Using plant extracts for the micropropagation of buckwheat
Автор: Borovaya S.A., Boginskaya N.G., Klykov A.G.
Журнал: Овощи России @vegetables
Рубрика: Селекция, семеноводство и биотехнология растений
Статья в выпуске: 5 (73), 2023 года.
Бесплатный доступ
Background. Various plant hormones are used (cytokinins, auxins) to increase the regeneration efficiency and the net reproduction rate of buckwheat in vitro. However, the growth and development rates of plantlets have been noted to be low under these conditions. For this reason, search for the plant extracts that are able to stimulate the regenerative ability of plants is a promising direction of biotechnological research. Materials and methods. Aseptic single-node cuttings of common buckwheat plantlets (varieties Dikul and Izumrud) were grown on MS nutrient media with plant extracts from Fagopyrum esculentum and Reynoutria japonica (0.1, 0.5, and 1%) for 21 days. The following morphobiological paramaters of the plantlets were evaluated: plant height, the number of internodes, the number of leaves, leaf length, and the number and length of roots. Results. Dealcoholized aqueous solutions of the extracts from F. esculentum and R. japonica in the studied concentrations (0.1-1%) significantly stimulated the growth and development of the buckwheat plantlets increasing their net reproduction rate (4.00-6.00) and rhizogenesis. The media with the plant extracts in concentrations of 0.1-0.5% were observed to produce the strongest positive effect. As the result, the morphobiological characteristics of the plantlets and the success rate of the micropropagation were the highest.
Fagopyrum esculentum moench, plant extracts, morphobiological parameters, in vitro
Короткий адрес: https://sciup.org/140301907
IDR: 140301907 | УДК: 633.12:573.6:632.437 | DOI: 10.18619/2072-9146-2023-5-37-42
Текст научной статьи Using plant extracts for the micropropagation of buckwheat
Поступила в редакцию: 02.06.2023
Принята к печати: 06.09.2023
Опубликована: 29.09.2023
Использование фитоэкстрактов для микроклонального размножения гречихи
Резюме
Актуальность. Для повышения эффективности регенерации и коэффициента размножения гречихи in vitro используют различные фитогормоны (цитокинины, ауксины). Однако при этом отмечаются невысокие темпы роста и развития регенерантов. Поэтому поиск фитоэкстрактов, способных стимулировать регенерационную способность растений, является перспективным направлением биотехнологических исследований.
Материалы и методы. На питательных средах МС с экстрактами растений Fagopyrum esculentum и Reynoutria japonica (0,1; 0,5 и 1%) в течение 21-х суток культивировали асептические одноузловые черенки регенерантов гречихи посевной сортов Дикуль и Изумруд. Оценку морфобиологических показателей микроклонов проводили по следующим показателям: высота растения, число междоузлий, число листьев, длина листовой пластинки, количество и длина корней.
Результаты. Добавление деалкоголизированных водных растворов экстрактов F. esculentum и R. japonica в питательную среду в диапазоне исследуемых концентраций 0,1-1% существенно стимулировало рост и развитие регенерантов гречихи, повышая коэффициент размножения (4,00-6,00) и ризогенез. Наибольший положительный эффект наблюдался на средах 0,1-0,5%-м содержанием фитоэкстрактов, где обнаружены максимальные значения морфобиологических характеристик и выхода черенков для микрокло-нального размножения.

Introduction urrently, various methods of plant micropropagation in vitro are gaining in recognition and popularity. An important step in the micropropagation is the selec tion of an optimal nutrient medium, which will enable the highest possible yield of experimental material for cultivation. This is especially relevant for cross-pollinated and rare plant species. Additionally, this method allows researchers to study the biological effect of plant extracts under laboratory conditions reducing the cost of field experiments.
Common buckwheat Fagopyrum esculentum Moench, 1794 is a species of herbaceous plants from the genus Fagopyrum of the family Polygonaceae . Preserving and multiplying valuable genotypes with the use of micropropagation methods is of considerable importance for this cross-pollinated crop [1]. According to А. Tomasiak et al. [2], buckwheat is sensitive to growing conditions in vitro , which is widely used for studying the regeneration of sprouts, the induction of callus formation, organogenesis, somatic embryogenesis, and the synthesis of phenolic compounds [3-5].
