Установление минимальных температурных и временных порогов образования ферритов-шпинелей CuFe2O4, NiFe2O4, CoFe2O4, ZnFe2O4 для методов твердофазного и жидкофазного синтеза
Автор: Зирник Глеб Михайлович, Чернуха Александр Сергеевич, Некорыснова Надежда Сергеевна, Вепрева Анастасия Владимировна, Матвеев Константин Витальевич, Смолякова Ксения Романовна, Винник Денис Александрович
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 4 т.14, 2022 года.
Бесплатный доступ
Определение минимальных температурных и временных порогов образования гомогенных образцов ферритных материалов для разных методов интересно с той точки зрения, что финальная температура ферритизации, а также время выдержки влияет на конечный размер частиц. С уменьшением температуры ферритизации и времени выдержки также снижается конечный размер частиц получаемого материала. Для гетерогенных катализаторов это является важным параметром, так как чем меньше размер частиц катализатора, тем выше его каталитическая активность. В статье исследованы керамический, золь-гель (цитратный) метод, метод соосаждения и гидротермальный метод на примере получения СuFe2O4, CoFe2O4, NiFe2O4, ZnFe2O4. Экспериментально определены нижние температурные пороги образования ферритных фаз для керамического, золь-гель (цитратного), гидротермального метода и метода соосаждения на примере получения CuFe2O4, NiFe2O4, СoFe2O4, ZnFe2O4. Температурный порог образования уменьшается в ряду: керамический метод (1000-1100 °С), метод соосаждения (400-800 °С), гидротермальный метод (180 °С), золь-гель (цитратный) метод (150-300 °С); при этом для каждого из методов выдержка может составлять от нескольких часов до суток. Используя данные порошковых дифрактограмм, методом полнопрофильного анализа по Ритвельду установлены параметры решетки для всей линейки образцов. Для СuFe2O4, полученного керамическим методом: a = 8,376 Å, V = 587,74 Å3; золь-гель (цитратным) методом: a = 8,270, V = 565,63 Å3; методом соосаждения: a = 8,392, V = 590,90 Å3; гидротермальным методом: a = 8,278, V = 567,24 Å3. Для СoFe2O4, полученного керамическим методом: a = 8,385, V = 598,49 Å3; золь-гель (цитратным) методом: a = 8,353, V = 582,82 Å3; методом соосаждения: a = 8,378, V = 588,04 Å3; гидротермальным методом: a = 8,393, V = 591,31 Å3. Для NiFe2O4, полученного керамическим методом: a = 8,338, V = 579,59 Å3; золь-гель (цитратным) методом: a = 8,357, V = 583,49 Å3; методом соосаждения: a = 8,347, V = 581,40 Å3; гидротермальным методом: a = 8,351, V = 582,38 Å3. Для ZnFe2O4, полученного керамическим методом: a = 8,439, V = 600,97Å3; золь-гель (цитратным) методом: a = 8,439, V = 8,439 Å3; методом соосаждения: a = 8,440, V = 601,10 Å3; гидротермальным методом: a = 8,453, V = 604,02 Å3.
Ферриты-шпинели, гетерогенные катализаторы, твердофазный синтез, золь-гель (цитратный) синтез, метод соосаждения, гидротермальный синтез, температурный порог образования, снижение размера частиц
Короткий адрес: https://sciup.org/147239531
IDR: 147239531 | УДК: 54.052+54.057+544.22+544.032.7+544.015 | DOI: 10.14529/chem220412
Текст научной статьи Установление минимальных температурных и временных порогов образования ферритов-шпинелей CuFe2O4, NiFe2O4, CoFe2O4, ZnFe2O4 для методов твердофазного и жидкофазного синтеза
Начиная с 50-х годов XX века по настоящий момент достаточно глубокого исследованы магнитные свойства разнообразных ферритов [1, 2]. На основе их магнитных свойств широкое распространение получили компоненты электроники (радарные модули, изоляторы, фазовозвраща-тели) [3], радиопоглощающие покрытия [4], постоянные магниты [5], устройства магнитной записи [6]. Число ферритов, чьи магнитные свойства используют на практике, достаточно велико: это могут быть ферриты-шпинели MeFe 2 O 4 [7] (иногда их называют моноферритами), гексаферриты M-типа MeFe 12 O 19 [8]; ферриты-гранаты Me 3 Fe 5 O 12 [9], а также ортоферриты, имеющие структуру перовскита состава MeFeO 3 [10].
В то же время ферриты могут быть применены в области органического синтеза, в частности, в процессах фотокатализа [11–14] и традиционного катализа [15–17]. С точки зрения электропроводности и зонной структуры ферриты являются полупроводниками [13, 18], в силу чего они могут эффективно преобразовывать кванты света в электронно-дырочные пары (e–/h+). Это позволяет использовать электроны (e–) в реакциях восстановления, а дырки (h+) – в реакциях окисления.
К примеру, ряд ферритов-шпинелей MeFe2O4 могут быть применены для окисления органических субстратов при облучении: фенола [11], метиленового голубого [13], изопропанола [14], атразина [16]; а также в реакциях восстановления с получением водорода из воды [14]. Также для фотокатализа используют и другие ферриты, в частности, ферриты-перовскиты MeFeO 3 применимы для получения водорода из воды [19] и окисления органических субстратов [20–24]. Несмотря на достаточно широкое использование ферритов в области фотокатализа, в литературе практически не сообщается о каталитических реакциях с их участием, и практически все ферри-ты-шпинели, кроме моноферрита меди [25–29], остаются слабо исследованными. Предполагается, что не только ферриты меди могут обладать каталитической активностью, тому в подтверждение есть ограниченное число работ, в которых изучали каталитическую активность моноферритов SrFe 2 O 4 [30], Ni 0,5 Zn 0,5 Fe 2 O 4 [31], Zn 1–x Mg x Fe 2 O 4 [32] в реакции переэтерификации. При этом авторы практически никогда не выделяют фактор, который бы исчерпывающе объяснял выбор метода синтеза ферритного материала. Иными словами, на данный момент не существует широко известных исследований с систематическим подходом к синтезу ферритов разными методами для области катализа.
