Virtual surgical planning in soft tissue reconstruction for oral cancer
Автор: Amiraliyev K.N., Rahimov Ch.R., Amiraliyev N.M., Farzaliyev I.M.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Опыт работы онкологических учреждений
Статья в выпуске: 6 т.22, 2023 года.
Бесплатный доступ
Introduction. Reconstruction of soft tissue defects after radical surgeries in cancer patients is important for early surgical rehabilitation and improving quality of life. Our study presents technologies for virtual surgical planning (VSP) of soft tissue defect reconstruction in patients with squamous cell carcinoma of the oral cavity. Case presentation. We described VSP in a report of a 54-year-old patient with locally advanced buccal mucosa cancer after extensive radical resection and reported the results. VSP was used to construct a 3D model from CT images, which was used to accurately assess the margin of radical surgical resection, as well as to develop individually based reconstruction of soft tissue defects. Next, we reported a series of cases of patients with oral cancer of various locations, who, after radical surgery, underwent reconstruction with using of VSP (n=7) or conventional reconstruction (n=10). A comparative analysis of intra and postoperative results was carried out.
Oral cancer, radical surgery, soft tissue reconstruction, virtual surgical planning
Короткий адрес: https://sciup.org/140303549
IDR: 140303549 | УДК: 616.31-006.6-089.844 | DOI: 10.21294/1814-4861-2023-22-6-121-129
Текст научной статьи Virtual surgical planning in soft tissue reconstruction for oral cancer
Oral squamous cell carcinoma (SCC) is by far the most common type of head and neck cancer worldwide [1]. Despite the fact that cancer of this localization can be detected at an early stage, patients are often diagnosed with it in the later stages. Radical surgical resection is a key component of interdisciplinary approaches to the treatment of this category of patients [2–5]. Both the tumor itself and extensive surgical resection can cause large and complex defects, which subsequently affect the functional and aesthetic results, so the reconstruction of defects is important to achieve an optimal quality of life in this group of cancer patients [6–8].
In the conventional surgical treatment of this group of patients, surgeons often rely on their own clinical experience for tumor resection, flap preparation, and defect reconstruction. Due to the complex anatomy and special functions of the affected areas, it is difficult to achieve accurate flap preparation and defect reconstruction, as well as to achieve ideal functional and aesthetic results.
Over the past few years, 3D virtual planning technology and individual patient planning have revolutionized head and neck bone reconstruction. This led to a reduction in the operation time and good functional and aesthetic results. Digital technology is increasingly being used to assist in the reconstruction of bones [9, 10]. Despite these achievements, the practice of 3D technology in the reconstruction of soft tissue defects of the head and neck is poorly understood. Due to the complex surfaces of this area, as well as soft tissue elasticity and irregular spatial structure, very few studies have used digital technologies to reconstruct soft tissue defects. In our opinion, this is an area where the same advantages can be applied. We are representing two clinical cases of implementation of VSP (virtual surgical planning) for preoperative planning of sub- mental flap (SMF) transfer in post resection buccal mucosa defect reconstruction and compared the clinical data of reconstruction for other localizations of soft tissue defects of the oral cavity in patients using VSP technology or without it.
Material and Methods
Current study was performed on 17 patients with SCC of the mouth and skin of the head and neck in period of 2020–23 in Departments of Oral and Maxillofacial surgery and Head and Neck Tumors surgery of Azerbaijan Medical University. After radical surgeries for locally advanced SCC, reconstruction of soft tissue defects was performed in 7 patients using VSP, and in the remaining 10 using the conventional method. In all cases, clinical, radiological and laboratory examinations were performed preoperatively. Moreover, in all cases, preoperative biopsy and subsequent pathological conformation of squamous cell carcinoma was obtained.
According to our department’s protocol for preoperative assessment of tumor cases, all patients underwent contrast CT scan of the head and neck for primary tumor and regional metastasis detection. Accumulated data were well documented and used for subsequent computer assisted preoperative planning. The data obtained were analyzed using Fishers exact test. The statistical processing of the obtained indicators was carried out using biometric methods: the average value (M) used in statistics of variations, the average error of the average value (m), the maximum (max) and minimum (min) values, and Student’s criterion (t). Statistical analysis was performed with SPSS version 23.0 for Windows (SPSS, Chicago, Illinois, USA). A two-sided p<0.05 was defined as stasticially significant.
