Upregulation of Chloroplast Antioxidant System to Alleviate Salt Dependent Oxidative System in Rice Varieties
Автор: Lins S., Yusuf A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.16, 2020 года.
Бесплатный доступ
The chloroplast antioxidant system in pokkali (vytilla-2), kaipad (ezhome-1) and upland (koduvelliyan) rice varieties showed upregulation of enzymatic and non- enzymatic antioxidants under salt stress. The levels of hydrogen peroxide (H2O2), malondialdehyde (MDA) with respect to the lipid peroxidation of thylakoid membrane, non-enzymatic antioxidants like ascorbate (AsA), glutathione (GSH) content, enzymatic antioxidants- superoxide dismutase (SOD) and ascorbate-glutathione cycle enzymes viz, ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) were quantified using the isolated chloroplast fraction on the 7,14 and 21 days of NaCl treatment. Chlorophyll in the NaCl treated plants decreased concomitantly with an increase in NaCl concentration. The H2O2 content and lipid peroxidation, proportionate to MDA content and the enzymatic antioxidants of the chloroplast fraction upregulated during NaCl treatment. The specific activity of the chloroplast antioxidant and ascorbate-glutathione cycle enzymes (SOD, APX, GR, MDHAR and DHAR) showed salt dependant upregulation consistently with NaCl application. Salinity induced oxidative stress alleviation by the antioxidant system in the chloroplasts of these varieties indicate the effective cooperation between antioxidant and ascorbate-glutathione cycle enzymes and holds a vital role in combating the ROS generation, thus protecting the chloroplast in the aerobic environment.
Antioxidant enzymes, Ascorbate-glutathione cycle, Chloroplast, Salt tolerant rice
Короткий адрес: https://sciup.org/143173842
IDR: 143173842
Текст научной статьи Upregulation of Chloroplast Antioxidant System to Alleviate Salt Dependent Oxidative System in Rice Varieties
Soil salinity is one of the most trenchant factors limiting crop production. In the arid and semi-arid regions of the world, crop productivity has been affected badly due to soil salinity. Under saline environment, the bioenergetics of photosynthesis is affected owing to the imbalance in Na:K ratios. Rice is one of the most important cereal crops in the world; however, it is very sensitive to salt stress and listed as the most saltsensitive cereal crop (Munns et al ., 2016) and the grain yield is more affected by salt stress than vegetative growth (Rao et al ., 2008). Increased soil salinity reduces water and nutrient uptake in rice plants leading to osmotic stress, ion toxicity, nutrient imbalance and water deficit resulting in reduced yield and restraining biomass accumulation (Singh et al ., 2018). Hyper-concentrations of salt also harm photosynthetically active leaves leading to chlorosis and early leaf senescence (Hanin et al ., 2016). Chloroplasts are the active centres of photosynthesis and generate a higher quantity of R S, during photosynthesis and respiration; however, the chloroplast antioxidant system in rice is not studied so far.
R S dependent oxidative stress leads to an increase in the levels of singlet oxygen (1 2); superoxide anion ( 2-), hydrogen peroxide (H2 2); and the hydroxyl ( H-), hydroperoxyl (H -), peroxyl (R -) and alkoxyl (R -) radicals that exceed the plant’s ability to maintain cellular homeostasis, causing lipid peroxidation and damage to cell membranes, proteins and nucleic acids (Vranova et al., 2002). The presence of physiologically bivalent oxygen, the requirement of high reduction power, the danger of excessive photosynthetic electron energy makes chloroplasts soft targets for oxidative damage compared to other organelles in the plant’s system (Bier and Dietz, 2005). Biotic and abiotic stress initiate disturbances in cellular R S balance by enhanced R S generation or reduced R S scavenging (Steffens et al., 2013). In order to maintain optimal growth, it is essential to scavenge the over produced R S effectively (Wang et al., 2010). Plants are equipped with an array of enzymatic and non-enzymatic antioxidant to scavenge R S (Foyer and Noctor, 2003). The antioxidant enzymes, superoxide dismutase (S D; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6) and ascorbate-glutathione cycle enzymes like ascorbate peroxidase (APX; EC 1.11.1.11), glutathione reductase (GR; EC 1.6.4.2), dehydroascorbate reductase (DHAR; EC 1.8.5.1) and monodehydroascorbate reductase (MDHAR; EC 1.6.5.4) are among the enzymes that scavenge 2- and H2 2 in plant cells (Toivonen, 2004). The prevention of H- radical generation by S D, CAT and APX stands in the front line defense mechanism against oxidative stress (Mittler, 2002). S D initially converts two molecules of 2- into H2 2; excess H2 2 is then converted into monohydro ascorbate and H2 by APX or into 2- and H2 by CAT whenever cellular concentrations reach the micromolar or millimolar range, respectively (Gill and Tuteja, 2010), however, CAT has not been observed in chloroplasts, hence its role is not investigated in this study. Many studies were conducted on the responses of salinity induced oxidative stress in leaf and roots of rice, however, organelle specific R S scavenging in the leaf sub-cellular compartments under salt stress is not reported so far.
The present study aimed at the quantitative determination of generated chloroplast R S and subsequent scavenging mediated by the enzymatic and non-enzymatic antioxidants in three rice varieties, under salinity stress.