В литературе достаточно подробно описаны методы получения ферритов твердофазными [33–35], жидкофазными [36–41] и газофазными [42–45] методами. Каждый из классов методов отражает агрегатное состояние реагирующих веществ. Для твердофазных методов характерна реакция между порошками (твердыми веществами), для жидкофазных – реакция в растворах, для газофазных – реакция реагентов в виде пара, плазмы или газа. В основном газофазные методы применяют для получения покрытий и тонких пленок [42]. К данным методам относят метод лазерного-импульсного нанесения [42, 43], метод химического осаждения паров [46], метод термического разложения спрея [44]. Твердофазные методы синтеза применяют для получения порошкообразных материалов и, как правило, такие реакции проводятся при высоких температурах – это методы СВС [47], метод твердофазного синтеза [35], флюсовый метод [48]. Жидкофазные методы применимы для получения нано и микрочастиц. Среди данных методов выделяют метод химического соосаждения [49–51], гидротермальный [41, 52], микроволновый [53], золь-гель (цитратный) метод [37, 38, 54] (иногда в литературе он описывается как self-combustion method, что можно перевести как «метод самовозгорания»; при этом его не следует путать с методом СВС), электрохимический [55], микро-эмульсионный метод [56], метод высокотемпературных растворов [57].
Как известно, размеры кристаллитов и частиц получаемого материала неразрывно связаны с финальной температурой спекания, соответственно, чем выше будет температура ферритизации (спекания), тем большей степенью кристалличности будет обладать материал [58]. Иными словами, чем выше температура спекания, тем больше становится «зерно» и тем более крупным будет размер кристаллитов и частиц получаемого материала. Таким образом, из утверждения выше можно сделать вывод: метод синтеза, обеспечивающий среди всех наименьшую температуру получения, будет обеспечивать и наименьший размер частиц получаемого материала. Среди прочего это же утверждение относится ко времени выдержки материала при спекании. Установление данных параметров является очень важным для гетерогенного катализа, поскольку каталитическая активность напрямую связана с размерами частиц: чем меньше будет размер частиц используемого катализатора, тем выше будет его каталитическая активность (ферриты являются именно гетерогенными катализаторами, так как они не растворимы в органических и прочих субстратах).
Данная работа ставит перед собой задачу исследования условий минимальных температур и времени выдержки, необходимых для образования гомогенных образцов СuFe 2 O 4 , CoFe 2 O 4 , NiFe2O4, ZnFe2O4 керамическим, а также золь-гель (цитратным), гидротермальным методом и методом соосаждения.
Экспериментальная часть
Как было описано во введении, в работе были применены четыре метода получения ферри-тов-шпинелей: керамический, золь-гель (цитратный), гидротермальный и метод соосаждения.
Для получения ферритов-шпинелей керамическим методом использовали оксид меди (II) (СuO, «х. ч.»), оксид кобальта II (CoO, «ч.»), оксид никеля II (NiO, «ч.»), оксид цинка (ZnO «ч.») и оксид железа III (Fe 2 O 3 , «х. ч.»).
Для получения ферритов-шпинелей золь-гель (цитратным) методом использовали девятиводный нитрат железа (III) (Fe(NO 3 ) 3 ·9H 2 O, «ч.»), шестиводный нитрат никеля (II) (Ni(NO 3 ) 2 ·6H 2 O, «ч.»), шестиводный нитрат кобальта (II) (Co(NO 3 ) 2 ·6H 2 O, «ч.»), трехводный нитрат меди (II) (Cu(NO 3 ) 2 ·3H 2 O, «ч.»), оксид цинка (ZnO «ч.») (который переводили в нитрат путем растворения в 65%-ной азотной кислоте), лимонную кислоту («ч.»), водный раствор аммиака (25 масс. %).
Для получения ферритов-шпинелей методом соосаждения использовали шестиводный хлорид железа (III) (FeСl 3 ·6H 2 O, «ч. д. а.»), четырехводный хлорид никеля (II) (NiСl 2 ·4H 2 O, «х. ч.»), шестиводный нитрат кобальта (II) (Co(NO 3 ) 2 ·6H 2 O, «ч.»), трехводный нитрат меди (II) (Cu(NO 3 ) 2 ·3H 2 O, «ч.»), оксид цинка (ZnO «ч.») (который был переведен в соль путем растворения в 65%-ной азотной кислоте), гидроксид натрия (NaOH, «ч. д. а.»), гидроксид калия (KOH, «ч. д. а.»).
Для получения феррито-шпинелей гидротермальным методом использовали шестиводный хлорид железа (III) (FeСl 3 ·6H 2 O, «ч.д.а.»), четырехводный хлорид никеля (II) (NiСl 2 ·4H 2 O, «х. ч.»), шестиводный нитрат кобальта (II) (Co(NO3)2·6H2O, «ч.»), трехводный нитрат меди (II) (Cu(NO 3 ) 2 ·3H 2 O, «ч.»), оксид цинка (ZnO «ч.») (который был переведен в соль путем растворения в 65%-ной азотной кислоте), гидроксид натрия (NaOH, «ч. д. а.»), гидроксид калия (KOH, «ч. д. а.»)
Навески реагентов отбирали на весах Сартогосм ЛВ 210-А, 4 знак точности (0,1 мг). Финальное спекание образцов производили в печи Naberthern P330 и муфельной печи (нихромовая спираль накала) с программируемым терморегулятором Варта ТП403. Отмывку от вспомогательных солей неферритизиванных шихт производили с использованием центрифуги Hermle LaborTechnic Z383. Перемешивание, нагрев, упаривание растворов и суспензий производили на магнитной плитке IKA ® C-MAG HS-7.
Методика получения ферритов-шпинелей керамическим методом широко известна и была использована без изменений [35]. В рамках данного метода готовится шихта, состоящая из оксидов железа (III) и нужного оксида металла (СuO для получения СuFe 2 O 4 , CoO для получения CoFe 2 O 4 , NiO для получения NiFe 2 O 4 и ZnO для получения ZnFe 2 O 4 соответственно), которая в дальнейшем тщательно перетирается и подвергается спеканию при нужной температуре.
Шихта оксидов составляется согласно уравнению реакции
MeO + Fe 2 O 3 ^ MeF e2O 4 . ( 1 )
Перетирание для синтеза проводили в яшмовой ступке в течение 10 минут. После перетирания шихту без дополнительной формовки переносили в корундовый тигель. Нагрев «сырой» шихты осуществляли с муфельной печью при скорости нагрева 12,5 °С/мин до 1100 °С (за исключением образцов ZnFe 2 O 4 , для них температура ферритизации составила 1000 °С). Выдержка в печи составляла от 1 до 6 часов для разных типов образцов (отражено в подписях к рентгенограммам), временной шаг составил 1 час. По истечении отведенного на спекание времени образцы извлекали из муфельной печи и остужали на воздухе при комнатной температуре.