The methods of buckwheat tissue culture are believed to be well developed in fundamental and applied aspects [6]. Different variants of the Murashige and Scoog nutrient medium [7] supplemented with plant hormones, vitamins, and minerals [8-14] are employed for the biotechnological propagation of buckwheat. All the developed methods for the micropropagation of buckwheat allowed researchers to prevent callus induction in explants and obtain clones that were genetically identical to their parents. However, if regeneration is stimulated with a high concentration of hormones, which cause cell dedifferentiation and prolonged cultivation, the occurrence frequency of genetic modifications rises [6]. For this reason, search for the plant extracts that are able to stimulate the regenerative ability of plant organisms is a promising direction of biotechnological research because the effect on their genetic apparatus is eliminated in this case.
There are limited data on the use of plant extracts as components of nutrient media for plant micropropagation i n vitro. To increase the efficiency of this process, I.M. Fardzinova suggested to use an infusion of magnolia vine [15] and tropical chestnut [16], extracts from aloe [17], rose root [18], and Siberian ginseng [19]. Seaweed concentrate «Kelpak» (0.25%) was added to a culture medium for potato and improved the quality of plantlets [20]. I.Yu. Solokhina [21] recommends extracts from common oat, buckwheat, and Jerusalem artichoke for the micropropagation of cucumber.
There is a hypothesis that phenolic compounds, e.g. phenolcarboxylic acids, increase the efficiency of plant propagation at the stages of proliferation, root formation, and adaptation in vitro [22]. The potential sources of phenolic compounds, including flavonoids, are plants from the buckwheat family F. esculentum and Reynoutria japonica Houtt. For example, the aboveground parts of buckwheat plants belonging to varieties Pri 7 and Izumrud were determined to contain rutin in a concentration of 12.5-21.7 mg/g of the dry matter [23]. A study on the flavonoid composition of extracts from R. japonica leaves identified eleven flavonoid compounds; the total amount of flavonoids was 3.66% on average, the rutin content was 1.28% and the content of quercetin was 0.53% [24]. There is some evidence that a solution of R. japonica extract has a stimulating effect on the germination rate and seed vigor of Triticum aes-tivum L., Hordeum vulgare L. and Glycine max (L.) Merr. [25], Tagetes erecta [24]. F. esculentum extracts demonstrate growth-stimulating activity during the germination of buckwheat seeds [26]. Thus, bioactive compounds contained in plant extracts, including flavonoids from the representatives of the buckwheat family, can produce growth-stimulating effect on test-tube buckwheat plantlets.
Our research goal was to study the effect of extracts from plants of the family Polygonaceae on the growth and development of buckwheat plantlets in vitro .
Materials and methods
Sterilizing the box, laboratory glassware, and instruments, preparing and autoclaving the nutrient media were performed according to generally accepted protocols [27].
Matured seeds of two common buckwheat varieties were used as starting explants. Variety Dikul was created in FSBSI “Federal Scientific Center of Lemunes and Groat Crops” and variety Izumrud was bred in FSBSI “Federal Scientific Center of Agricultural Biotechnology of the Far East named after A.K. Chaiki”. Previously reported methods [28] were employed to sterilize the plant material, introduce it into the in vitro culture, and obtain the needed quantity of plantlets for the experiment.
Preparing aqueous-alcoholic extracts from F. esculen-tum and R. japonica
Plant leaves were dried by the method of air drying in shade to achieve a moisture content of 12% and then ground to 1 mm fractions using a laboratory LZM mill. The extraction was performed employing a reflux reaction apparatus with C 2 H 5 OH 70% (t 90°С) and vacuum filtration; the obtained extracts were transferred into a volumetric flask. Before use, the necessary amount of the extracts was dealcoholized by evaporation until the smell of alcohol disappeared and reconstituted to the initial volume with distilled water.
Studying the growth-stimulating activity of the plant extracts for the micropropagation of buckwheat in vitro
The MS culture media were supplemented with the dealcoholized extracts from F. esculentum and R. japonica in a concentration of 1 ml, 5 ml, and 10 ml per 1 l (Table 1).