MATERIALS AND METHODS
Plant material and growth conditions
Three best performing salt-tolerant varieties ezhome-1 (kaipad), koduvelliyan (upland) and vytilla-2 (pokkali) from different agroecological zones were selected based on our previous studies. Twenty-one-day old seedlings were planted in pots containing soil mixture and submerged in tanks containing different concentrations of NaCl incremented with 25mM NaCl per day to attain a final concentration of 25-150mM in individual tanks. The pots containing the seedlings were placed in tanks with water up to the upper rim of the pots. Leaf samples were collected from the plants after 7,14 and 21 days of salt treatment and used for the isolation of chlorophyll and chloroplasts.
Chlorophyll content
Total chlorophyll content was measured according to Arnon (1949). The leaf tissue (0.05g) from each treatment was homogenised in 10ml 80% (v/v) chilled acetone and centrifuged at 250 x g for 10 min at 4ºC and the supernatant was collected. The procedure was repeated until the pellet became white in colour. The final volume was adjusted to 20 ml with 80% (v/v) acetone. The absorbance was read at 645 and 663nm against the blank (80% v/v acetone). The quantity of total chlorophyll, chl a and chl b were calculated using the equations;
Chl a = 0.0127 A 663 - 0.00269 A 645
Chl b = 0.0229 A 645 - 0.00468 A 663
Total chl = 0.0229 A 645 + 0.00802 A 663
Where, A 663 = Absorbance of sample at 663 nm
A 645 = Absorbance of sample at 645 nm
Isolation and purification of chloroplasts
Chloroplast was isolated from leaf samples collected from the salt-treated rice varieties during the 7th, 14th and 21st day of salt stress. Leaf samples were powdered in liquid nitrogen (LN 2 ) and homogenised in 10ml chloroplast isolation buffer (CIB) containing 0.3M sucrose, 50mM Na H (pH 7.8), 10mM NaCl, 5mM MgCl 2 and 0.1% BSA. The homogenate was filtered through two layers of muslin cloth and the filtrate was centrifuged at 370 x g for 10 minutes. The pellet was resuspended in 15ml of CIB (without BSA) and was centrifuged at 1000 x g for 10min. The crude chloroplast fraction was purified using 10-80% percoll gradient in a swinging bucket rotor at 2700 x g for 15min. The intactness of the chloroplasts was determined using ferricyanide reduction assay (Wood et al., 1966). Thylakoid membranes were obtained by resuspending the chloroplast pellet in 5ml 50mM Tricine (pH 8.0) and centrifuged at 2000 x g thrice for 5 minutes and resuspended in the tricine buffer (pH 8.0: Covello et al ., 1989).
Hydrogen peroxide
Hydrogen peroxide (H 2 2 ) content was determined according to the method proposed by Velikova et al. (2000). A standard curve was prepared using a known concentration of H 2 2 and the quantity was calculated. Lipid peroxidation
The isolated thylakoid membrane was used for determining lipid peroxidation by quantifying the quantity of malondialdehyde (MDA, Ɛ= 155mM-1 cm-1), a product of lipid peroxidation (Stewart and Bowley, 1980). AsA content
Ascorbic acid (AsA) content was determined using the method explained by Chen and Wang (2002). A standard curve was prepared using gradient concentrations of AsA at an D of 525nm.
GSH content
The quantity of total GSH and the reduced glutathione were determined using previously described methods (Srivastava et al ., 1968). Leaf samples (0.5g) were macerated in 4 ml 5% (v/v) trichloroacetic acid and centrifuged at 11,000 x g at 4ºC for 15 min. Supernatant (1ml) was mixed with 2.5ml 100mM phosphate buffer (pH 7.7) and 0.2ml 0.6mM DTNB. The D was measured at 412nm and the GSH was quantified. Total GSH was assayed using 1ml supernatant, 100mM phosphate buffer (pH 7.7), 0.6mM DTNB (0.2ml) along with 1U glutathione reductase. Quantity of the oxidised glutathione (GSSG) was calculated using the formula: total GSH – reduced GSH.
Enzyme Assays
Superoxide dismutase (S D, EC 1.15.1.1) activity was measured according to the modified protocol of Giannopolitis and Ries (1977). ne unit S D was calculated as the enzyme activity required for 50% photoreduction of nitrobluetetrazolium to blue formazan.
Ascorbate peroxidase (APX, EC 1.11.1.11) activity was measured according to Nakano and Asada (1981). The assay mixture contained 0.5mM ascorbate in phosphate buffer (1.66ml, pH 7.0), purified chloroplast fraction (0.08ml) and 2mM H 2 2. (0.26ml). Immediately after the addition of H 2 2, D was measured at 290nm for 2min at 15sec intervals and the specific activity was calculated and expressed as µmole mg protein-1 min -1.
Glutathione reductase (GR, EC 1.6.4.2) activity was determined using the method of Smith et al . (1989). The reaction mixture contained 100mM potassium phosphate buffer (pH 7.6), 1mM EDTA, 5mM NADPH, 6mM 5.5’- dithio-bis (2-nitrobenzoic acid) [DTNB] and 0.2mM oxidised glutathione (GSSG) and 100µl purified chloroplast fraction. After the addition of NADPH dependant change in D was measured at 412nm for
2min at 14sec intervals. The specific activity was calculated and expressed as µmole mg protein-1 min-1.
Monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) activity was assayed according to Hossain et al . (1984). The reaction mixture contained Tris-HCl buffer (pH 8.0), 0.2mM NADH, 2.5mM ascorbic acid and 49.6µl purified chloroplast. The reaction was initiated by the addition of 0.4 units of ascorbate oxidase. Specific activity was determined by monitoring the oxidation of NADH at 340nm and expressed as µmole mg protein-1 min -1.
