Water cleaning from metal ions by electrochemical treatment by using the diaphragm using of a diagram electrolyzer for cleaning sewage from hexavalent chromium
Автор: Shestakov I. Y., Vasilyeva Y. A.
Журнал: Siberian Aerospace Journal @vestnik-sibsau-en
Рубрика: Technological processes and material science
Статья в выпуске: 4 vol.20, 2019 года.
Бесплатный доступ
In the production of space rocket technology, electrochemical processes are used, as a result there is pollution of sewage by metal ions. The strict requirements of environmental authorities do not allow sewage, containing metal ions with concentration exceeding the maximum permissible values, to be discharged directly into reservoir or sewers. The greatest difficulties are caused by the purification of water from hexavalent chromium. The proposed methods for purifying from hexavalent chromium, electrocoagulation method, galvanocoagulation method, sorption methods, combined methods, have some disadvantages, such as: significant energy consumption, significant consumption of soluble metal anodes, passivation of the anodes, need for large excesses of reagent (iron salts), large amounts of precipitate and the complexity of its dehydration, high cost and scarcity of sorbents, high consumption of reagents for the regeneration of sorbents, and others. This work shows equipment for experiments, including a diaphragm electrolyzer with a coaxial arrangement of electrodes. Formulas for calculating the chromium ions flux due to migration and diffusion are presented. The difference between the calculated amperage from the practical one is 25 %, and the theoretical degree of purification from the real one is 4 %, which confirms the effectiveness of the proposed cleaning method. The concentration of chromium anions was determined by atomic absorption spectroscopy. The degree of purification of water from chromium ranged from 84 to 96 %. The highest degree of purification (96 %) was obtained with an electrolysis duration of 29 minutes.
Electroplatings wastes, hexavalent chromium, diaphragm electrolyzer, electrochemical effect, direct current.
Короткий адрес: https://sciup.org/148321711
IDR: 148321711 | УДК: 628.16.087 | DOI: 10.31772/2587-6066-2019-20-4-497-501
Текст научной статьи Water cleaning from metal ions by electrochemical treatment by using the diaphragm using of a diagram electrolyzer for cleaning sewage from hexavalent chromium
Introduction. Due to the constantly increasing requirements for standards for the concentration of harmful substances (in particular, for substances of the first hazard class – hexavalent chromium) in industrial effluents (in particular, in electroplatings wastes), interest in various cleaning methods has always been relevant.
In the production of aircraft parts, galvanic technologies are used, as a result of which electroplatings wastes of electroplating shop contaminated with metal ions are formed. The requirements of environmental authorities do not allow direct discharge into reservoirs or sewers of electroplatings wastes containing high concentration of chromium, for example, in the form of chromic acid, metal chromates, etc. In addition, chromium is an expensive metal and its extraction from chromium-containing electroplatings wastes is also desirable from an economic point of view. For a long time there is a need for an economical and efficient way to remove chromium from industrial wastewater and its subsequent regeneration [1–7].
Currently, there are a large number of ways to purify industrial water from chromium – mechanical, chemical, electrical, physical, biological, combined, etc. The proposed methods for purifying hexavalent chromium – electrocoagulation method, galvanocoagulation method, sorption methods, combined methods have their drawbacks, such as: high power consumption, significant consumption of soluble metal anodes, passivation of the anodes, the need to use a reagent (iron salts), the formation of a large amount of precipitate and the complexity of its subsequent dehydration, the high cost and scarcity of sorbents, the use of reagents for the regeneration of sorbents, and others [8; 9]. SibSAU staff have developed a combined method of purifying water from anions and cations, including hexavalent chromium [10; 11]. However, the proposed method requires prolonged water precipitation (8–10 hours), which is not always possible under production conditions. Therefore, the development of an effective method of purifying water from hexavalent chromium is an urgent problem.
Diaphragm electrolyzers are used to change the active reaction and redox potential of the medium [12]. In this work, it is noted that when using a diaphragm electrolyzer due to the migration of hexavalent chromium ions from the cathode chambers, their concentration in catholyte decreases. However, this phenomenon has not been investigated with the aim of using chromium ions to purify water.
Work Description. Fig. 1 shows the experimental setup with the help of which water was purified from hexavalent chromium.