Table 1. Variants of the content of the extracts from F. esculentum and R. japonica in the MS nutrient medium Таблица 1. Варианты содержания экстрактов F. esculentum и R. japonica в питательной среде МС
|
Component of the nutrient medium |
Content of the extract in the nutrient medium, ml/L |
|||
|
Control |
0.1% |
0.5% |
1% |
|
|
MS1. Extract from F. esculentum |
- |
1 |
5 |
10 |
|
MS2. Extract from R. japonica |
- |
1 |
5 |
10 |
Statistical analysis
Microsoft Excel 2010 programms were used for data input, processing of the original data, and statistical analysis. Software Statistica 6 was used to perform single-factor dispersion analysis. The results are expressed as means ± standard deviation.
The nutrient medium without extracts served as the control variant. The morphobiological parameters of the plantlets (plant height, the number of internodes, the number of leaves, leaf length, and the number and length of roots) were determined on the 21 st day of cultivation. The length of roots was measured using a web cam on a stand and software IC Measure 1.0. The experiment was conducted with three repetitions.
Results and discussion
As the result of the conducted research, it was discovered that the nutrient media with the extracts had a stimu- lating effect on the development of the buckwheat plantlets already at the first stages of cultivation in vitro. The fast growth of microshoots and the appearance of leaves were observed (fig. 1).
The analysis of the obtained data showed that a significant positive effect was achieved on the MS1 media with the extract from F. esculentum on the 21 st day of cultivation. The morphobiological parameters of the plantlets were considerably higher in all experimental variants compared to the control (table 2).
The number of internodes is an important parameter determining the efficiency of micropropagation through the use of microcuttings. The media with the extracts increased their number up to 5.67-6.00 and the net reproduction coefficient was 4.67-5.67 exceeding the control by more than two times. The highest number of leaves and leaf length were noted in the variants with the use of the extracts.

Fig.1.BuckwheatplantletsofvarietyIzumrud on the nutrientmediawith the extractfrom
F.esculentum (MS1) on the 4th dayofcultivation
Рис.1.Микроклоны гречихи сорта Изумруд на питательных средах с экстрактом
F.esculentum(МС1) на 4-е сутки культивирования
The key moment in micropropagation is rhizogenesis in vitro . A well-developed root system produces a beneficial effect on the growth of test-tube plant-let. The root formation was improved in the variants with the use of the extracts, which facilitated an increase in the regenerative ability of the plantlets. Variety Izumrud had a higher number of roots than the control (by 4.5-5.5 times on average, 3.00-3.67 pcs.); the maximal observed root length was 35.1289.55 mm (table 2, fig. 2). Variety Dikul formed a lower number of shorter roots (2.003.33 pcs. on average, 14.8874.95 mm).
Table 2. Effect of the nutrient medium with the extract from F. esculentum (MS1) on the growth and development of buckwheat in vitro (on the 21st day of cultivation) Таблица 2. Влияние питательной среды с экстрактом F. esculentum(МС1) на рост и развитие гречихи invitro (21-е сутки культивирования)
|
Control |
2.30±1.13 |
3.33±2.08 |
5.00±3.6 |
6.33±4.73 |
0±0 |
0±0 |
2.66 |
|
0.1% |
11.03±2.22* |
6.00±2.00* |
8.00±1.00* |
13.67±1.53* |
2.00±1.0* |
59.97±16.01* |
5.67 |
|
0.5% |
13.90±4.01* |
6.00±2.65* |
9.00±4.58* |
14.33±2.08* |
3.33±2.08* |
74.95±49.67* |
5.00 |
|
1% |
4.33±0.45* |
5.67±0.57* |
8.67±1.53* |
8.67±2.52 |
2.00±1.73* |
14.88±13.89* |
5.67 |
Note. Differences are relevant at *р < 0.05 compared to the control.