Dehydroascorbate reductase (DHAR, EC 1.8.5.1) activity was assayed following glutathione dependant generation of AsA at 265nm for one min in a 2ml reaction mixture containing phosphate buffer (1.4ml, pH 7.0), 20mM reduced glutathione (0.2ml) (GSH) in phosphate buffer (pH 7.0), 2mM (0.2ml) DHA and the chloroplast fraction (0.2ml) (Hossain et al ., 1984). ne unit DHAR activity was expressed as the quantity of enzyme required for the formation of 1.0µM ascorbate at 265nm.
Total protein
Total protein was quantified according to the protocol explained by Lowry et al. (1951), using BSA as standard and the absorbance was measured at 660nm using UV-VIS spectrophotometer. All the enzymatic and non-enzymatic assays were repeated thrice and the values were subjected to statistical analysis. As the highest expressions of R S, enzymatic and non-enzymatic antioxidants were observed during the 21st day of stress, these results are presented and the data obtained on the 7th and 14th day are presented as supplementary data.
RESULTS
Chlorophyll content
The chlorophyll content was affected by the increased concentration of NaCl in all three varieties (Fig. 1). Less chlorophyll damage was observed in vytilla-2, followed by ezhome-1 in 150mM NaCl.
H2O2 content and lipid peroxidation
Among the three varieties, the highest H 2 2 content was detected in the chloroplasts of upland varieties and the lowest quantity was observed in vytilla-2 treated with 150mM NaCl on the 21st day (Fig. 2.a). The increase in H 2 2 content in all the varieties compared to the control in 150mM NaCl on the 21st day was vytilla-2 (1.28 fold), ezhome-1 (1.88 fold) and koduvelliyan (1.8 fold).
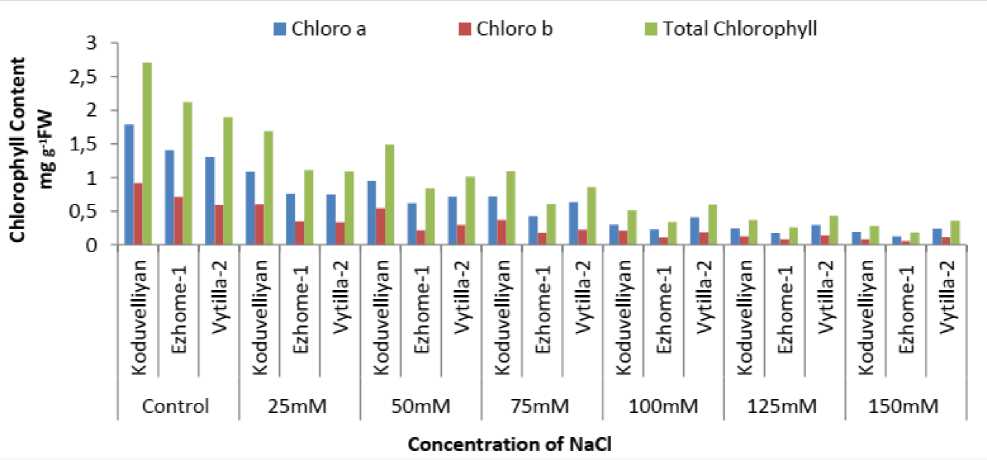
Figure 1: Chlorophyll a,b and total chlorophyll content in the leaves of koduvelliyan (upland), ezhome-1 (kaipad) and vytilla-2 (pokkali) varieties treated with different concentrations of NaCl on the 21st day.
Lipid peroxidation of the thylakoid membrane was under stress, showed similarity to the H2 2 content in measured as the quantity of malondialdehyde produced the control and the three varieties during salt stress (Fig.
2.b). The lipid peroxidation measured using the MDA level increased in vytilla-2 (1.27 fold), ezhome-1 (1.86 fold) and in the koduvelliyan (1.1 fold), in 150mM NaCl treatment on the 21st day.
AsA content in the chloroplast of the three varieties was consistent irrespective of the NaCl concentration and exposure period (Fig. 2.c). In the control the AsA content was higher in koduvelliyan variety followed by vytilla-2. From 50mM to 150mM NaCl the AsA content remained stable with higher AsA content in koduvelliyan and vytilla-2 varieties.
