence of alkali. It has been observed to form complex compounds with salts of divalent and trivalent metals [3].
Chemical structure of the well-known representatives of tropolones
One of the earliest identified tropolones was β-thujaplicin, also known as hinoktiol, which was isolated in 1936 by the Japanese chemist T. Nozoe from the wood of the Formosan cypress (C10H12O2) [6]. Subsequent studies revealed the presence of this compound in other species of trees belonging to the cypress family. From a chemical perspective, it is classified as 2-hydroxy-4-isopropylcyclohepta-2,4,6-trien-1-one, a monoterpenoid that possesses notable aromatic characteristics (Fig. 1). Two additional isomers of thujaplicin, α-thujaplicin and γ-thujaplicin, exist, distinguished by the positioning of the isopropyl group relative to the two oxygen atoms surrounding the aromatic ring [7].
Another notable and extensively studied tropolone is colchicine (C22H25NO6 – 5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzene α-heptalen-7-yl). Its initial isolation was conducted in 1820 by the renowned French chemists P.S. Pelletier and J.B. Caventou, who extracted it from the plant Colchicum autumnale (autumn crocus) [8]. Subsequently, in 1959, colchicine was synthesized by the pioneering Swiss organic chemist A. Eschenmoser [9]. Colchicine comprises three hexameric rings: an aromatic ring A, an aminated cycloheptadiene ring B, and a tropolone ring C. The latter is capable of binding to the tubulin molecule and inhibiting its ability to polymerize, that is, to form microtubules, which in turn leads to the cessation of cell division [3]. The anti-inflammatory effect of this substance is associated with this mechanism. The anti-inflammatory effect of colchicine is associated with the disruption of neutrophil microtubules, which hinders their migration, a process
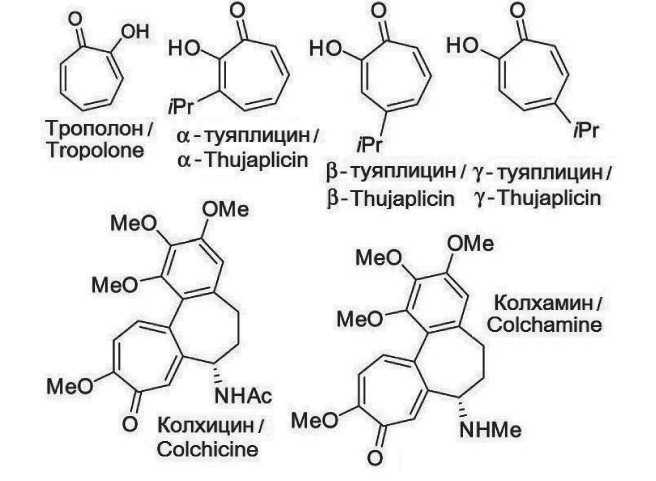
Fig. 1. Structural formula of tropolone and its derivatives. Note: created by the authors
Рис. 1. Структурная формула трополона и его производных. Примечание: рисунок выполнен авторами that is subject to chemotaxis. Consequently, colchicine impedes the migration of neutrophils to the lesion, thereby preventing the progression of inflammation. Beyond its anti-inflammatory properties, colchicine has also been observed to influence protein metabolism, the synthesis of membrane lipids, and the transport of amino acids through the cell membrane. Its therapeutic applications are evident in the domains of rheumatology, cardiology, dermatology, and neurology. However, the presence of serious side effects limits the range of its clinical application [9].
The alkaloid colchamine (C21H25NO5), which was first isolated in the USSR in 1952 by V.V. Kiselev, G.P. Menshikov and A.A. Beer from the bulbs of the magnificent crocus (Colchicum speciosum), is less toxic. A structural analysis reveals that colchamine contains three hexameric rings, similar to colchicine. However, it lacks an acetamide group (NH – COCH3) and instead possesses an aminomethyl group (NH – CH3). Both tropolones are characterized as finely crystalline powders of white or white-yellowish color and possess analogous pharmacological properties [10].
Methods of obtaining tropolones
As previously mentioned, tropolones are isolated from various plants and fungi. However, the limited availability of natural sources and the challenges associated with utilizing these compounds in their original, pure form have led to the development of methods for synthesizing tropolones and their analogues with enhanced efficiency and selectivity. These methods are designed to exhibit properties that are novel within this series [11].