The experimental procedure was similar to that described in [13]. The difference is that the number of holes in a small tank (3) is increased and the water of the electroplating shop taken from the bath with rinsing water following the chromium bath with chromium-containing solution in the manufacture (coating) of aircraft parts was cleaned.
Based on the calculations given in article [13], the electrolysis time is 29 minutes. In this case, the degree of purification should be equal to 100 %.
The degree of purification was calculated by the formula
Y =
(с )
| С о С к 1 100,
I Со J where Со , Ск – initial and final concentration of the removed metal ion, mg/l;
Y = ( 7.0 - 0.29 ^ 1оо % = 96 %
( 7.0 )
where Со [CrO 4 ]2- = 7.0 mg/l; Ск [CrO 4 ]2- = 0.29 mg/l.
The results of the experiments are presented in fig. 2.
Thus, the discrepancy with the estimated degree of purification is 4 %.
It is known that the flow of dissolved particles in the electrolyzer consists of three terms [14; 15]:
Ni = -Zi • Ui • F • Ci • VФ - Di • VCi + Ci • v, flow migration diffusion convection where Ni – component i flow, mol/(cm2 s); Zi – ion charge in proton charge units; Ui – component mobility i, cm2mol/(J s); F – Faraday constant, C/mol; Ci – concentration of component i, mol/cm3; VФ - voltage between electrodes, V; Di - diffusion coefficient, cm2/s; VCi - concentration gradient, mol/cm3.
We neglect the third term since the electrolysis mode is stationary and, accordingly, the convective component is negligible.
After data substitution, the migration flow will be The diffusion component is determined: equal to:
–Z i ∙ U i ∙ F ∙ C i ∙ ∇ Ф = 7.655 ∙ 10–10 mol . D i ∙ ∇ C i = 26.77 ∙ 10–11 mol .
s ⋅ cm2 s ⋅ cm2
Fig. 1. Experimental setup:
1 – fluoroplastic tank; 2 – cathode (stainless steel 12Х18Н10Т);
3 – fluoroplastic glass (small tank) with holes; 4 – tarpaulin diaphragm;
5 – graphite anode; 6 – DC source; 7 – multimeter
Рис. 1. Экспериментальная установка:
1 – ёмкость из фторопласта; 2 – катод (нержавеющая сталь 12Х18Н10Т);
3 – фторопластовый стакан (малая ёмкость) с отверстиями; 4 – диафрагма из брезентовой ткани; 5 – анод из графита; 6 – источник постоянного тока; 7 – мультиметр
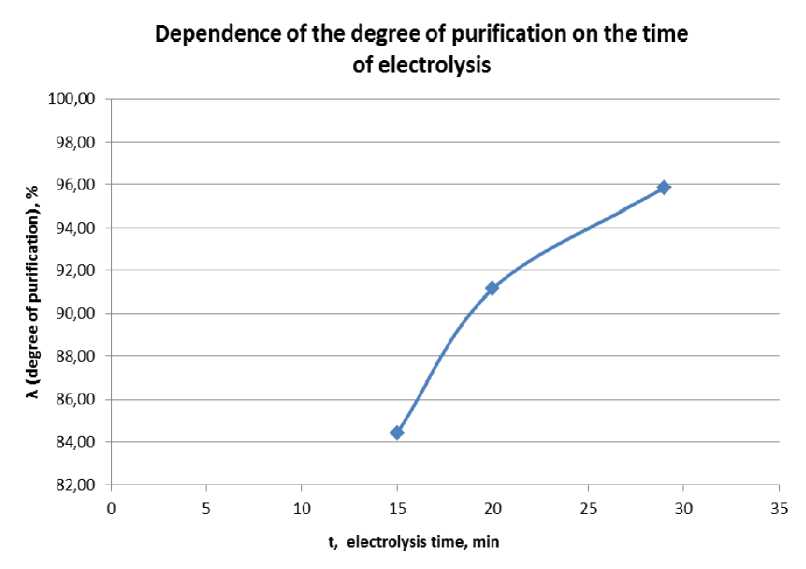
Fig. 2. Dependence of the degree of purification on the time of electrolysis
Рис. 2. Зависимость степени очистки от времени проведения электролиза
Thus, the flux of chromium ions will be equal to:
N i =10.3 ∙ 10–10
mol s ⋅ cm2
The amperage will be:
I calc = N i ∙ F ∙ S = 993 950 ∙ 10–10 А ∙ 323.106 cm2 = cm2
= 3.2 ∙ 10–2A = 0.032 A, where Ni – component i flow, mol/(cm2*s); F – Faraday constant, C/mol; S – surface area of the cathode (anode) involved in electrolysis, cm2.