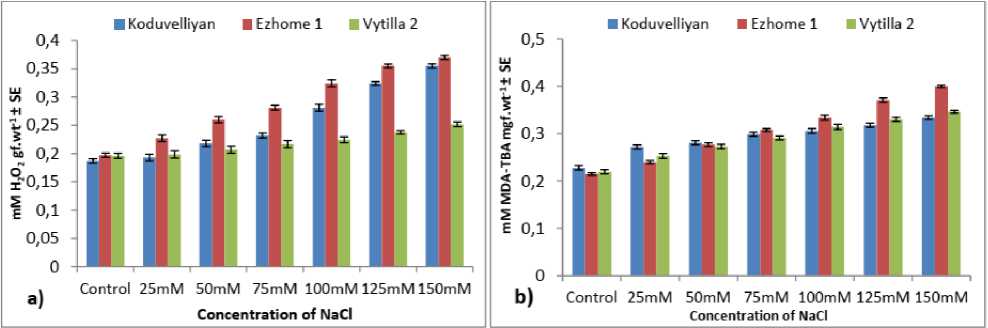
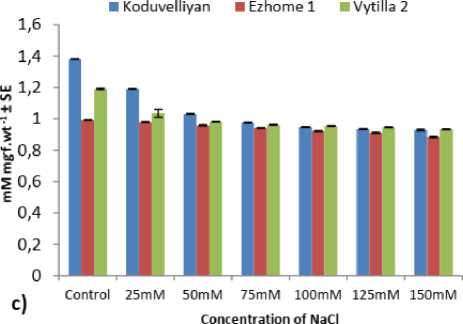
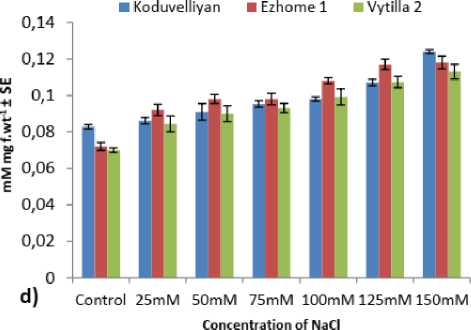
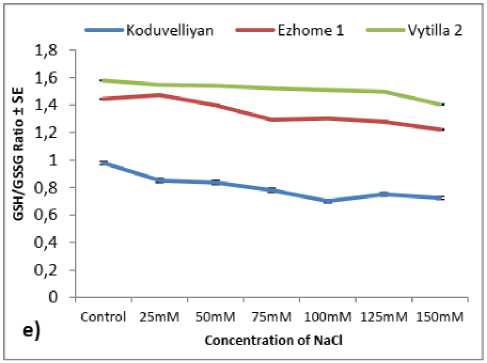
Figure 2: The quantity of a) Hydrogen peroxide, b) MDA, c) Ascorbate, d) Glutathione and e) GSH/GSSG ratio in the chloroplast fraction of koduvelliyan (upland), ezhome-1 (kaipad) and vytilla-2 (pokkali) varieties treated with different concentrations of NaCl on the 21st day.
The chloroplast GSH content increased steadily in all the three varieties upon NaCl treatment. Koduvelliyan variety showed the highest GSH content and in ezhome-1 the GSH content was at par with the koduvelliyan variety. GSH content in the ezhome-1 increased in 125mM NaCl, and decreased in 150mM NaCl (Fig. 2.d).
The redox potential determined by the GSH/GSSG ratio of the vytilla-2 and ezhome-1 chloroplasts had better redox potential with a higher GSH/GSSG ratio (Fig. 2.e). The redox potential of the koduvelliyan variety was much lower than the other two varieties, with a progressive increase in 150 mM NaCl treatment.
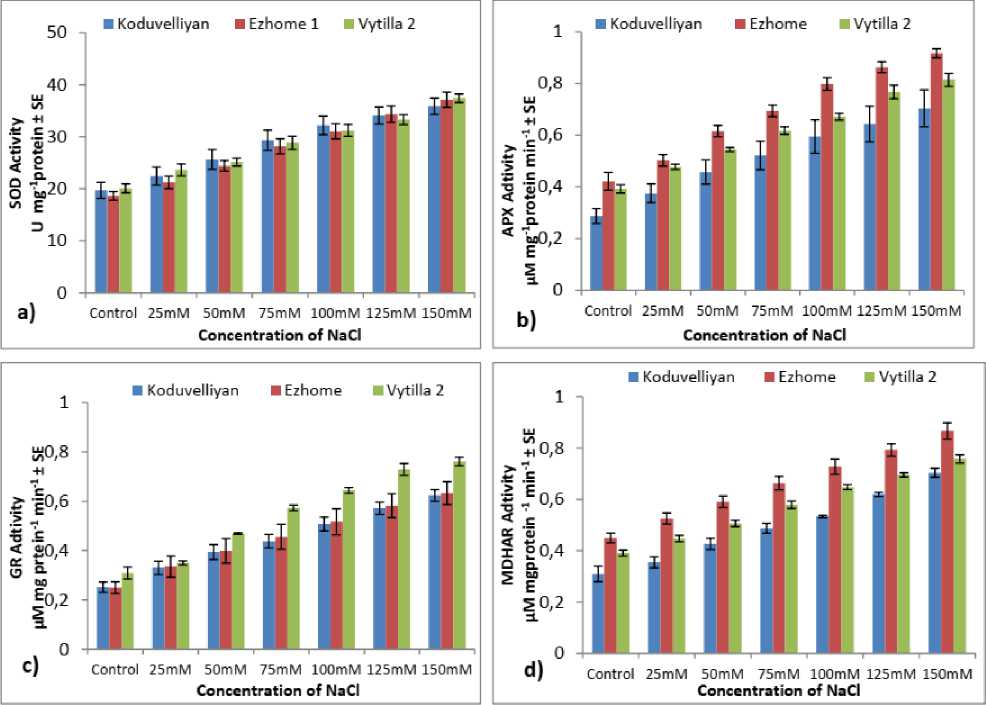
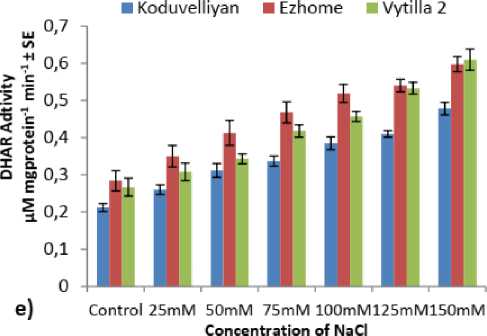
Figure 3: a) Superoxide dismutase, b) Ascorbate peroxidase, c) Glutathione reductase, d) Monodehydroascorbate reductase, e) Dehydroascorbate reductase activity in the chloroplast fraction of koduvelliyan (upland), ezhome-1 (kaipad) and vytilla-2 (pokkali) varieties treated with different concentrations of NaCl on the 21st day.