Методика получения ферритов-шпинелей золь-гель (цитратным) методом ранее описана в литературе и была применена без изменений [59]. Суть данного метода – получение смеси-хелатированных ионов металлов, гомогенно распределенных на ионном уровне, которые далее путем реакции самовозгорания превращаются либо в феррит, либо в продукт, предшествующий ферриту. Навески нитратов железа (III), нитрата металла (II) и лимонной кислоты, взятых в соотношении Me2+/Fe3+/C6H8O7 = 1:2:9, растворяли в дистиллированной воде. Важно, чтобы на каждый ион металла приходилось по три молекулы лимонной кислоты. После отбора навесок и растворения в дистиллированной воде растворы двух нитратов и лимонной кислоты смешивали в фарфоровой чашке с получением прозрачного цветного раствора. Раствор перемешивали на магнитной плитке в течение 5 минут, после чего добавляли аммиак до того момента, пока среда не станет слабощелочной (pH~ 8 по индикаторной бумаге). При этом цвет раствора изменяется (образуется золь). После добавления аммиака раствор еще некоторое время перемешивают, после чего упаривают на плитке при постоянном перемешивании до образования медообразной массы (геля). Полученный гель помещают в муфельную печь, предварительно разогретую до температуры 150 °С. Выдержку осуществляют в течение 12–20 часов. В том случае, если визуально ксерогеля не образовалось, имеющуюся не вздувшуюся черную массу (полуксерогель) механически измельчают, переносят в фарфоровый тигель и спекают при 300 °С в течение нескольких часов с получением ксерогеля либо феррита. Ксерогель представляет из себя смесь аморфного феррита, промежуточных оксидных фаз, а также несгоревшего в ходе реакции углерода. По этой причине для удаления остаточного углерода необходима длительная выдержка полученного ксерогеля в печи при температуре выше 150 °С. Установление момента полного избавления образца от остаточного углерода определяют путем фиксирования его массы на этом этапе. В том случае, если на этапе получения ксерогеля с его последующим отжигом от остаточного углерода гомогенного образца феррита не образовалось, проводят ферритизацию по методике, изложенной для керамического метода, а в качестве «сырой» шихты используют полученный на этапе синтеза ксерогеля материал. Температурный шаг для финальной стадии спекания составил 100 °С (от 800 °С до 1000 °С), временной шаг – 1 час (от 1 до 4 часов). Возможная реакция (2) образования феррита представлена ниже:
9Me(N0 3 ) 2 + 18Fe(N0 3 ) 3 + 81C 6 H 8 0 7 + 274,5 0 2 ^ 9MeFe2O4 + 36N 2 + 486CO 2 + 324H 2 O . (2)
Важно подчеркнуть, что температура печи 150–300 °С в действительности не отражает локальной температуры в шихте, развивающейся в ходе экзотермической реакции горения органических прекурсоров и разложения нитратов. Так, визуально было обнаружено, что в ходе синтеза отдельные части шихты при горении светятся красно-оранжевым светом, что явно свидетельствует о локально и кратковременно достигаемых температурах в ~ 600–700 °C.
Методика получения ферритов-шпинелей методом химического соосаждения гидроксидов также широко описана в литературе и была применена без изменений [40]. Суть метода заключается в получении мелкодисперсных осадков ферритных прекурсоров, осажденных друг на друге. Хлорид железа (III), хлорид нужного металла (II) (либо его нитрат) растворяли в дистиллированной воде, растворы переносили в стеклянный стакан, после чего по каплям при постоянном перемешивании через капельную воронку приливали раствор щелочи (KOH либо NaOH). Минимально необходимое количество щелочи определяют из уравнения
MeCl2 + FeCl3 + 5Na0H ^ Me(0H )2 + Fe(0H )3 + 5NaCl . (3) Однако для полного осаждения гидроксидов из раствора были использованы массы щелочи на 10–15 % выше расчетного значения.
После полного добавления раствора щелочи и получения осадка гидроксидов суспензию разливали по пробиркам для центрифугирования и производили отмывку от вспомогательных водорастворимых солей путем центрифугирования суспензии (7000–8000 об/мин при 3–5 минутах центрифугирования для одного цикла) и декантации раствора над осадком. Отмывку производили промывкой дистиллированной водой не менее 3 раз без контроля pH среды. После получения отмытой от солей суспензии «сырой» шихты суспензия фильтровалась через фильтр Шота, высушивалась на воздухе и подвергалась финальной ферритизации либо сама суспензия упаривалась в керамической чашке на плитке (см. ниже). Температурный шаг финальной стадии спекания составил 200 °С (от 400 до 1000 °С), временной шаг – 1 час (от 1 до 4 часов). Возможная реакция (2) образования феррита представлена выше.
Также было установлено, что нагрев отмытой от вспомогательных солей водной суспензии шихты феррита кобальта на плитке IKA ® C-MAG HS-7 в режиме нагрева 270 °С в течение ~ 3 часов с целью упаривания раствора до момента образования сухого, рассыпчатого материала приводит к образованию гомогенного, высококристалличного моноферрита кобальта СoFe2O4.
Методика получения ферритов-шпинелей методом гидротермального синтеза также широко известна и описана в литературе [41]. Во многом этот метод схож с методом химического соосаждения, описанным выше. Суть данного метода – это получение суспензии осажденных друг на друге гидроксидов с последующей гидротермальной обработкой материала.
Методика синтеза до этапа получения суспензии гидроксидов аналогична методу химического соосаждения и описана выше. После получения суспензии гидроксидов ее количественно переносили во вкладыш к автоклаву (40 мл), после чего вкладыш помещали в автоклав и плотно герметизировали (важно подчеркнуть, что для удобства работы прекурсоры (соли) предварительно стоит растворять в минимальном количестве воды для полного переноса суспензии во вкладыш к автоклаву без упаривания раствора). Автоклав помещали в предварительно разогретую до 180 °С муфельную печь. Выдержка в печи составила 24 часа. После гидротермальной обработки автоклав остужали на воздухе, полученную суспензию распределяли по пробиркам для центрифугирования и отмывали дистиллированной водой от вспомогательных солей путем
3 циклов центрифугирования (7000–8000 об/мин при 3–5 минутах центрифугирования для одного цикла) с последующей декантацией раствора над осадком. «Сырую» в виде пасты шихту переносили в керамическую чашку и высушивали на плитке IKA ® C-MAG HS-7 в режиме нагрева 150180 °С с получением сухого, порошкообразного остатка. При необходимости для некоторых образцов может проводиться ферритизация с температурным шагом в 100 °С (от 700 до 900 °С) и выдержки в печи от 1 до 4 часов с временным шагом в 1 час.
Анализ полученных ферритов проводили на многофункциональном рентгеновском дифрактометре Rigaku Ultima IV (Cu Kα, 40 кВ, 30 мА; λ = 0,1542 нм) при скорости записи 5°/мин. Определение параметров решетки и размеров элементарной ячейки для полученных гомогенных образцов проводили в программном комплексе PDXL с использованием полнопрофильного анализа по Ритвельду. Нормализация фона и построение рентгенограмм проводили в программном пакете Origin ® 2018.