Computed assisted simulation was performed on Materialise Mimics Research 21.0 software. The data
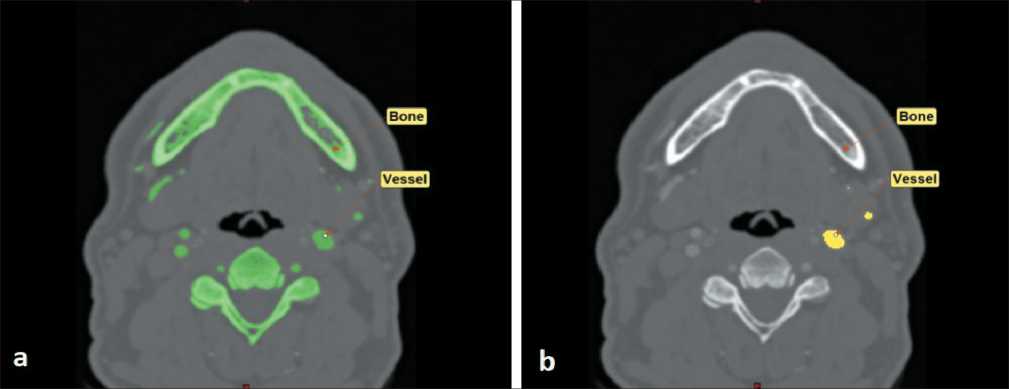
Fig 1. Segmentation of anatomical structures: a) general segmentation of bones and contrasted arteries; b) extraction of external carotid artery. Note: created by the authors
Рис. 1. Сегментация анатомических структур: а) общая сегментация костей и контрастированных артерий; б) идентификация наружной сонной артерии. Примечание: рисунок выполнен авторами
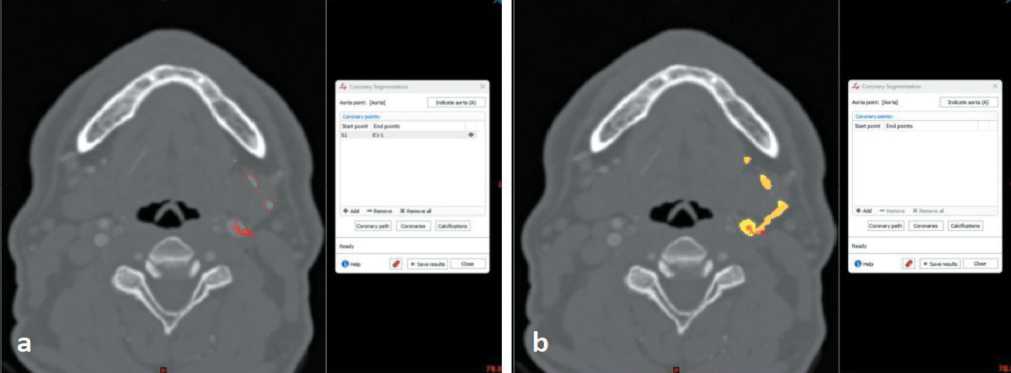
Fig 2. Advanced coronary segmentation: a) detection of submental artery on axial slices; b) 3D reconstruction of submental artery. Note: created by the authors
Рис. 2. Расширенная сегментация коронарных сосудов: а) обнаружение подбородочной артерии на аксиальных срезах; б) 3D-реконструкция подбородочной артерии. Примечание: рисунок выполнен авторами
obtained from VSP were also well documented and used as a reference during surgery.
Case presentation
The 54-year-old male patient visited our clinic with a tumor on the mucosa of the left cheek. As a result of the examination, the patient was diagnosed with squamous cell carcinoma of the left cheek mucosa (T3N0M0). At the consultation, it was decided to perform a radical resection of the tumor at the first stage of treatment. SF was chosen as a reconstructive material.
The general idea of presented method comes from capabilities of Materialise Mimics Reasearch 21.0 software, which allows advanced segmentation of heart and coronary arteries. CT scan data were obtained in DICOM format and uploaded to specified software. On the first step of initial segmentation, by the means of “Bone scale Thresholding” and “Region growth”, virtual functions were done. This maneuver allows segmentation of both facial skeleton and contrasted arteries of the region of interest. Then, by implication of “Crop mask” and additional implication of “Region growth” functions, segmentation of external carotid artery was achieved (Fig. 1).