Enzymatic antioxidants
The S D activity in the chloroplasts of the three varieties increased with NaCl stress and the magnitude of upsurge varied with NaCl concentration and exposure period (Fig. 3.a). At the highest salinity level (150mM NaCl), the S D activity in all the three varieties increased two-fold compared to non-stressed plants. Higher S D activity in all the NaCl concentrations was expressed in vytilla-2 (37.42 U/mg protein) followed by ezhome-1 (37.12 U/mg protein) and in koduvelliyan (35.87 U/mg protein). Salt treatment markedly increased the S D activity in all the varieties.
Chloroplast APX specific activity increased significantly in all the three varieties in NaCl concentrations during the 7th, 14th and 21st days of stress (Fig. 3.b). In 150mM NaCl, ezhome-1 showed higher APX activity (0.917 µMmg-1min-1), than in the control. An increase in NaCl concentration synergistically increased the APX activity and the exposure period affected the level of expression.
The chloroplast GR specific activity significantly increased in all three varieties (Fig. 3.c). The increase in GR activity was dependent on the stress intensity and duration in all the varieties. The GR activity was in the decreasing order in vytilla-2 (0.23 µMmg-1min-1 in control and 0.761 µMmg-1min-1 in 150mM NaCl) followed by koduvelliyan (0.183 µMmg-1min-1 in control and 0.623 µMmg-1 min-1 in 150mM NaCl) and ezhome-1 (0.168 µMmg-1min-1 in control and 0.663 µMmg-1min-1 in 150mM NaCl). The GR activity in vytilla-2 showed a threefold increase at 150mM NaCl during the 21st day, whereas, it was approximately two fold in koduvelliyan and ezhome-1 in the same concentration of NaCl.
The specific activity of chloroplast MDHAR in all the three varieties increased with increase in NaCl concentration and exposure period. In 150mM NaCl, higher MDHAR activity was observed in ezhome-1 (Fig. 3.d), with a two fold increase in enzyme activity compared to control. The other two varieties showed an increase in the MDHAR activity with respect to NaCl concentration compared to control. The DHAR activity was uniform in the control as well as NaCl stress (Fig. 2.e). In ezhome-1 and vytilla-2 the DHAR activity increased two fold (Fig. 3.e) in 150mM NaCl on 21st day compared to the control. In the koduvelliyan variety also the DHAR specific activity increased proportionate with the NaCl concentration.
DISCUSSION
Soil salinity affects approximately 20% of irrigated land and reduces crop yield significantly. The physiological responses of plants to salinity are often complex and multi-faceted, which makes experiments difficult to design and interpret (Qadir et al., 2014). The varieties used for the present study are, a released salt tolerant pokkali variety (vytilla-2), a traditional cultivar grown in salt affected ‘kaipad system’ (ezhome-1) and an upland farmer variety (koduvelliyan), whose salt tolerance potential is unknown; selected by screening nine pokkali, three kaipad and three upland varieties compared to a salt susceptible variety IR-28 through enzymatic and non-enzymatic antioxidants (Lins and Yusuf, 2020). Chloroplasts are the major organelle responsible for increased metabolic activity and increased electron flow leading to the generation of R S, facilitated by triplet chlorophyll and electron transport chain in PSI and PSII. In addition to inducing oxidative stress, salt stress alters several physiological mechanisms causing an osmotic and ionic imbalance (Hasegawa, 2000). The upregulation of chloroplast enzymatic and non-enzymatic antioxidant system in these rice varieties provide a potential clue for their salt tolerance. The intactness of the chloroplasts determined using ferricyanide assay shows a decline in the total chlorophyll content under salt stress, reaffirms the reduction in the photosystem II activity, however, better recovery of the intact chloroplasts in the salt- treated rice varieties suggest their survival potential under salt stress, though chloroplasts are the major R S production centre in plants. The effective scavenging of R S determine the survivability of salt- tolerant rice, however, the chloroplast antioxidant system and their expression levels are not characterised in these rice varieties. Chl a and chl b levels are reduced drastically under salt stress in all these varieties, attributed to the inhibition of chlorophyll synthesis coupled with chlorophyllase activity under oxidative stress (Hassan et al., 2014). The increased total chlorophyll content in the upland variety compared to other tolerant varieties suggest their inherent chloroplast protection capacity and the reduction in chlorophyll pigments correlated to the accumulation of Na+ in the leaf tissues that may inhibit the strength of firm binding of complex compartments of the chlorophyll together. The reduction in photosynthetic efficiency, compounded by the deficient C 2 absorption contributes to the slow growth of plants under salt stress (Kohan et al., 2018).