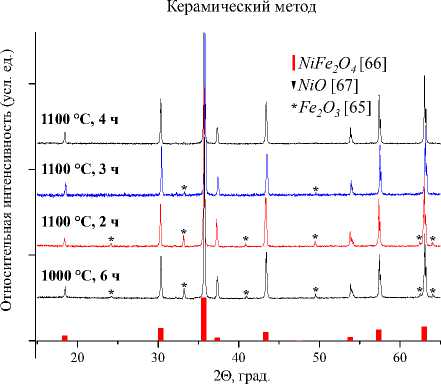
Значения установленных минимальных температур и времен выдержки образцов СuFe2O4, CoFe 2 O 4 , NiFe 2 O 4 , ZnFe 2 O 4 , а также их параметры решетки и размер элементарной ячейки [60–63] указаны в таблице. Результаты рентгеновского фазового анализа для образцов СuFe 2 O 4 , CoFe 2 O 4 , NiFe 2 O 4 , ZnFe 2 O 4 , полученных керамическим, золь-гель (цитратным), гидротермальным методом и методом соосаждения, представлены на рис. 1–4.
Керамический метод
Метод соосаждения
CuFe2O4 [63] CuO [64]
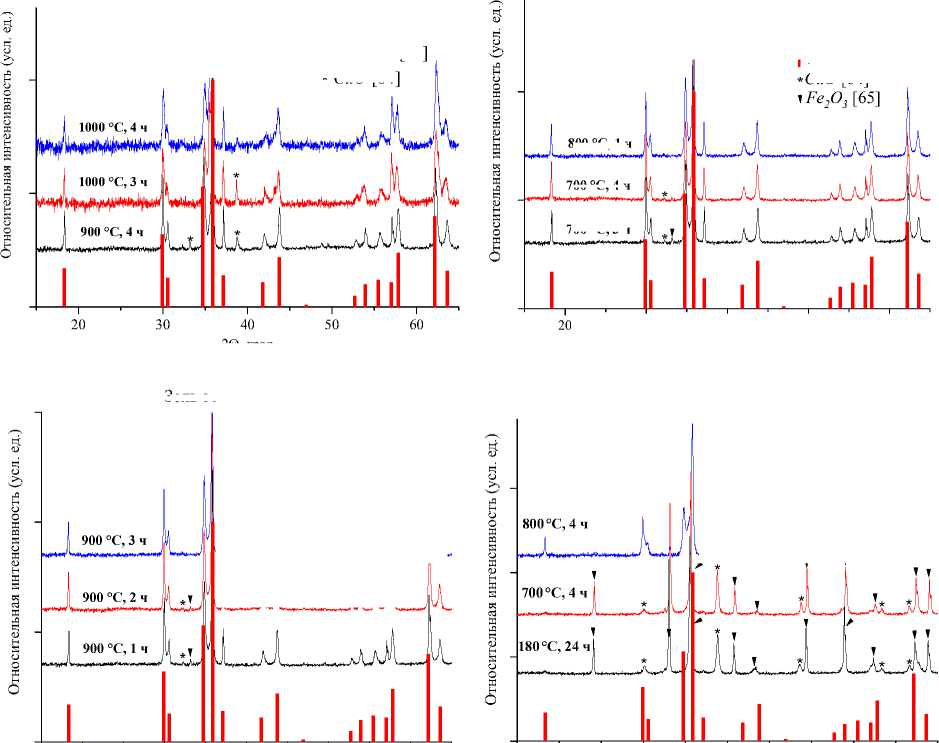
800 °C, 1 ч
700 °C, 4 ч
Fe 2 O 3 [65] > * 1
CuFe2O4 [63]
* CuO [64]
40 50
2 Θ , град.
б)
Гидротермальный метод
40 50
2 Θ , град.
в)
40 50
2 Θ , град.
г)
Рис. 1. Порошковые дифрактограммы образцов СuFe 2 O 4 , полученных: а) керамическим методом;
б) методом соосаждения; в) золь-гель (цитратным методом); г) гидротермальным методом. Символами в легенде к рисункам обозначены: I – СuFe 2 O 4 [63] (также и в виде штрихграммы), * – CuO [64], ▼– Fe 2 O 3 [65]
CuFe2O4 [63]
* CuO [64]
700 °C, 3 ч
2 Θ , град.
а)
Золь-гель (цитратный) метод
CuFe2O4 [63]
* CuO [64] ।
Fe 2 O 3 [65]
а)
Золь-гель (цитратный) метод
500 °С, 1 ч
NiFe2O4 [66]

150 °С, 35 ч

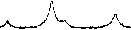
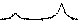
Метод соосаждения
700 °С, 2 ч
600 °С, 2 ч к = к
NiFe2O4 [66]
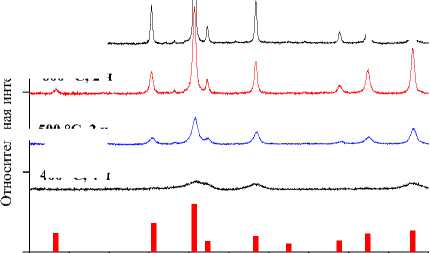
40 2 Θ , град. б)
500 °С, 2 ч
400 °С, 4 ч
Гидротермальный метод
180 °С, 24 ч

NiFe2O4 [66]
I, Il , I ■ , I I , I
20 30 40 50 60
2 Θ , град.
2 Θ , град.
в)
г)
Рис. 2. Порошковые дифрактограммы образцов NiFe 2 O 4 , полученных: а) керамическим методом; б) методом со-осаждения; в) золь-гель (цитратным методом); г) гидротермальным методом. Символами в легенде к рисункам обозначены:*– Fe 2 O 3 [65], I – NiFe 2 O 4 [66](также и в виде штрихграммы), ▼ – NiO [67]
Керамический метод
Метод соосаждения
*
1100 °С, 4 ч
1100 °С, 3 ч
1100 °С, 2 ч
CoFe2O4 [68]
Fe2O3 [65]
CoO [69]
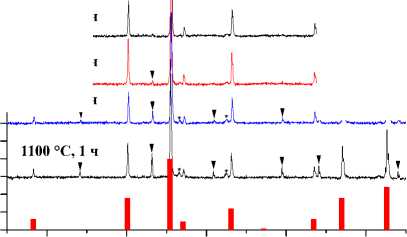
20 30 40 50 60
2 Θ , град.