Next to this step one used “Advanced coronary segmentation” tool in order to trace the course of submental artery, which was well contrasted on CT scan data. After tracing of the artery in the axilla, sliced 3D reconstruction of its course was also achieved (Fig. 2). The next step was associated to 3D reconstruction of basic anatomical structures, which should be used in such reconstructive procedure. Therefore, additional segmentation followed by 3D virtual reconstruction was done for artery, bones and skin of the patient (Fig. 3).
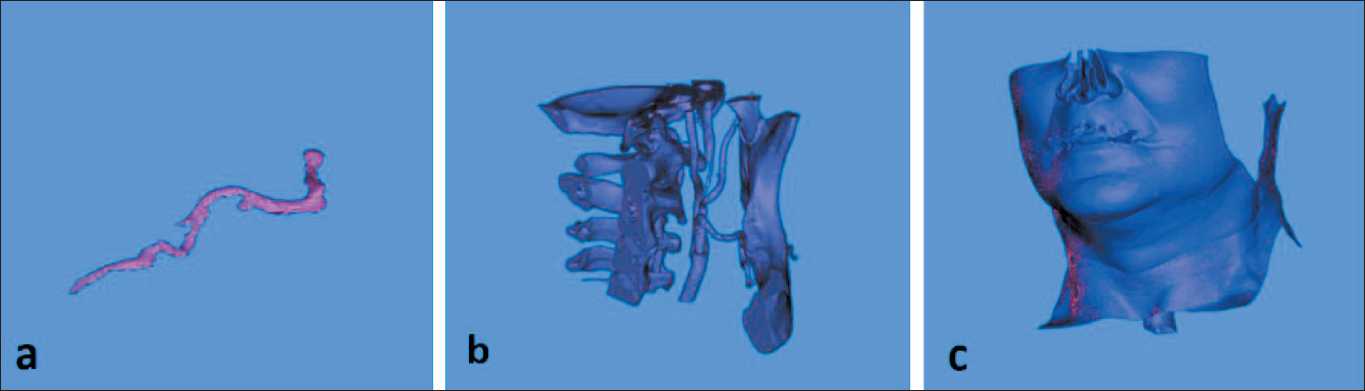
Fig 3. 3D reconstruction step: a) 3D reconstruction of submental artery; b) 3D reconstruction of facial skeleton; c) 3D reconstruction of the skin. Note: created by the authors
Рис. 3. Этап 3D-реконструкции: a) 3D-реконструкция подбородочной артерии; b) 3D-реконструкция лицевого скелета; c) 3D-реконструкция кожи. Примечание: рисунок выполнен авторами
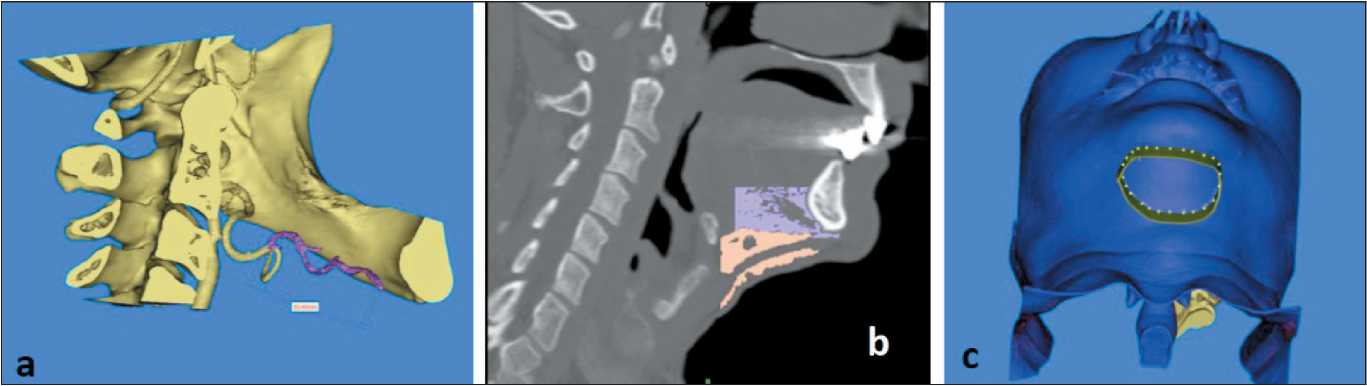
Fig 4. Prefabrication of submental flap raising scenario: a) determination of the length of submental artery; b) segmentation of anterior belly of digastric muscle and submental fat pad; c) outlining of proposed submental flap’s skin component. Note: created by the authors Рис. 4. Готовый сценарий поднятия подбородочного лоскута: а) определение длины подбородочной артерии; б) сегментация переднего брюшка дигастральной мышцы и подбородочной жировой площадки;
-
в) контур предполагаемого кожного компонента подбородочного лоскута. Примечание: рисунок выполнен авторами
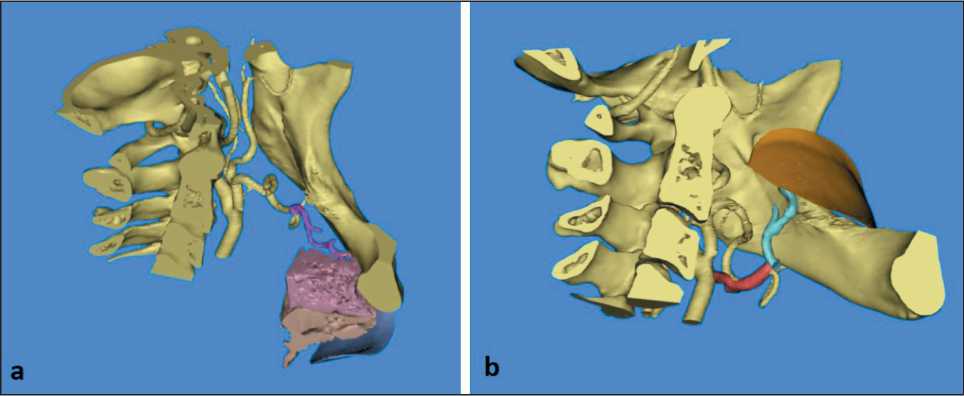
Fig 5. 3D simulation of flap’s transfer: a) complete 3D reconstruction of all components of the flap; b) virtual transfer of submental flap to defected region. Note: created by the authors