Conclusion. The highest degree of purification (96 %) was obtained with an electrolysis duration of 29 minutes. The process was carried out at a voltage of 50 V.
The difference between the calculated amperage from the practical one is about 25 %, and the theoretical degree of purification from the real one is 4 %, which confirms the effectiveness of the proposed cleaning method and the correct calculation method.
Further experiments are required, followed by practical testing under industrial conditions with an increased rate of electrochemical reactions in order to reduce electrolysis time.
Список литературы Water cleaning from metal ions by electrochemical treatment by using the diaphragm using of a diagram electrolyzer for cleaning sewage from hexavalent chromium
- Khalemskiy A. M. [Toxic industrial sewage treatment of chrome compounds, arsenic, and organic substances by electrocoagulation and ferrate methods]. Ekologiya proizvodstva. Metallurgiya i mashinostroenie. 2006, No. 3(4), P. 15 (In Russ.).
- Zvyagintseva A. V., Boldyreva O. N. [Sewage neutralization of galvanizing room is one of environmental safety methods]. Mashinostroitel'. 2003, No. 2, P. 48–52 (In Russ.).
- Skovronek E. [Sewage treatment in galvanotechnics]. Gal'vanotekhnika i obrabotka poverkhnosti. 2002, Vol. 10, No. 4, P. 55–61.
- Verbol' S. V., Zapariy M. M., Kozlov V. V. [Method of galvanic sewage treatment]. Ekologiya i promyshlennost' Rossii. 2001, P. 7–8.
- Stuart F. E. Electronic water purification progress report on the electronic coagulator – a new device which gives promise of unusually speedy effective results. Water Sewage. 1946, No. 84, P. 24–26.
- Bonilla C. F. Possibilities of the electronic coagulator for water treatment. Water Sewage. 1947. No. 85, P. 21–45.
- Chen G. Electrochemical technologies in wastewater treatment. Separation and Purification Technology. 2004, No. 38, P. 11–41.
- Yakovlev S. V., Krasnoborod'ko I. G., Rogov V. M. Tekhnologiya elektrokhimicheskoy ochistki vody [Technology of water electrochemical purification]. Leningrad, Stroyizdat Publ., 1987, P. 312.
- Rogov V. M., Filipchuk V. L. Elektrokhimicheskaya tekhnologiya izmeneniya svoystv vody [Electrochemical technology of change in water properties]. L'vov, Vishcha shkola Publ., 1989, P. 128.
- Shestakov I. Ya., Raeva O. V. Sposob ochistki vody i vodnykh rastvorov ot anionov i kationov [Water and water solutions treatment of anions and cations]. Patent RF, No. 2519383, 2014.
- Shestakov I. Ya., Raeva O. V. [Water treatment of metal ions by AC electrochemical action during air barbotage and follow coagulation and sedimentation]. Vestnik SibGAU. 2014, No. 2(54), P. 148–154.
- Pas'ko O. A., Semenov A. V., Smirnov G. V., Smirnov D. G. Bytovoy diafragmennyy elektrolizer [Common diaphragm electrolyzer]. Patent RF, No. 2344996, 2006.
- Dobosh D. Elektrokhimicheskie konstanty [Electrochemical constants]. Moscow, Mir Publ., 1980, P. 365.
- Shestakov I. Ya., Vasilyeva E. A., Remizov I. A. [Water purification from chromium ions in a diaphragm electrolyzer]. Vestnik SibGAU. 2016, Vol. 17, No. 2, P. 498–501 (In Russ.).
- Newman J. Elektrokhimicheskie sistemy [Electrochemical systems]. Moscow, Mir Publ., 1977, P. 245–249.