Щ m s
Щ s
t;
s
Щ
500 °С, 1 ч
После просушки на плитке
CoFe2O4 [68]
V^W^Vw*-*^ Vj^^v^^Wh^A^^ ‘‘‘t***^/ V*.

^-L
2 Θ , град.
а)
б)
Рис. 3. Порошковые дифрактограммы образцов СоFe 2 O 4 , полученных: а) керамическим методом; б) методом со-осаждения; в) золь-гель (цитратным методом); г) гидротермальным методом. Символами в легенде к рисункам обозначены: ▼ – Fe 2 O 3 [65], I – СoFe 2 O 4 [68] (также и в виде штрихграммы), * – CoO [69] (см. также с. 133)
Золь-гель (цитратный) метод
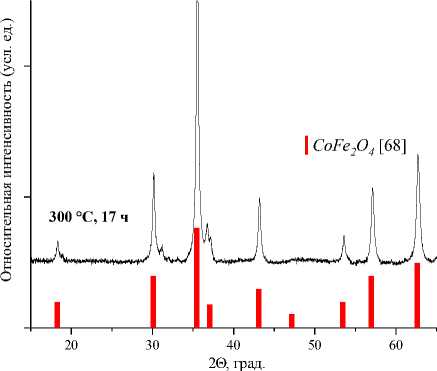
в)
Гидротермальный метод
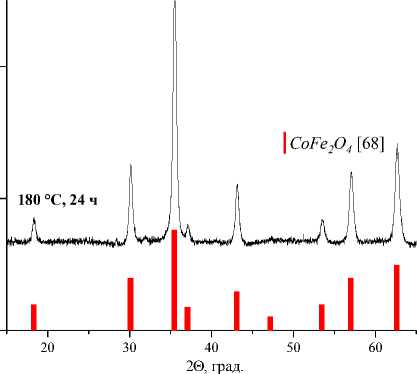
г)
Рис. 3. Окончание
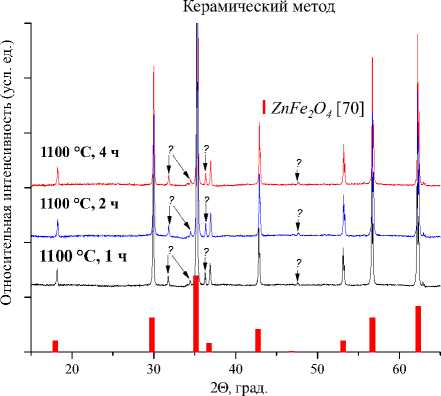
а)
Метод соосаждения
?
I ZnFe2O4 [70]
Золь-гель (цитратный) метод
150 °С, 14 ч fi^WMwwMM* *w^ Ч^нД*
I ZnFe2O4 [70]
д#*1*
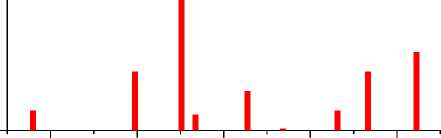
20 30 40 50 60
2 Θ , град.
?
500°С, 1 ч
~ ^*,1^^*^^^
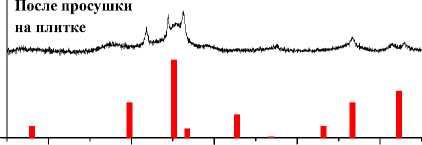
20 30 40 50 60
2 Θ , град.
б)
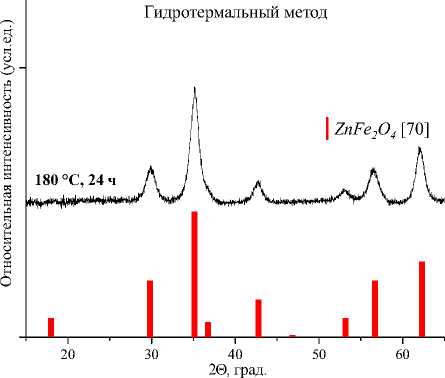
г)
в)
Рис. 4. Порошковые дифрактограммы образцов ZnFe 2 O 4 , полученных: а) керамическим методом; б) золь-гель (цитратным методом); в) методом соосаждения; г) гидротермальным методом. Символами в легенде к рисункам обозначены: I – ZnFe 2 O 4 [70] (также и в виде штрихграммы)
Параметры решетки, объем элементарной ячейки, минимальные температуры и временные пороги для полученных гомогенных образцов ферритов-шпинелей
|
Тип феррита и его метод получения |
a , Å |
V , Å3 |
Время выдержки и температура |
|
CoFe 2 O 4 [60] |
8,392 |
591,01 |
– |
|
CoFe2O4, керамический |
8,385 |
598,49 |
1100 °С, 4 ч |
|
CoFe2O4, золь-гель (цитратный) |
8,378 |
588,04 |
300 °С, 17 ч |
|
CoFe2O4, соосаждения |
8,353 |
582,82 |
Прокаливание на плитке, в режиме нагрева 270 °С, ~ 3 ч |
|
CoFe2O4, гидротермальный |
8,393 |
591,31 |
180 °С, 24 ч |
|
NiFe 2 O 4 [61] |
8,337 |
579,47 |
– |
|
NiFe2O4, керамический |
8,338 |
579,59 |
1100 °С, 4 ч |
|
NiFe 2 O 4 , золь-гель (цитратный) |
8,357 |
583,49 |
150 °С, 20 ч |
|
NiFe2O4, соосаждения |
8,347 |
581,40 |
400 °С, 2 ч |
|
NiFe2O4, гидротермальный |
8,351 |
582,38 |
180 °С, 24 ч |
|
ZnFe 2 O 4 [62] |
8,416 |
596,10 |
– |
|
ZnFe2O4, керамический |
8,439 |
600,97 |
1100 °С, 1 ч |
|
ZnFe2O4, золь-гель (цитратный) |
8,439 |
601,00 |
150 °С, 14 ч |
|
ZnFe2O4, соосаждения |
8,440 |
601,10 |
500 °С, 1 ч |
|
ZnFe2O4, гидротермальный |
8,453 |
604,02 |
180 °С, 24 ч |
|
CuFe 2 O 4 [63] |
8,394 |
591,44 |
– |
|
CuFe2O4, керамический |
8,376 |
587,74 |
1000 °С, 4 ч |
|
CuFe2O4, золь-гель (цитратный) |
8,270 |
565,63 |
900 °С, 4 ч |
|
CuFe2O4, соосаждения |
8,392 |
590,90 |
800 °С, 1 ч |
|
CuFe2O4, гидротермальный |
8,278 |
567,24 |
800 °С, 4 ч |
Заключение
К наиболее эффективным методам (с точки зрения минимальной температуры и времени выдержки) можно отнести золь-гель (цитратный) метод, гидротермальный метод. Установлено, что температурный порог образования уменьшается в следующем ряду: керамический метод (1000– 1100 °С), метод соосаждения (400–800 °С), гидротермальный метод (180 °С), золь-гель (цитратный) метод (150–300 °С). Дальнейшие исследования позволят количественно связать размер частиц и кристаллитов с условиями получения материала для конкретного метода.