The H2 2 content in the chloroplast of the pokkali variety exhibited a progressive increase under stress. In the koduvelliyan and ezhome-1, the increase was twofold corroborating the earlier reports on increase in H2 2 under salt stress. H2 2 act as a potent inhibitor of photosynthesis and the survival of salt-tolerant varieties under salt stress is coordinated by the efficient detoxification of H2 2. Salt tolerant varieties perceive H2 2 as a stress signal during the early stages of stress and upregulate efficient H2 2 detoxification mechanisms (enzymatic and non-enzymatic antioxidant) to decrease the H2 2 levels on signal completion (Bose et al., 2014), though there is a possibility of chloroplastic H2 2 leakage, due to the liquid phase isolation of chloroplasts. It may be speculated that the measured quantity of H2 2 in the membranes won’t provide the exact measure of H2 2 in the membranes as H2 2 will readily cross the membranes and the values obtained will not reflect the exact quantitative measures. In the case of the salt tolerant rice, the salt dependant chloroplast H2 2 content reflect the in vivo qualitative levels not the quantitative level has borne by the incidences of salt stress reported in the chloroplasts of tomato and pea (Mittova et al., 2002b; Gomez et al., 1999). It has been suggested that the channelisation of H2 2 across the membrane is mediated by specific aquaporin proteins (Bienert et al., 2006).The chloroplast MDA content, though an indirect method for determining lipid peroxidation, increased in ezhome-1 and reduced in the other two varieties, similar results were observed in vivo in other plant species (Abdelzawed, 2016). The irregularities in electrochemical Mehler’s reaction under salt stress resulted in the generation of R S and membrane disorganisation leading to the production of H2 2 and MDA (Chawla et al., 2013). Lipid hydroperoxides are produced by the oxidation of unsaturated fatty acids by the R S can be deternined by the MDA content in the salt-tolerant varieties is attributed to the higher H2 2 levels and the downregulation of the antioxidant system as observed in tomato (Mittova et al., 2002). The higher inherent chloroplast H2 2 and MDA in the salt-tolerant varieties, under control conditions, suggest that the upregulation of the enzymatic antioxidants is controlled by the salt dependent activation of the antioxidant system.
The maintenance of steady-state AsA in the chloroplast of these varieties indicating their salt tolerance potential as the AsA level is correlated with R S scavenging and higher Na+ accumulation in many plants (Akram et al ., 2007, Abdelzawed, 2016). The increased GSH content in the chloroplasts indicates the modulation of the photosystem II, thereby increasing the efficiency of these varieties under salt stress corroborating the results obtained in salt-stressed tomato (Cui et al 2018). The salt induced damage to the thylakoid membrane is alleviated by the increased GSH level and its redox state. The exogenous application of GSH protected epidermal cells in onion, suggesting the survival of plant cells under oxidative stress (Hoque et al . 2008). The higher GSH/GSSG ratio in the koduvelliyan variety suggests the existence of an operational redox buffering mechanism in this variety and the higher redox potential created under increased salt stress offers better stability for the cellular membranes as reported in Solanum lycopersicum cv. Rheinlands Ruhm (Poór et al ., 2019).
The elevated chloroplast H2 2 and MDA in the salt-tolerant varieties during the prolonged exposure to higher NaCl concentrations are proportionate with the S D and APX activities. The highly stable R S, H2 2 serves as a signalling molecule that plays a crucial role in environmental stress responses (Mittler et al., 2011 Suzuki et al., 2012), produced by superoxide dismutase (S D) by scavenging the superoxide and its by-products. Among the three varieties, the highest specific activity of chloroplast S D was observed in the salt-tolerant vytilla-2 followed by ezhome-1 and koduvelliyan. The higher chloroplast R S produced under salt stress in the salt-tolerant varieties is proportionate with the S D specific activity incongruent with the results obtained in salt tolerant rice (Rosatto, 2017; Hervera et al., 2018). The constitutive operation of water-water cycle in chloroplasts, instantly scavenges R S from their site of production (Asada 1999), prior to their interaction with the target molecules and the regeneration of oxidized ascorbate and glutathione by the action of enzymatic antioxidants, including S D, APX and ascorbate generating enzymes like MDHAR, DHAR, and GR (Lee et al., 2007). The S D activity also protects many vital organelles from serious superoxide mediated damage with a key role in the physico-chemical processes and prevents damages to cell membranes. Reduction in oxidative damage due to increased S D activity under higher salt concentrations was observed in many plants (Candan and Tarhan, 2003; Zhao et al., 2006).
APX is the major enzyme responsible for H 2 2 detoxification in the chloroplast mediated by AsA and two chloroplast genes that predominantly assists in antioxidant signaling (Teixeira et al ., 2006). The chloroplast of the three rice varieties maintained an increased APX activity, with the highest activity in the kaipad variety, this increase in GR activity in the chloroplasts of the rice varieties affirms its role in establishing the reduced status of GSH, in the ascorbate-glutathione cycle and also providing the sulfhydryl groups to act as a substrate for glutathione-s-transferase (Yousuf et al ., 2012). The removal of photosynthetically evolved H 2 2 , in the chloroplast is assisted by GSH and glutathione reductase, mediated by ascorbate and ascorbate peroxidase. The MDHAR and DHAR activities under stress regulate the reduced AsA content and their correlation with DHAR and MDHAR activities in the chloroplast fraction of the salt-tolerant varieties suggest the role of upregulated enzymes in maintaining the AsA content as reported in tobacco (Chang et al ., 2017) Similar results were obtained in the pokkali variety (Hossain et al ., 2015), however, chloroplast MDHAR and DHAR activities showed a coordinated increase in the salt-tolerant varieties used in the present study. Thus the redox status maintained by the activities of AsA and ensued H 2 2 detoxification plays a prominent role in the salt tolerance mechanism of the selected varieties contributed by the redox homeostasis and the coactivation of AsA-GSH cycle enzymes in the salt-tolerant rice varieties.
The GR activity reduces the oxidised glutathione (GSSG) into GSH maintaining the redox homeostasis in the chloroplasts. The upregulation and the coordinated activity of chloroplast APX, MDHAR, DHAR, and GR, played vital roles in oxidative stress alleviation in the rice varieties in synchrony with the results obtained in other plant species (Chao et al ., 2010; Anjum et al ., 2011).