Рис. 5. 3D-моделирование переноса лоскута: a) полная 3D-реконструкция всех компонентов лоскута; b) виртуальный перенос подбородочного лоскута в область дефекта. Примечание: рисунок выполнен авторами
Additionally, we performed analysis of the relationship of submental artery and mandible and measure the length of artery, that was calculated as 55.40 mm (Fig. 4a). In order to complete full thickness virtual submental flap one performed additional segmentation of anterior belly of digastric muscle and submental fat pad, as well as final outlining of skin part of the flap (Fig 4b, c).
After all necessary preparation, final design of flap was achieved. Thus, based on clinical data related to suspected defect anatomy and size, final virtual transfer of the flap was done (Fig. 5a, b). It should be noted that no significant bending or twisting of submental artery was noted on this step, that theoretically confirmed availability of this flap for proposed reconstructive procedure. Finally, all virtual data was well documented and used intraoperatively as additional guidelines.
Results
The case series were compared on the clinico-pathological characteristics of the patients and on the intra- and postoperative results. Clinical and pathological features of patients with SCC of the oral cavity in the VSP and conventional groups are presented in Table 1. There were no significant differences in patient age, male/female ratio, defect location, and TNM stages between the two groups.
Patients in the VSP group had significantly shorter operative time (190 ± 7.8 vs 235 ± 8.3 min) and reduced post-operative hospital stay time (7.6 ± 2.9 vs 13.1 ± 3.5 days) compared to the conventional group. Patients in both groups had similar intraoperative blood loss (Table 2).