Данная работа выполнена при финансовой поддержке грантового конкурса «УМНИК» в рамках проекта № 16800ГУ/2021 и гранта Президента Российской Федерации для государственной поддержки молодых российских ученых – докторов наук (МД-5612.2021.4).
Список литературы Установление минимальных температурных и временных порогов образования ферритов-шпинелей CuFe2O4, NiFe2O4, CoFe2O4, ZnFe2O4 для методов твердофазного и жидкофазного синтеза
- Brockman F.G., Dowling P.H., Steneck W.G. Dimensional effects resulting from a high dielectric constant found in a ferromagnetic ferrite. Phys. Rev. 1950;77(1):85-93. DOI: 10.1103/PhysRev.77.85
- Vinnik D.A., Sherstyuk D.P., Zhivulin V.E., Zhivulin D.E. Impact of the Zn-Co content on structural and magnetic characteristics of the Ni spinel ferrites. Ceram. Int. 2022;48(13): 18124-18133. DOI: 10.1016/j.ceramint.2022.03.070
- Pardavi-Horvath M. Microwave applications of soft ferrites. J. Magn. Magn. Mater. 2000;215:171-183. DOI: 10.1016/S0304-8853(00)00106-2
- Meshrama M.R., Agrawala N.K., Sinhaa B., Misra P.S. Characterization of M-type barium hexagonal ferrite-based wide band microwave absorber. J. Magn. Magn. Mater. 2004;271(2-3):207-214. DOI: 10.1016/j.jmmm.2003.09.045
- Mohsen Q. Factors affecting the synthesis and formation of single-phase barium hexaferrite by a technique of oxalate precursor. Am. J. Appl. Sci. 2010;7(7):901-908. DOI: 10.3844/ajassp.2010.914.921
- Kubo O., Ido T., Yokoyama H. Properties of Ba ferrite particles for perpendicular magnetic recording media. IEEE Trans. Magn. 1982;18(6):1122-1124. DOI: 10.1109/TMAG.1982.1062007
- Fairweather A., Roberts F.F., Welch A.J.E. Ferrites. Rep. Prog. Phys. 2019;15:142.
- Carol Trudel T.T., Mohammed J., Hafeez H.Y., Bhat.Structural B.H. Dielectric, and magneto-optical properties of Al-Cr substituted M-type barium hexaferrite. Phys. Status Solidi Appl. Mater. Sci. 2019;216(16):1-9. DOI: 10.1002/pssa.201800928
- Smolenskij G.A., Lemanov V.V. Ferrity i ikh tekhnicheskoe primenenie. [Ferrites and their technical applications]. Leningrad, Nauka. 1975:219.
- Belov K.P., Kadomtseva A.M. Magnetoelastic properties of rare-earth orthoferrites. Sov. Phys. Usp. 1971 ;14(2): 154-162. DOI: 10.1070/PU1971v014n02ABEH004455
- Meng W., Li F., Evans D.G., Duan X. Photocatalytic activity of highly porous zinc ferrite prepared from a zinc-iron (III)-sulfate layered double hydroxide precursor. J. Porous Mater. 2004;11(2):97-105. DOI: 10.1023/B:JOPO.0000027365.89103.f1
- Chung Y.S., Bin P.S., Kang D.W. Magnetically separable titania-coated nickel ferrite photocatalyst.Mater. Chem. Phys. 2004;86(2-3):375-381. DOI: 10.1016/j.matchemphys.2004.03.027
- Padmapriya G., Manikandan A., Krishnasamy V., Jaganathan S.K. Spinel NixZni.xFe2O4 (0.0 < x < 1.0) nano-photocatalysts: Synthesis, characterization and photocatalytic degradation of methylene blue dye. J. Mol. Struct. 2016;1119:39-47. DOI: 10.1016/j.molstruc.2016.04.049
- Kim H.G., Borse P.H., Jang J.S., Jeong E.D. Fabrication of CaFe2O4/MgFe2O4 bulk heterojunction for enhanced visible light photocatalysis. Chem. Commun. 2009;39:5889-5891. DOI: 10.1039/b911805e
- Zhang S., Zhao X., Niu H., Shi Y. Superparamagnetic Fe3O4 nanoparticles as catalysts for the catalytic oxidation of phenolic and aniline compounds. J. Hazard. Mater. 2009;167(1-3):560-566. DOI: 10.1016/j.jhazmat.2009.01.024
- Guan Y.H., Ma J., Ren Y.M., Liu Y.L. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res. 2013;47(14):5431-5438. DOI: 10.1016/j.watres.2013.06.023
- Ren Y., Lin L., Ma J., Yang J. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M=Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B Environ. 2015;165:572-578. DOI: 10.1016/j.apcatb.2014.10.051
- Gul I.H., Ahmed W., Maqsood A. Electrical and magnetic characterization of nanocrystalline Ni-Zn ferrite synthesis by co-precipitation route. J. Magn. Magn. Mater. 2008;320(3-4):270-275. DOI: 10.1016/j.jmmm.2007.05.032
- Tijare S.N., Joshi M.V., Padole P.S., Mangrulkar P.A. Photocatalytic hydrogen generation through water splitting on nano-crystalline LaFeO3 perovskite. Int. J. Hydrogen Energy. 2012;37(13): 10451-10456. DOI: 10.1016/j.ijhydene.2012.01.120
- Li S., Lin Y.H., Zhang B.P., Wang Y. Controlled fabrication of BiFeO3 uniform microcrystals and their magnetic and photocatalytic behaviors. J. Phys. Chem. C. 2010;114(7):2903-2908. DOI: 10.1021/jp910401u
- Thirumalairajan S., Girija K., Hebalkar N.Y., Mangalaraj D. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activities. RSC Adv. 2013;3(20):7549-7561. DOI: 10.1039/c3ra00006k
- Feng Y.N., Wang H.C., Luo Y.D., Shen Y. Ferromagnetic and photocatalytic behaviors observed in Ca-doped BiFeO3 nanofibres. J. Appl. Phys. 2013;113(14):3-6. DOI: 10.1063/1.4801796
- Mohan S., Subramanian B., Bhaumik I., Gupta P.K. Nanostructured Bi(i_x)Gd(x)FeO3_a multiferroic photocatalyst on its sunlight driven photocatalytic activity. RSC Adv. 2014;4(32):16871-16878. DOI: 10.1039/c4ra00137k
- Tang P., Chen H., Cao F., Pan G. Magnetically recoverable and visible-light-driven nanocrystalline YFeO3 photocatalysts. Catal. Sci. Technol. 2011;1(7): 1145-1148. DOI: 10.1039/c1cy00199j