CONCLUSION
In conclusion, our results clearly illustrate the role of the chloroplast antioxidant system in scavenging the reactive oxygen species and the upregulation of the chloroplast antioxidant defense system against salinity stress in the rice varieties and impart knowledge regarding the mechanism involved in effective protection from oxidative stress.
ACKNOWLEDGEMENTS
The authors are grateful to the Director, Interuniversity Centre for Plant Biotechnology for providing facilities and support. Financial support from Kerala State Council for Science Technology and Environment for this study under the SRS scheme No. 001/SRSLS/2013/CSTE is acknowledged.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interests.
Список литературы Upregulation of Chloroplast Antioxidant System to Alleviate Salt Dependent Oxidative System in Rice Varieties
- Abdeljawad, S. M., Zaqoot, H. A., Aish, A. M. (2016) Evaluation of Small and Large Scale Reverse Osmosis Desalination Plants Performance in the Gaza Strip During 2013. Nature Environment and Pollution Technology, 15(2), 693.
- Akram, M., Malik, M.A., Ashraf, M.Y., Saleem, M. F. and Hussain, M. (2007) Competitive seedling growth and K+/Na+ ratio in different maize (Zea mays l.) Hybrids under salinity stress. Pak. J. Bot. 39(7), 2553-2563.
- Anjum, S.A., Xie, X.Y., Wang, L.C., Saleem, M.F., Man, C., Lei, W. (2011) Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research, 6(9), 2026-2032.
- Arnon, D.I. (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiology, 24(1), 1.
- Asada, K. (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual review of plant biology, 50(1), 601-639.
- Baier M., Dietz K-J. (2005) Chloroplasts as source and target of cellular redox regulation: a discussion on chloroplast redox signals in the context of plant physiology. Journal of Experimental Botany, 56 (416), 1449–1462.
- Bienert, G.P., Schjoerring J. K.,Jahn T.P. (2006) Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1758(8), 994-1003.
- Bose, J., Rodrigo-Moreno, A., Shabala, S. (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 65, 1241–1257.
- Candan, N., Tarhan, L. (2003) Changes in chlorophyllcarotenoid contents, antioxidant enzyme activities and lipid peroxidation levels in Zn-stressed Mentha pulegium. Turkish Journal of Chemistry, 27: 21-30.
- Chao, Y.Y., Hong, C.Y., Kao, C.H. (2010) The decline in ascorbic acid content is as-sociated with cadmium toxicity of rice seedlings. Plant Physiol. Biochem, 48, 374–381.
- Chawla, S., Naraghi, M., Davoudi, A. (2013) Effect of twist and porosity on the electrical conductivity of carbon nanofiber yarns. Nanotechnology, 24(25), 255708.
- Chang, L., Sun, H., Yang, H., Wang, X., Su, Z., Chen, F., Wei, W. (2017) Over-expression of dehydroascorbate reductase enhances oxidative stress tolerance in tobacco. Electronic Journal of Biotechnology, 25, 1-8.
- Chen, J. X., Wang, X. F. (2002) Guide to plant physiological experiments. Guange hou: South China University of Technology Press, pp.123-127.
- Covello, P. S., Chang, A., Dumbroff, E. B., Thompson, J. E. (1989) Inhibition of photosystem II precedes thylakoid membrane lipid peroxidation in bisulfitetreated leaves of Phaseolus vulgaris. Plant physiology, 90(4), 1492-1497.
- Cui, J., Jiang, N., Zhou, X., Hou, X., Yang, G., Meng, J., Luan, Y. (2018) Tomato MYB49 enhances resistance to Phytophthora infestation and tolerance to water deficit and salt stress. Planta, 248(6), 1487-1503.
- Foyer, C. H., Noctor, G. (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiologia plantarum, 119(3), 355-364.
- Giannopolitis, C.N., Reis, S.K. (1977) Superoxide dismutases: II. Purification and quantitative relationship with water soluble protein in seedlings. Plant Physiol, 59: 315–318.
- Gill, S.S., Tuteja, N. (2010) Reactive oxygen species and antioxi-dant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry, 48, 909–930.
- Gomez J.M.. Hernandez J.A.,Jimenez A., del Rio L.,A., Sevilla F. (1999) Differential responses of antioxidant system of chloroplast and mitochondria to long- term NaCl stress on pea plants. Free Radicals Research, 31, 11-18.
- Hanin, M., Ebel, C., Ngom, M., Laplaze, L., Masmoudi, K. (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Frontiers in Plant Science, 7, 1787.
- Hasegawa, P.M., Bressan, R.A., Zhu, J. K., Bohnert, H.J. (2000) Plant cellular and molecular responses to high salinity. Annual review of plant biology, 51(1), 463-499.
- Hassan, M., Mansoor, S. (2014) Oxidative stress and antioxidant defense mechanism in mung bean seedlings after lead and cadmium treatments. Turkish Journal of Agriculture and Forestry, 38(1), 55-61.
- Hervera, A., De Virgiliis, F., Palmisano, I., Zhou, L., Tantardini, E., Kong, G., Kapustin, A. N. (2018) Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nature cell biology, 20(3), 307.
- Hoque, M.A., Banu, M.N., Nakamura, Y., Shimoishi, Y., Murata, Y. (2008) Proline and glycinebetaine enhance antioxidant and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. Plant Physiol., 165: 813–882.