In addition, patients in the two groups had comparable postoperative complications. In the VSP group,
|
table 1/Таблица 1 Clinical and pathological characteristics of patients in the VsP and conventional groups Клинико-патологическая характеристика пациентов в ВХП и обычной группах |
||||
|
Parameteres/Параметры |
VSP group/Группа ВХП (n=7) |
Conventional group/ Группа сравнения (n=10) |
p |
|
|
Gender/Пол |
||||
|
Male/Мужской Female/Женский |
5 2 |
7 3 |
0.65 |
|
|
Age (years)/Возраст (лет) |
60.1 ± 7.2 |
58.2 ± 9.6 |
0.30 |
|
|
T caterory/Критерий Т |
||||
|
T3 T4 |
4 3 |
6 4 |
0.54 |
|
|
Lymphnodes/Лимфоузлы |
||||
|
N0 N+ |
5 2 |
7 3 |
0.62 |
|
|
Soft tissue defect location/Локализация дефекта мягких тканей |
||||
|
Tongue/Язык |
2 |
3 |
||
|
Buccal mucosa/Слизистая оболочка щеки |
2 |
3 |
||
|
Floor of mouth/Дно полости рта |
1 |
2 |
1.00 |
|
|
Alveolar ridge mucosa/ |
1 |
1 |
||
|
Слизистая оболочка альвеолярного отростка |
||||
|
Retromolar region/Ретромолярная область |
1 |
1 |
||
|
Note: created by the authors. |
||||
|
Примечание: таблица составлена авторами. |
||||
|
table 2/Таблица 2 |
||||
|
Comparison of surgical outcomes between the VsP and conventional group Сравнение хирургических результатов между ВХП и обычной группой |
||||
|
Results/Результаты |
Conventional group/ VSP group/Группа ВХП Группа сравнения (n=7) (n=10) |
p |
||
|
Operative time (min)/ Продолжительность операции (мин) |
190 ± 7.8 |
235 ± 8.3 |
0.02 |
|
|
Intraoperative blood loss (ml)/ Интраоперационная кровопотеря (мл) |
215 ± 8.0 |
230 ± 8.7 |
0.90 |
|
|
Post-operational hospital stay (days)/ |
7. 6 ± 2.9 |
13. 1 ± 3.5 |
0.04 |
|
|
Послеоперационное пребывание в стационаре (дни) |
||||
|
Note: created by the authors. |
||||
|
Примечание: таблица составлена авторами. |
||||
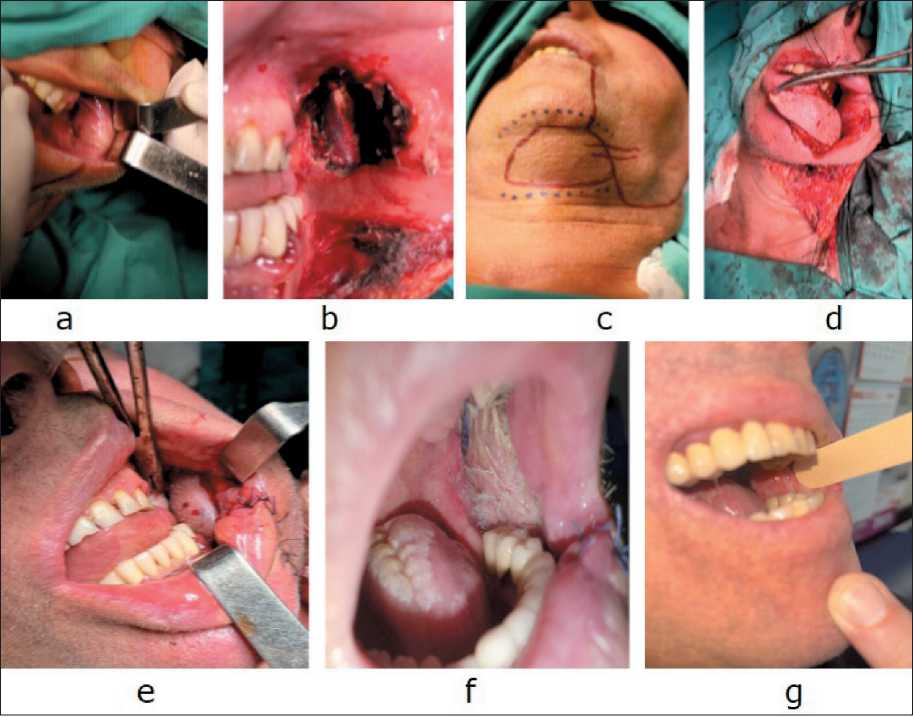
Fig. 6. a) SCC of the left buccal mucosa; b) defect after radical resection; c) design of the submental flap; d) adaptation of the formed flap to the defect area; e) submental flap sutured in place; f) 3 months after surgery; g) 1 year after surgery. Note: created by the authors Рис. 6. a) плоскоклеточный рак слизистой оболочки левой щеки; b) дефект после радикальной резекции; c) дизайн подбородочного лоскута; d) адаптация сформированного лоскута к области дефекта; e) подбородочный лоскут ушит на место; f) вид раны через 3 мес после операции; g) вид раны через 1 год после операции. Примечание: рисунок выполнен авторами table 3/Таблица 3
Comparison of postoperative complications between the VsP and conventional groups Сравнение послеоперационных осложнений в ВХП и обычной группе
|
Parameteres/Параметры |
VSP group/Группа ВХП (n=7) |
Conventional group/ Группа сравнения (n=10) |
p |
|
Post-operative complications/Послеоперационные осложнения |
|||
|
No/Нет |
5 |
6 |
0.28 |
|
Yes/Да |
2 |
4 |
|
|
Type of complications/Тип осложнений |
|||
|
Wound dehiscence/Расхождение краев раны |
1 |
2 |
|
|
Marginal necrosis/Краевой некроз |
1 |
1 |
|
|
0.15 |
|||
|
Partial necrosis/ Частичный некроз |
0 |
1 |
|
|
Total necrosis/Тотальный некроз |
0 |
0 |
|
Note: created by the authors.