The synthesis of tropolones and their derivatives can be achieved through biosynthesis. For instance, colchicine and its related alkaloids are formed by the method proposed by A.R. Battersby. These compounds are obtained from L-tyrosine and L-phenylalanine by a series of oxidation and rearrangement reactions mediated by CYP450 [12]. However, chemical synthesis of tropolones is more prevalent and can be carried out from commercially available six- and sevenmembered carbon rings, as well as other compounds, by various cyclization and cycloaddition reactions [13]. For instance, J.W. Cook has proposed a method for the synthesis of halogenated derivatives of tropone and tropolone. The reaction utilizes cycloheptanones, cycloheptenones, and cycloheptadienones as substrates. These compounds undergo halogenation (most often with bromine) followed by elimination [14]. In 1950, W.V.E. Doering and L.H. Knox reported a novel method for the preparation of tropolones and its derivatives by oxidation of cyclohepta-1,3,5-triene and various substituted cycloheptatrienes in the presence of potassium permanganate. However, the method was characterized by a low yield of the desired product, ranging from 4 % to 6 % [15]. Subsequent studies revealed that the oxidation of cycloheptatrienes with singlet oxygen results in the formation of isomeric endoperoxides, which undergo reduction to tropolones during the subsequent stage of the synthesis in the presence of thiourea [16]. In 2012, C. Meck et al. developed a method for synthesizing various types of polysubstituted α-hydroxytropolones (α-HT, compounds with two hydroxyl groups adjacent to the central ketone) [17, 18]. This method is based on the reaction of oxidopyrylium dipolar cycloaddition between an alkyne and a triflate salt, followed by ring opening, aromatization, and demethylation in the presence of boron trichloride.
D.V. Schiavone et al. (2022) described the synthesis of polyoxygenated tropolones, namely 3,7-dimethoxy (dMT) and 3,7-dihydroxytropolones (dHT). The initial step involves the cycloaddition reaction of iodalkynes and the oxidopyryl ylide dimer, yielding cycloadducts. Subsequent to the solvolysis of methanol in the presence of 4-dimethylaminopyridine, intermediate products are formed, which, subsequent to a ringopening reaction mediated by boron trichloride, yield dMT. Subsequent heating of the compounds in the presence of hydrogen bromide in acetic acid as catalysts leads to the formation of dHT. Utilizing a similar approach, the authors successfully obtained novel 3-hydroxy-7-methoxytropolones by substituting methanol with benzyl alcohol during solvolysis [11].
E.A. Gusakov et al. (2024) have thoroughly delineated the interaction of 4-chloro-2,7-dimethyl-1,8-naphthyridine and 4,6-di-tert-butyl-3-nitro-1,2-benzoquinone. In the presence of an acid, a reaction occurs between the two compounds, resulting in the expansion of the o-quinone ring to form 1,3-tropolone and bis-1,3-tropolones [19].
The employment of diverse methodologies, encompassing the modification of side chains or the condensation of tropolone with other functional groups, has culminated in the identification of numerous novel compounds exhibiting a variety of characteristics. The investigation of the structureactivity relationship, coupled with the accumulation of information concerning the chemical and physical properties of the obtained substances, has resulted in the development of more effective synthesis methods and the production of tropolone series compounds with enhanced efficiency and selectivity, thereby increasing their value for various drug development studies [11].
Biological activity of tropolones and their use in medicine
Antiviral activity
One potential medical application of tropolone compounds is the treatment of infectious diseases caused by various viruses.
Acquired immunodeficiency syndrome (AIDS) is a complex disease caused by the human immunodeficiency virus (HIV). The high morbidity and mortality rates, compounded by the absence of effective prevention methods, underscore the grave public health threat posed by AIDS. The limited efficacy of existing anti-retroviral drugs for AIDS therapy is a matter of significant concern, as it is compounded by the rapid emergence of drug-resistant HIV strains [20]. Consequently, there is an urgent need to explore new therapeutic options, and tropolones are considered a promising class of molecules for the treatment of this disease. Tropolones have been shown to target ribonuclease H (RNase H), a Mg2+-dependent enzyme that plays a pivotal role in the viral life cycle, responsible for the degradation of RNA from the RNA-DNA intermediate during replication. The ability of tropolones to bind metals leads to the chelation of the cations at the active center of RNase H, resulting in the loss of its enzymatic activity [21].