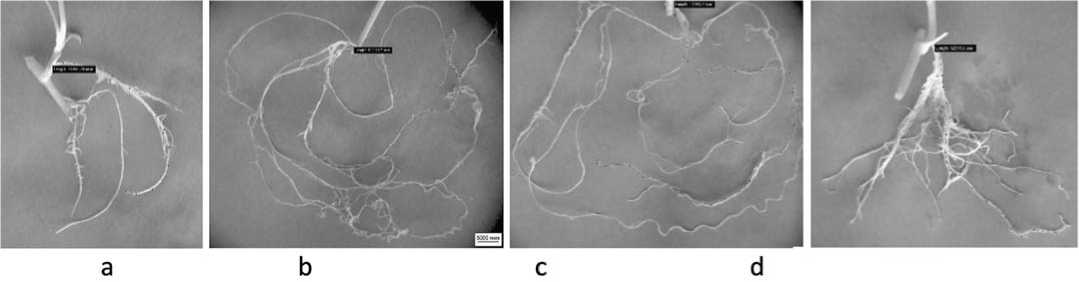
Fig.2.Rootdevelopmentin the buckwheatplantletsofvarietyIzumrud on the MS nutrientmediacontaining the extractfrom F.esculentum (а – control,b – 0.1%,c
Рис.2.Корнеобразование регенерантов гречихи сорта Изумруд на питательных средах МС1,содержащих экстракты F.esculentum (а – контроль; b
– 0.5%,d – 1%)
The strongest positive effect was observed on the media supplemented with the F. Esculentum extract in a concentration of 0.1-0.5%; the regeneration of plants was the most intensive in these variants. The morphobiological parameter values were as follows: plant height – 11.03-14.37 cm, the number of internodes – 5.67-6.00 pcs., the number of leaves – 7.33-9.00 pcs., leaf length – 12.00-14.33 mm, the number of roots – 2.00-3.33 pcs., root length – 59.97-89.55 mm, and the net reproduction rate – 5.00-5.67.
Our study on the regenerative ability of the buckwheat plantlets growing on the nutrient m edia with the extract from R. japonica (MS2) showed that the plant height was by 2.3-7.5 times higher in the experimental variants than in the control (11.27-17.27 cm on average) (table 3). The exception was the variant with variety Izumrud and the nutrient media with the extract in a concentration of 1%. No significant differences in the m orphobiological param eters from the
– 0,1%; c – 0,5%; d – 1%)
control group were detected in this case, probably, due to the fact that the high concentration of the active ingredient had a slight inhibitory effect on the growth of this variety.
The number of internodes (4.67-7.33 pcs.) and the net reproduction rate increased; the latter was 4.00-6.33 exceeding the control by 1.7-2.7 times. The extract did not affect the number of leaves significantly but the leaf length increased to 9.00-13.67 mm.
The medium supplemented with the extract considerably stimulated the rhizogenesis in the plantlets (table 3, fig. 3). Thus, the number and length of roots were higher (2.33-4.33 pcs. and 30.23-110.33 mm, respectively) on average than the control (0.00-0.65 pcs. and 0.00-12.31 mm, respectively).
The buckwheat plantlets developed better on the nutrient media with concentrations of the R. japonica extract within 0.1-0.5 %. The observed morphological parameter
Table 3. Effect of the nutrient medium with the extract from R. japonica (MS2) on the growth and development of buckwheat in vitro (on the 21st day of cultivation) Таблица 3. Влияние питательной среды с экстрактом R. japonica (МС2) на рост и развитие гречихи in vitro (21-е сутки культивирования)
|
Variant |
Plant height, cm |
Number of internodes, pcs. |
Number of leaves, pcs. |
Leaf length, mm |
Number of roots, pcs. |
Root length, mm |
Net reproduction rate |
|
variety Izumrud |
|||||||
|
Control |
7.46±2.32 |
4.00±0.58 |
6.33±0.57 |
8.67±3.21 |
0.65±0.60 |
12.31±12.00 |
2.33 |
|
0.1% |
17.27±1.79* |
7.00±1.00* |
7.33±0.58 |
13.33±0.58* |
3.67±0.58* |
110.33±42.14* |
6.33 |
|
0.5% |
17.23±1.94* |
7.33±1.15* |
8.33±3.06 |
13.67±3.21* |
4.33±2.89* |
92.65±35.54* |
6.00 |
|
1% |
6.70±4.46 |
4.67±0.58 |
7.00±1.00 |
12.00±2.65 |
3.67±1.53* |
30.23±23.41* |
4.00 |
|
variety Dikul |
|||||||
|
Control |
2.30±1.13 |
3.33±2.08 |
5.00±3.6 |
6.33±4.73 |
0±0 |
0±0 |
2.67 |
|
0.1% |
15.77±0.61* |
5.67±0.58* |
7.33±3.21 |
12.33±2.08* |
3.33±0.58* |
74.70±32.43* |
4.66 |
|
0.5% |
11.67±6.98* |
5.67±0.58* |
6.67±1.53 |
12.00±1.00* |
3.33±0.58* |
31.37±10.53* |
5.33 |
|
1% |
11.27±5.39* |
5.33±1.15* |
8.00±2.00 |
9.00±3.46* |
2.33±1.15* |
38.19±14.73* |
5.33 |
Note. Differences are relevant at *р < 0.05 compared to the control.