Примечание: таблица составлена авторами.
-
2 cases had postoperative complication with wound dehiscence. Postoperative complications occurred in 4 cases of the conventional reconstruction group, which included 2 wound dehiscence, 1 marginal and 1 partial necroses of the flap (Table 3). Tough the results were not significantly different, the VSP group tended to have fewer and milder complications.
Discussion
In reconstructive surgery, preoperative planning is essential for an optimal functional and aesthetic outcome. Creating a 3D model has been used in industrial design for a decade, but only recently has it taken hold in medicine. 3D printing is one such technology, which is fast, convenient and relatively affordable [9, 11–13]. The current standard of care for locally advanced head and neck tumors is radical surgery and appropriate reconstruction followed by adjuvant, radiation, or chemoradiotherapy [2–4]. Reconstruction should be aimed at maximum accuracy and achieving optimal function, as this will help the patient’s rehabilitation and improve his quality of life [1, 6–8, 12].
Digital technologies are increasingly being used to help reconstruct the mandibular bone after surgical resection of tumors with satisfactory results [9–11]. Due to complex head and neck surfaces, soft tissue elasticity, and irregular spatial patterns, very little research has been done on digital technologies for the reconstruction of head and neck soft tissue defects.
-
H. Koumoullis et al. [13] presented the first report on personalized individual planning for soft tissue reconstruction. They described the case of a patient with oral cancer who underwent tongue reconstruction after a hemiglossectomy using a 3D template. The operation was successful, the postoperative period without complications. The oncological, functional and aesthetic results were excellent. This report confirms that patient- specific 3D planning can be used in the reconstruction of soft tissue defects of the head and neck. J. Xu et al. [14] report a case series study in patients with oral and oropharyngeal squamous cell carcinoma who were randomly assigned to receive computerized soft tissue reconstruction (CARST) (n=15) or conventional soft tissue reconstruction (n=15). The results of the study indicated that the CARST group had significantly shorter operative and postoperative hospital stays, higher flap utilization, and a trend towards fewer and milder postoperative complications. S. Battalgia et al. [11] applied the appropriate protocol to 42 consecutive patients requiring composite mandibular reconstruction using perforated skin for soft tissue reconstructive surgery. Before surgery, all patients underwent CTA to assess the position of the primary vessels. The authors conclude that this protocol appears to be a valuable approach to the evaluation and virtual modeling of compound mandibular reconstructions. It is an important tool for intraoperative reproduction of the planned soft tissue area to be reconstructed.
Список литературы Virtual surgical planning in soft tissue reconstruction for oral cancer
- Tranby E.P., Heaton L.J., Tomar S.L., Kelly A.L., Fager G.L., Backley M., Frantsve-Hawley J. Oral Cancer Prevalence, Mortality, and Costs in Medicaid and Commercial Insurance Claims Data. Cancer Epidemiol Biomarkers Prev. 2022; 31(9): 1849-57. https://doi.org/10.1158/1055-9965. EPI-22-0114.