- Dandia A., Jain A.K., Sharma S. CuFe2O4 nanoparticles as a highly efficient and magnetically recoverable catalyst for the synthesis of medicinally privileged spiropyrimidine scaffolds. RSC Adv. 2013;3(9):2924-2934. DOI: 10.1039/c2ra22477a
- Tamaddon F., Amirpoor F. Improved catalyst-free synthesis of pyrrole derivatives in aqueous media. Synlett. 2013;24(14): 1791-1794. DOI: 10.1055/s-0033-1339294
- Yang S., Xie W., Zhou H., Wu C. Alkoxylation reactions of aryl halides catalyzed by magnetic copper ferrite. Tetrahedron. 2013;69(16):3415-3418. DOI: 10.1016/j.tet.2013.02.077
- Nguyen A.T., Pham L.T., Phan N.T.S., Truong T. Efficient and robust superparamagnetic copper ferrite nanoparticle-catalyzed sequential methylation and C-H activation: Aldehyde-free propargylamine synthesis. Catal. Sci. Technol. Royal Society of Chemistry. 2014;4(12):4281-4288. DOI: 10.1039/c4cy00753k
- Nguyen A.T., Nguyen L.T.M., Nguyen C.K., Truong T. Superparamagnetic copper ferrite nanoparticles as an efficient heterogeneous catalyst for the a-arylation of 1,3-diketones with C-C cleavage. ChemCatChem. 2014;6(3):815-823. DOI: 10.1002/cctc.201300708
- Gon?alves A., Mares M.K.L., Zamian E.R., Filho J.N. Statistical optimization of biodiesel production from waste cooking oil using magnetic acid heterogeneous catalyst MoO3/SrFe2O4. Fuel. 2021;304:121463. DOI: 10.1016/j.fuel.2021.121463
- Da Silva A.L., Farias A.F.F., Pontes J.R.M., Rodrigues A.M. Synthesis of the ZnO-Ni0.5Zna5Fe2O4-Fe2O3 magnetic catalyst in pilot-scale by combustion reaction and its application on the biodiesel production process from oil residual. Arab. J. Chem. KingSaud University. 2020;13(11):7665-7679. DOI: 10.1016/j.arabjc.2020.09.003
- Ashok A., Ratnaji T., Kennedy L.J., Vijaya J.J. Magnetically recoverable Mg substituted zinc ferrite nanocatalyst for biodiesel production: Process optimization, kinetic and thermodynamic analysis. Renew. Energy. Elsevier Ltd. 2021;163:480-494. DOI: 10.1016/j.renene.2020.08.081
- Sainz M.A., Mazzoni A.D., Aglietti E.F., Caballero A. Thermochemical stability of spinel (MgOAl2O3) under strong reducing conditions. Mater. Chem. Phys. 2004;86(2-3):399-408. DOI: 10.1016/j.matchemphys.2004.04.007
- Liu G.Q., Wen L., Wang X., Ma B.Y. Effect of the impurity LixNii_xO on the electrochemical properties of 5V cathode material LiNi0.5Mn1.5O4. J. Alloys Compd. 2011;509(38):9377-9381. DOI: 10.1016/j.jallcom.2011.07.045
- Zhao Q., Feng G., Jiang F., Lan S. Comparison of Fe2TiO5/C photocatalysts synthesized: Via a nonhydrolytic sol-gel method and solid-state reaction method. RSC Adv. Royal Society of Chemistry. 2020;10(71):43762-43772. DOI: 10.1039/d0ra07884k
- Fan L., Zheng H., Zhou X., Zhang H. A comparative study of microstructure, magnetic, and electromagnetic properties of Zn2W hexaferrite prepared by sol-gel and solid-state reaction methods. J. Sol-Gel Sci. Technol. Springer US. 2020;96(3):604-613. DOI: 10.1007/s10971-020-05364-2
- Kaykan L., Sijo A.K., Zywczak A., Mazurenko J. Tailoring of structural and magnetic properties of nanosized lithium ferrites synthesized by sol-gel self-combustion method. Appl. Nanosci. Springer International Publishing. 2020;10(12):4577-4583. DOI: 10.1007/s13204-020-01413-y
- Naamoune F., Messaoudi B., Kahoul A., Cherchour N. A new sol-gel synthesis of Mn3O4 oxide and its electrochemical behavior in alkaline medium. Ionics (Kiel). 2012;18(4):365-370. DOI: 10.1007/s11581-011-0621-8
- Singh, R.N. Pandey J. P., Singh N.K., Lal B. Sol-gel derived spinel MxCo3_xO4 (M = Ni, Cu; 0 < x < 1) films and oxygen evolution. Electrochim. Acta. 2000;45(12): 1911-1919. DOI: 10.1016/S0013-4686(99)00413-2
- Dutta S.K., Akhter M., Ahmed J., Amin M.K. Synthesis and catalytic activity of spinel ferrites: A brief review. Biointerface Res. Appl. Chem. 2021;12(4):4399-4416. DOI: 10.33263/BRIAC124.43994416
- Khalid N.R., Nadeem Y., Muhammad B.T., Zainab I. Facile hydrothermal synthesis of 3D flower-like La-MoS2 nanostructure for photocatalytic hydrogen energy production. Int. J. Energy Res. 2019;43(1):491-499. DOI: 10.1002/er.4286
- Araujo C., Almeida B.G., Aguiar M., Mendes J.A. Structural and magnetic properties of CoFe2O4 thin films deposited by laser ablation on Si (001) substrates. Vacuum. 2008;82(12): 1437-1440. DOI: 10.1016/j.vacuum.2008.03.014
- Parmar S., Biswas A., Sachin K.S., Ray B. Coexisting 1T/2H polymorphs, reentrant resistivity behavior, and charge distribution in MoS2-hBN 2D/2D composite thin films. Phys. Rev. Mater. 2019;3(7). DOI: 10.1103/PhysRevMaterials.3.074007
- Serrar H. Effect of water and methanol solvents on the properties of CuO thin films deposited by spray pyrolysis. Thin Solid Films. 2019;686:1-27. DOI: 10.1016/j.tsf.2019.05.001
- Patil P.S. Versatility of chemical spray pyrolysis technique. Mater. Chem. Phys. 1999;59(3): 185-198. DOI: 10.1016/S0254-0584(99)00049-8
- Park J. Chemical Vapour Deposition of Polymers: Principles, Materials and Applications. Chemical Vapour Deposition: Surface Engineering Series Volume 2. 2001;2:243-246.