- Hossain, Z., Mustafa, G., Komatsu, S. (2015) Plant responses to nanoparticle stress. Int. J. Mol. Sci., 16, 26644–26653.
- Hossain, M.A., Nakano, Y., Asada, K. (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol., 25: 385-395.
- Kohan-Baghkheirati, E., Bagherieh-Najjar, M., Abdolzadeh, A., Geisler-Lee, J. (2018) Altered DREB1A Gene Expression in Arabidopsis thaliana Leads to Change in Root Growth, Antioxidant Enzymes Activity, and Response to Salinity but Not to Cold. Journal of Genetic Resources, 4(2), 90-104.
- Lee, Y. P., Kim, S.H., Bang, J.W., Lee, H.S., Kwak, S.S., Kwon, S.Y. (2007) Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant cell reports, 26(5), 591-598.
- Lowry, Ο.Η., Rosebrough, Ν.J., Farr, A.L., Randall, R.J. (1951) Protein measurement with the folin phenol reagent. J. Biol. Chem, 193, 265-275.
- Mittler, R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Science, 7, 405–410.
- Mittler, R., Vanderauwera, S., Suzuki, N., Miller, G.A.D., Tognetti, V.B., Vandepoele, K., Van Breusegem, F. (2011) ROS signaling: the new wave?. Trends in plant science, 16(6), 300-309.
- Mittova, V., Tal, M., Volokita, M., Guy, M. (2002) Salt stress induces up‐regulation of an efficient chloroplast antioxidant system in the salt‐tolerant wild tomato species Lycopersicon pennellii but not in the cultivated species. Physiologia Plantarum, 115(3), 393-400.
- Munns, R., James, R. A., Gilliham, M., Flowers, T. J., Colmer, T.D. (2016) Tissue tolerance: an essential but elusive trait for salt-tolerant crops. Functional Plant Biology, 43(12), 1103- 1113.
- Nakano, Y., Asada, K. (1981) Hydrogen peroxide is scavenged by ascobate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol., 22: 867–880.
- Poór, P., Borbély, P., Czékus, Z., Takács, Z., Ördög, A., Popović, B., Tari, I. (2019) Comparison of changes in water status and photosynthetic parameters in wild type and abscisic aciddeficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. Journal of plant physiology, 232, 130-140.
- Qadir, M., Quillérou, E., Nangia, V., Murtaza, G., Singh, M., Thomas, R. J., Noble, A. D. (2014) Economics of salt‐induced land degradation and restoration. In Natural Resources Forum. 38(4), pp. 282-295.
- Rao, S.P., Mishra, B., Gupta, S.R., Rathore, A. (2008) Reproductive stage tolerance to salinity and alkalinity stresses in rice genotypes. Plant Breeding, 127: 256–261.
- Rossatto, T., do Amaral, M. N., Benitez, L. C., Vighi, I. L., Braga, E. J. B., de Magalhaes Júnior, A. M., da Silva Pinto, L. (2017) Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiology and Molecular Biology of Plants, 23(4), 865-875.
- Singh, A.K. (2018) The physiology of salt tolerance in four genotypes of chickpea during germination. Smith, I. K., Vierheller, T.L., Thorne, C. A. (1989) Properties and functions of glutathione reductase in plants. Physiologia Plantarum, 77(3), 449-456.
- Srivastava, S. K., Beutler, E. (1968) Accurate measurement of oxidized glutathione content of human, rabbit, and rat red blood cells and tissues. Analytical biochemistry, 25, 70-76.
- Steffens, B., Steffen-Heins, A., Sauter, M. (2013) Reactive oxygen species mediate growth and death in submerged plants. Front. Plant Sci., 4: 179.
- Stewart, R. R., Bewley, J. D. (1980) Lipid peroxidation associated with accelerated aging of soybean axes. Plant physiology, 65(2), 245-248.
- Suzuki, N., Miller, G., Sejima, H., Harper, J., Mittler, R. (2012) Enhanced seed production under prolonged heat stress conditions in Arabidopsis thaliana plants deficient in cytosolic ascorbate peroxidase 2. Journal of experimental botany. 64(1), 253-263.
- Teixeira, F. K., Menezes-Benavente, L., Galvão, V. C., Margis, R., Margis-Pinheiro, M. (2006) Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular compartments. Planta, 224(2), 300.
- Toivonen, P. M. (2004) Postharvest storage procedures and oxidative stress. Hort. Science. 39(5), 938-942.
- Velikova, V., Yordanov, I. and Edreva, A.(2000) Oxidative Stress and Some Antioxidant Systems in Acid RainTreated Bean Plants: Protective Role of Exogenous Polyamines. Plant Science. 151, 59-66.
- Vranová, E., Inzé, D., Van Breusegem, F. (2002) Signal transduction during oxidative stress. Journal of experimental botany, 53(372), 1227-1236.
- Wang, G. P., Zhang, X. Y., Li, F., Luo, Y., Wang, W. (2010) Overaccumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica. 48(1), 117-126.
- Wood, P. M., Bhagavan, H. N., Crane, F. L. (1966) Requirement for plastoquinone A in the Hill reaction of isolated chloroplasts. Plant physiology, 41(4), 633-640.
- Yousuf, P. Y., Hakeem, K. U. R., Chandna, R., Ahmad, P. (2012) Role of glutathione reductase in plant abiotic stress. In Abiotic Stress Responses in Plants. Springer, New York, NY. pp. 149-158.
- Zhao, Y., Truhlar, D. G. (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. The Journal of chemical physics, 125(19), 194101.