- Pfster D.G., Spencer S., Adelstein D., Adkins D., Anzai Y., Brizel D.M. Bruce J.Y., Busse P.M., Caudell J.J., Cmelak A.J., Colevas A.D., Eisele D.W., Fenton M., Foote R.L., Galloway T., Gillison M.L., Haddad R.I., Hicks W.L., Hitchcock Y.J., Jimeno A., Leizman D., Maghami E., Mell L.K., Mittal B.B., Pinto H.A., Ridge J.A., Rocco J.W., Rodriguez C.P., Shah J.P., Weber R.S., Weinstein G., Witek M., Worden F., Yom S.S., Zhen W., Burns J.L., Darlow S.D. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. JNCCN. 2020; 18(7): 873-98. https://doi.org/10.6004/jnccn.2020.0031.
- Alterio D., Marvaso G., Ferrari A., Volpe S., Orecchia R., JereczekFossa B.A. Modern radiotherapy for head and neck cancer. Semin Oncol. 2019; 46(3): 233-245. https://doi.org/10.1053/j.seminoncol.2019.07.002.
- Gyawali B., Shimokata T., Honda K., Ando Y. Chemotherapy in locally advanced head and neck squamous cell carcinoma. Cancer Treat Rev. 2016; 44: 10-6. https://doi.org/10.1016/j.ctrv.2016.01.002.
- Neligan P.C. Head and neck reconstruction. Plast Reconstr Surg. 2013: 131(2): 260-9. https://doi.org/10.1097/PRS.0b013e3182778938.
- Forner D., Phillips T., Rigby M., Hart R., Taylor M., Trites J. (2016). Submental island fap reconstruction reduces cost in oral cancer reconstruction compared to radial forearm free fap reconstruction: a case series and cost analysis. J Otolaryngol Head Neck Surg. 2016; 45. https://doi.org/10.1186/s40463-016-0124-8.
- Rogers S.N., Lowe D., Yueh B., Weymuller E.A. The physical function and social-emotional function subscales of the University of Washington Quality of Life Questionnaire. Arch Otolaryngol Head Neck Surg. 2010; 136(4): 352-7. https://doi.org/10.1001/archoto.2010.32.
- Semple C.J., McKenna G., Parahoo R., Rogers S.N., Tiblom Ehrsson Y. Factors that afect quality of life for older people with head and neck cancer: A systematic review. Eur J Oncol Nurs. 2023; 63. https://doi.org/10.1016/j.ejon.2023.102280.
- Chang E.I., Boukovalas S., Liu J., Largo R.D., Hanasono M.M., Garvey P.B. Reconstruction of Posterior Mandibulectomy Defects in the Modern Era of Virtual Planning and Three-Dimensional Modeling. Plast Reconstr Surg. 2019; 144(3): 453-62. https://doi.org/10.1097/PRS.0000000000005954.
- Kumar B.P., Venkatesh V., Kumar K.A., Yadav B.Y., Mohan S.R. Mandibular Reconstruction: Overview. J Maxillofac Oral Surg. 2016; 15(4): 425-41. https://doi.org/10.1007/s12663-015-0766-5.
- Battaglia S., Ricotta F., Maiolo V., Savastio G., Contedini F., Cipriani R., Bortolani B., Cercenelli L., Marcelli E., Marchetti C., Tarsitano A. Computer-assisted surgery for reconstruction of complex mandibular defects using osteomyocutaneous microvascular fbular free faps: Use of a skin paddle-outlining guide for soft-tissue reconstruction. A technical report. J Craniomaxillofac Surg. 2019; 47(2), 293-9. https://doi.org/10.1016/j.jcms.2018.11.018.
- Chae M.P., Lin F., Spychal R.T., Hunter-Smith D.J., Rozen W.M. 3D-Printed haptic “Reverse” models for preoperative planning in soft tissue reconstruction: A case report. Microsurgery. 2015; 35: 148-53. https://doi.org/10.1002/micr.22293.
- Koumoullis H., Burley O., Kyzas P. Patient-specifc soft tissue reconstruction: an IDEAL stage I report of hemiglossectomy reconstruction and introduction of the PANSOFOS fap. Br J Oral Maxillofac Surg. 2020; 58(6), 681-6. https://doi.org/10.1016/j.bjoms.2020.04.017.
- Xu J., Lai F., Liu Y., Tan Z., Zheng C., Wang J., Guo H., Jiang L., Ge X., Lan X., Chen C., Ge M. Novel computer-aided reconstruction of soft tissue defects following resection of oral and oropharyngeal squamous cell carcinoma. World J Surg Oncol. 2022; 20(1): 196. https://doi.org/10.1186/s12957-022-02654-7.