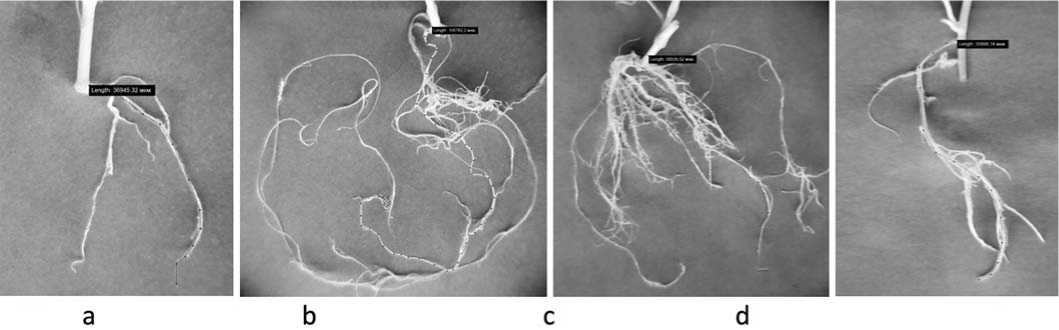
Fig.3.Rootdevelopmentin the buckwheatplantletsofvarietyIzumrud on the MS nutrientmediacontaining the extractfrom R.japonica (а – control,b – 0.1%,c – 0.5%,d – 1%) Рис.3.Корнеобразование регенерантов гречихи сорта Изумруд на питательных средах МС, содержащих экстракты рейнутрии японской (а – контроль; b – 0,1%; c – 0,5%; d – 1%)
values were the highest: plant height – 11.67-17.27 cm, the number of internodes – 5.67-7.33 pcs., leaf length – 12.0013.67 mm, the number of roots – 3.33-4.33 pcs., root length – 74.7-110.33 mm, and the net reproduction rate – 5.33-6.33.
Extracts from common buckwheat have been reported to produce a growth-stimulating effect by some authors. It was discovered that bioflavonoids from plant extracts were able to activate the growth of roots and shoots in potato under in vitro conditions on ½ В5 nutrient medium [29] and Gamborg’s medium [30]. A considerable growth rate of roots was noted in plantlets on a MS medium supplemented with this extract. According to B.A. Kapusina et al. [31], the addition of extracts from buckwheat and common oat to a culture medium might stimulate the growth and development of potato plantlets and decrease fungal infection.
Conclusions
-
1. The extracts from plants of the family Polygonaceae ( F. esculentum and R. japonica ) stimulated the growth and development of the buckwheat plantlets in vitro considerably increasing the net reproduction rate (up to 4.67-6.33) and facilitating the root development.
-
2. The increase in the concentration of the extract to 1% in the nutrient medium did not produce a significantly stronger effect.
Aboutthe Authors:
Svetlana A. Borovaya – Postgraduate Student, Researcher, the Laboratory of Breeding and Genetic Research on Field Crops, ,
Natalia G. Boginskaya – Junior Researcher, the Laboratory of Breeding and Genetic Research on Field Crops, ,
Aleksei G. Klykov – Doc. Sci. (Biology),
Academician of RAS,
Head of the Department of the Breeding
and Biotechnology of Agricultural Crops, ,
Об авторах:
University. 6 p. (In Russ.)