Hepatitis B virus (HBV) is carried hematogenously directly to the liver and multiplies in hepatocytes, causing liver damage and intoxication of the body (with or without jaundice (20-30 % of cases). In severe cases, it can lead to cirrhosis and liver cancer (hepatocellular carcinoma), from which, according to statistics, approximately 1.1 million people died in 2022 [22]. Like HIV, HBV has its own RNase H, which is also necessary for it to degrade viral RNA to facilitate the formation of double-stranded DNA, which can be inhibited by various tropolone compounds. In the work of M.E. Woodson et al. (2023), 36 structurally diverse tropolones from 6 subclasses (α-HT, sulfur-based α-HT, amide-attached α-hydroxytropolones (amide α-HT), 3,7-methoxyhydroxytropolones (MHT), dMT, dHT) on HepDES19, a HepG2-derived cell line that carries a stably transfected HBV genome. 22 tested compounds inhibited HBV replication with EC50 values < 5 μM. The most effective were α-HT and amide α-HT [23].
D.V. Schiavone et al. (2022) also evaluated the synthesized tropolones mentioned above for their antiviral activity on the HepDES19 culture. 3-hydroxy-
7-methoxytropolones showed the most effective effect (EC50 < 5 μM) compared to other compounds. In addition, the authors studied the suppression of the replication of the herpes simplex virus-1 (HSV-1) [13], which causes a disease in approximately two out of three people worldwide that cannot be completely cured [24]. According to the results of the work, among the synthesized tropolones, substances containing biphenyl ketone in their structure had significant activity [11]. In this case, two magnesium-dependent enzymes can serve as a target for tropolones: UL12 and UL15. The first isalkalinenuclease, which repairs genomic breaks and creates suitable DNA forms for encapsi-dation [25]. The second is thought to be responsible for cleavage and packaging of the viral genome into pre-assembled capsids [26].
Antimicrobial activity
Due to their potent biological activity and the gradual development of bacterial resistance, tropolo-nes and their derivatives possess considerable promise for biomedical applications. In a study by F. Cao et al. (2018), the antibacterial activity of 18 troponols, 26 tropones, and 48 α-HT at three concentrations (5.8, 20.4, and 71.4 μM) was investigated for four clinically significant bacterial species for humans: Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii. None of the compounds were found to inhibit the growth of Pseudomonas aeruginosa, and the studied substances demonstrated the most effective activity against S. aureus, with four tropolones, nine tropones, and six α-HT exhibiting minimum inhibitory concentration (MIC) values of 80 % (MIC80) of less than 20 μM [27]. One of the potential targets for tropolones in bacterial cells is CapF, a bifunctional metalloenzyme of S. aureus. CapF is required for the biosynthesis of capsular polysaccharide, the mucous layer that covers the bacterial surface, and functions in the presence of Zn2+ [28].
The data from the study of F. Cao et al. [27] are of great importance for medicine, since there is a problem of microorganism resistance to antibiotics, including tetracyclines – antimicrobial drugs used to treat infections caused by S. aureus. This fact, as well as the toxicity of tetracyclines, has limited their use in the clinic.
A study by C.Y. Le (2023) evaluated the antimicrobial efficacy of β-thujaplicin against several strains of S. aureus in comparison with various antibiotics used. Although tropolone demonstrated antibacterial activity on the studied strains, both sensitive and resistant to tetracycline, its MIC80 were higher than those of commercial drugs. From this we can conclude that β-thujaplicin can be used in combination with other antibiotics for more successful treatment of S. aureus infection, which was confirmed at the next stage of the work. With the combined effect of tropolone with tetracycline antibiotics, a synergistic interaction of the substances was observed, and the corresponding MIC80 values were several times lower than in the absence of β-thujaplicin. At the same time, the intracellular concentration of tetracycline increased by approximately 30 %. This effect may be due to the inhibition of the internal efflux pump of bacteria by tropolone, for example, Tet38, which is responsible for the removal of active and dangerous substances from the cell [29].