- Amosov A.P., Borovinskaya I.P., Merzhanov A.G. Poroshkovaya tekhnologiya samorasprostranyayushchegosya vysokotemperaturnogo sinteza materialov: Uchebnoe posobie [Powder technology of self-propagating high-temperature synthesis of materials: Tutorial] Moscow, Mashinostroenie-1. 2007:471.
- Al-Mamun M., Su X., Zhang H., Yin H. Strongly coupled CoCr2OVcarbon nanosheets as high performance electrocatalysts for oxygen evolution reaction. Small. 2016;12(21):2866-2871. DOI: 10.1002/smll.201600549
- Qiu F., Wang Z., Liu M., Wang Z. Synthesis, characterization and microwave absorption of MXene/NiFe2O4 composites. Ceram. Int. 2021;47(17):24713-24720. DOI: 10.1016/j.ceramint.2021.05.194
- Gao X., Bi J., Wang W., Liu H. Morphology-controllable synthesis of NiFe2O4 growing on graphene nanosheets as advanced electrode material for high performance supercapacitors. J. Alloys Compd. 2020;826:154088. DOI: 10.1016/j.jallcom.2020.154088
- Ayyappan S., Paneerselvam G., Antony M.P., Philip J. Structural stability of ZnFe2O4 nanoparticles under different annealing conditions. Mater. Chem. Phys. 2011;128(3):400-404. DOI: 10.1016/j.matchemphys.2011.03.012
- Lei W., Nie L., Liu S., Zhuo Y. Influence of annealing temperature on microstructure and lithium storage performance of self-templated CuxCo3_xO4 hollow microspheres. RSC Adv. 2016;6(67):62640-62646. DOI: 10.1039/c6ra10215h
- Shukla A., Bhardwaj A.K., Singh S.C., Uttam K.N. Microwave assisted scalable synthesis of titanium ferrite nanomaterials. J. Appl. Phys. 2018;123(16):161411. DOI: 10.1063/1.5008733
- Cady C.W. Tuning the electrocatalytic water oxidation properties of AB2O4 spinel nanocrystals: A (Li, Mg, Zn) and B (Mn, Co) site variants of LiMn2O4. ACS Catal. 2015;5(6):3403-3410. DOI: 10.1021/acscatal.5b00265
- Mellsop S.R., Gardiner A., Marshall A.T. Electrocatalytic Oxygen Evolution on Electrochemically Deposited Cobalt Oxide Films: Comparison with Thermally Deposited Films and Effect of Thermal Treatment. Electrocatalysis. 2014;5(4):445-455. DOI: 10.1007/s12678-014-0212-3
- Garg N., Menaka, Ramanujachary K.V., Lofland S.E. Nanostructured dimagnesium manganese oxide (Spinel): Control of size, shape and their magnetic and electro catalytic properties. J. Solid State Chem. Elsevier. 2013;197:392-397. DOI: 10.1016/j.jssc.2012.08.063
- Sun S., Zeng H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002;124(28):8204-8205. DOI: 10.1021/ja026501x
- Arzamasov B.N., Makarova V.I., Muhin G.G. Materialovedenie: Uchebnik dlya vuzov. 8-e izd. [Materials Science: Textbook for universities. 8th ed.]. Moscow, BMSTU, 2008. 648 p.
- Khort A., Hedberg J., Mei N., Romanovski V. Corrosion and transformation of solution combustion synthesized Co, Ni and CoNi nanoparticles in synthetic freshwater with and without natural organic matter. Sci. Rep. Nature Publishing Group UK. 2021;11(1):1-14. DOI: 10.1038/s41598-021-87250-7
- Swanson H.E., McMurdie H.F., Morris M.C., Evans E.H., Paretzkin B. Standard X-ray diffraction powder patterns Section 9 - Data for 63 Substances. Monogr. 25. Nat. Bur. Stand. (U.S.). 1971;9:22.
- Hastings J.M., Corliss L.M. Neutron diffraction studies of zinc ferrite and nickel ferrite. Rev. Mod. Phys. 1953;25(1): 114-119. DOI: 10.1103/RevModPhys.25.114
- Verwey E.J.W., Heilmann E.L. Physical properties and cation arrangement of oxides with spinel structures I. Cation arrangement in spinels. J. Chem. Phys. 1947;15(4): 174-180. DOI: 10.1063/1.1746464
- Morris M.C., McMurdie H.F., Evans E.H., Paretzkin Z., Parker H.S., Pyrros N.P. Standard X-ray Diffraction Powder Patterns Section 20 - Data for 71 Substances. Monogr. 25. Natl. Bur. Stand. (U.S.) 1983;20:47.
- Brese N.E., O'Keeffe M., Ramakrishna B.L., Von Dreele R.B. Low-tempetature structure of CuO and AgO and their relationships to those of MgO and PbO. J. Solid State Chem. 1990;89:184. DOI: 10.1016/0022-4596(90)90310-T
- Morris M.C., McMurdie H.F., Evans E.H., Paretzkin B., Parker H.S., Panagiotopoulos N.C. Standard X-ray diffraction powder patterns section 18 — Data for 58 Substances. Monogr. 25. Natl. Bur. Stand. (U.S.) 1981;18:37.
- Subramanyam, K.N. Neutron and X-ray diffraction studies of certain doped nickel ferrites. J. Phys. C: Solid State Phys. 1971;4(15):2266. DOI: 10.1088/0022-3719/4/15/012
- Cairns R.W., Ott E. X-Ray studies of the system nickel-oxygen-water. I. Nickelous oxide and hydroxide. J. Am. Chem. Soc. 1933;55(2):527-533. DOI: 10.1021/ja01329a013
- Swanson H.E., McMurdie H.F., Morris M.C., Evans E.H., Paretzkin B. Standard X-ray diffraction powder patterns Section 9 - Data for 63 Substances. Monogr. 25. Nat. Bur. Stand. (U.S.). 1971;9:22.
- Schmahl N.G., Eikerling G.F.Z. Über kryptomodifikationen des Cu(II)-oxids. Phys. Chem. Neue Folge. (Wiesbaden). 1968;62:268-279. DOI: 10.1524/zpch.1968.62.5_6.268
- Waerenborgh J.C., Figueiredo M.O., Cabral J.M.P., Pereira L.C.J. Temperature and composition dependence of the cation distribution in synthetic ZnFeyAl2_yO4 (0 < y < 1) Spinels. J. Solid State Chem. 1994; 111(2):300-309. DOI:10.1006/jssc.1994.1231