Список литературы Using plant extracts for the micropropagation of buckwheat
- Neskovic M., Srejovic V. Vujicic R. Buckwheat (Fagopyrum esculentum Moench). Cropsi Berlin. 1986:579-596.
- Tomasiak А., Zhou М., BetekhtinА. Buckwheat in Tissue Culture Research: Current Status and Future Perspectives. International Journal of Molecular Sciences. 2022;23(4):2298. https://doi.org/10.3390/ijms23042298
- Tumova L., Píchová M., Dušek J. Fagopyrum esculentum in vitro. Ceska Slov Farm. 2007;56(3):125-128.
- Барсукова Е.Н., Клыков А.Г., Чайкина Е.Л. Использование метода культуры ткани для создания новых форм Fagopyrum esculentum Moench. Российская сельскохозяйственная наука. 2019;(5):3-6. https://doi.org/10.31857/S2500-2627201953-6
- Borovaya S.A. Study of the effect of selective media with high zinc doses on the survival, growth and development of common buckwheat in vitro. Plants. 2022;(11):264. https://doi.org/10.3390/plants11030264
- Suvorova G. Buckwheat Tissue Cultures and Genetic Transformation. In: Molecular breeding and nutritional aspects of buckwheat; Zhou M. et al., Eds. Elsevier, Academic Press: London. 2016;365-375. https://doi.org/10.1016/B978-0-12-803692-1.00029-8
- Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tussue cultures. Physiologia Plantarum. 1962;(15):473- 497. https://doi.org/10.1098/rstb.2000.0713
- Takahata Y. Plant regeneration from cultured immature inflorescence of Common Buckwheat (Fagopyrum esculentum Moench) and Perennial Buckwheat (F. cymosum Meisn.). Japan J. Breed. 1988;38(4):409-413.
- Румянцева Н.И., Сергеева Н.В., Хакимова Л.Э. и др. Органогенез и соматический эмбриогенез в культуре двух видов гречихи. Физиология растений. 1989;36(1):187-194. EDN DJWCXS.
- Румянцева Н.И. Морфогенез в культуре тканей гречихи: теоретические и прикладные аспекты. Дис. … канд. биол. наук. Казань. 1990. 217 с.
- Klcovа L., Gubišovа M. Evaluation of different approaches to buckwheat (Fagopyrum esculentum Moench) micropropagation. Czech J. Genet. Plant Breed. 2008;44(2):66-72. https://doi.org/10.17221/2677-CJGPB
- Dobránszki J. Role of cytokinins and explant type in shoot multiplication of buckwheat (Fagopyrum esculentum Moench) in vitro. Eur. J. Plant Sci. Biotechnol. 2009;3(1):66-70.
- Slavinska J., Kantartsi K., Obendorf R. In vitro organogenesis of Fagopyrum esculentum Moench (Polygonaceae) as a method to study seed set in buckwheat. Eur. J. Plant Sci. Biotechnol. 2009;3:75-78.
- Барсукова Е.Н. Повышение эффективности микроклонального размножения гречихи. Аграрная Россия. 2014;(11):21-23. EDN SZAWZT.
- Фардзинова И.М. Патент № 2141524 РФ, МПК C12N5/04, A01H4/00. Питательная среда для микроклонального размножения груши: № 96116867/13; заявлено 09.08.1996: опубликовано 20.11.1999а. Горский государственный аграрный университет. 6 с.
- Фардзинова И.М. Патент № 2134506РФ, МПК А01Н4/00. Питательная среда для регенерации растений яблони из семядолей: № 96116750/13; заявлено 09.08.1996: опубликовано 20.08.1999b. Горский государственный аграрный университет. 6 с.
- Фардзинова И.М. Патент № 2045891РФ, МПК А01Н3/00. Питательная среда для микроклонального размножения косточковых культур: № 5046484/13; заявлено 08.06.1992: опубликовано 20.10.1995. Научно-производственное объединение «Горное». 5 с.
- Фардзинова И.М. Патент № 2111652РФ, МПК А01Н4/00. Питательная среда для микроклонального размножения подвоев яблони: № 96116869/13; заявлено 09.08.1996: опубликовано27.05.1998. Горский государственный аграрный университет. 5 с.