As described above, the biological effects of tropo-lone compounds are usually mediated by coordination of cations at the active sites of metalloenzymes, but the exact mechanism(s) of inhibition are unknown.
Prospects for the use of tropolones in oncology
Cancer is a multifactorial disease characterized by uncontrolled cell growth and has more than 200 different types. Despite the enormous progress in cancer treatment, it remains the second leading cause of death in the world after cardiovascular diseases [30]. The problem in cancer treatment is the ineffectiveness of chemotherapy, the acquisition of multiple drug resistance and the occurrence of relapses. Therefore, for many researchers and oncologists around the world, the task of finding promising candidates with antitumor efficacy is relevant [31].
One of the many biological activities of tropolones is antitumor. Colchicine has not found its application in the treatment of oncological diseases due to its high toxicity. Colchamine is used locally in ointments for skin cancer, and in combination with sarcolysin – internally for esophageal and gastric cancer [32]. However, side effects of known antitumor alkaloids remain, including damage to the peripheral nervous system, alopecia, tachycardia, arterial hypotension, hepatotoxicity, and others. Therefore, there is an active search, synthesis, and research of new derivatives of the tropolone series as potential antitumor agents.
In progress S.L. Haney et al. (2024) studied the α-substituted tropolone MO-OH-Nap in five human osteosarcoma cell lines (CAL-72, HOS, 143B, SaOS-2, MG-63). Results72-hour incubation with the tested tropolone demonstrated a dose-dependent effect on all cell lines studied, with SaOS-2 proving to be the least sensitive to therapy (IC50= 5.93 μM), and HOS was the most sensitive (IC50= 0.67 μM). The authors believe thatMO-OH-Nap induces activation of the unfolded protein response (UPR) pathway, followed by induction of caspase-dependent cell death [33]. In another study, Y.F. Chiang et al. (2024) evaluated the anticancer effect of hinokitiol at different concentrations (0, 1, 10, 50, 75 and 100 μM) and times (24, 48 and 72 h) on three breast cancer cell lines: MCF-7, T47D and MDA-MB-231. The results showed time-and dose-dependent suppression of tumor cell viability. When studying the potential mechanisms of the antiproliferative action of tropolone, scientists found that exposure to hinokitiol increased the expression of p53 and PARP proteins, which led to increased apoptosis in breast cancer cells [34]. M. Haas et al. (2022) studied the effectβ-thujaplicin on squamous cell carcinoma of the head and neck. The study was conducted on CAL27 and FADU cell lines with increasing doses of tropolone (from 2.5 to 40 μM). β-thujaplicin decreased cell viability in a dose-dependent manner (3IC values50were 15.1 μM for CAL27 and 8.5 μM for FADU), andsuppressed tumor cell migration in both lines. When studying the effect on cell proliferation, it was found that tropolone led to cell cycle arrest.in the S-phase at concentrations of 5 and 10 μM [35]. According to the results of other studies, β-thujaplicin is able to suppress homologous recombination and DNA methylation that occur during the S-phase, as well as cause DNA fragmentation, which ultimately leads to apoptosis [36].
The in vitro data obtained suggest that tropolones are promising candidates for further testing in vivo models as antitumor agents. This is confirmed in the work of E.F. Komarova et al. (2023), which was aimed at studying the possible mechanisms of the antitumor action of tropolones by assessing the expression level of immunohistochemical tumor markers. During the study, subcutaneous PDX models (Patient-Derived Xenograft) of human squamous cell lung cancer were orally administered 2-(6,8-dimethyl-5-nitro-4-chloro-quinolin-2-yl)-5,6,7-trichloro-1,3-tropolone. After 36 days from the start of the substance administration, the animals were euthanized, tumor tissue was isolated and the expression level of Ki67, Bcl2, b-catenin, connexin 32, connexin 43 and P53 was assessed. Analysis of the protein expression level showed a dose-dependent antitumor activity of the studied substance: with an increase in the dose of tropolone, the expression of Ki67, b-catenin, Bcl2 decreased and connexin 43, P53 increased. The data obtained indicate that a possible mechanism of the antitumor action of the studied tropolone is a decrease in the proliferative activity of tumor cells and activation of apoptosis [37]. In another