- Фардзинова И.М. Патент № 2198505 РФ, МПК А01Н 4/00//С12N5/04. Питательная среда для регенерации растений абрикоса из незрелых зародышей: № 96116751/13: заявлено 09.08.1996: опубликовано 20.02.2003. Горский государственный аграрный университет. 6 с.
- Kowalski В., Jäger А.К., VanStaden J. The effect of a seaweed concentrate on the in vitro growth and acclimatization of potato plantlets. Potato Research. 1999;(42):131-139. https://doi.org/10.1007/BF02358403
- Солохина И. Ю. Исследование ростостимулирующих свойств БАВ растительного происхождения на овощных культурах in vitro. Рациональное использование сырья и создание новых продуктов биотехнологического назначения: материалы Международной научнопрактической конференции по актуальным проблемам в области биотехнологии. Орел, 06 июня 2018 года. Орел: ООО ПФ Картуш. 2018:86- 89.
- Упадышев М.Т. Патент № 2063682РФ, МПК А01Н4/00. Питательная среда для размножения ягодных и плодовых культур: № 93027859/13; заявлено 19.05.1993: опубликовано 20.07.1996. Всероссийский селекционно-технологический институт садоводства и питомниководства. 9 с.
- Клыков А.Г., Барсукова Е.Н. Биотехнология и селекция гречихи на Дальнем Востоке России. Владивосток: Дальнаука. 2021. 352 с.
- Зорикова С.П. Рейнутрия японская (Reynoutria japonica Houtt.) в Приморском крае (биология развития, флавоноидный состав, биологическая активность). Дисс. … канд. биол. наук. ДВО РАН Горнотаежная станция им. В.Л. Комарова. 2011;120 с.
- Borovaya S., Lukyanchuk L., Manyakhin A., Zorikova O. Effect of Reynoutria japonica extract upon germination and upon resistance of its seeds against phytopathogenic fungi Triticumaestivum L.,Hordeum vulgare L. and Glycine max (L.) Merr. Org. Agr. 2020;10(1):89-95. https://doi.org/10.1007/s13165-019-00254-6.
- Клыков А.Г., МоисеенкоЛ.М., Горовой П.Г. Биологические ресурсы видов рода Fagopyrum Mill. (Гречиха) на российском Дальнем Востоке. Владивосток: Дальнаука. 2018;360 с.
- Дунаева С.Е., Пендинен Г.И., Антонова О.Ю., Швачко Н.А., Ухватова Ю.В., Шувалова Л.Е., Волкова Н.Н., Гавриленко Т.А. Сохранение вегетативно размножаемых культур в in vitro и криоколлекциях: методические указания. 2-е изд, расш. и доп. Санки-Петербург: ВИР. 2017.
- Borovaya S.A., Klykov А.Г., Barsukova Е.Н., Chaikina Е.Л. Study of the effect of selective media with high zinc doses on the survival, growth and development of common buckwheat in vitro. Plants. 2022;(11):264. https://doi.org/10.3390/plants11030264
- Гаврилова А.Ю., Горькова И.В., Гагарина И.Н., Павловская Н.Е. Использование биофлавоноидов в in vitro технологиях. Передовые научно-технические проекты в биотехнологии: Материалы I Национальной научно-практической Интернет-конференции по актуальным проблемам в области биотехнологии, Орел 19 мая 2022 года. Орел: Орловский государственный аграрный университет имени Н.В. Парахина, 2022.
- Гаврилова А.Ю. Влияние биофлаваноидов на морфогенез картофеля при микроклональном размножении. Продовольственная безопасность как фактор повышения качества жизни: материалы Национальной (Всероссийской) научно-практической конференции, Орел 29 сентября 2021 года. Орел: Орловский государственный аграрный университет имени Н.В. Парахина, 2021.
- Капустин Б.А., Солохина И.Ю. Влияние растительных БАВ на рост и развитие овощных культур в условиях in vitro. Аграрная наука - сельскому хозяйству: Сборник материалов XIV Международной научно-практической конференции. В 2-х книгах, Барнаул, 07-08 февраля 2019 года. Книга 1. Барнаул: Алтайский государственный аграрный университет, 2019. С. 196-198.


