Water status and protein pattern changes towards salt stress in cotton
Автор: Saleh Basel
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.9, 2013 года.
Бесплатный доступ
A pot experiment was conducted to evaluate performance of four upland cotton ( Gossypium hirsutum L.) varieties, Deir-Ezzor22 (DE22), Niab78 (N78), Aleppo118 (A118) and Deltapine50 (DP50) grown under non-saline conditions (control) and salt stress (200 mM NaCl) for 7 weeks. During the course of the experiment, potential osmotic (ψ), leaf relative water content (RWC), water saturation deficit (WSD), membrane stability index (MSI), and salt tolerance index (STI) were measured. Moreover, protein pattern changes were detected under salt application. Data indicated that potential osmotic (ψ) considerably decreased under saline condition. Where, the lowest value was recorded for DP50 and A118, while the highest one was recorded for N78 and DE22. Whereas, RWC was strongly reduced for DP50 and A118, while, it was slightly increased for N78 and DE22 varieties. However, it was noticed that WSD showed an inverse trend of RWC. In contrast to DP50 and A118 varieties, both the estimated membrane stability index (MSI) and salt tolerance index (STI) were higher in N78 and DE22. In addition, salt application induced changes in protein pattern including decrease, increase or induction of some polypeptides bands. According to our results, N78 and DE22 varieties showed a better protection mechanism against salinity damage than the other two tested varieties.
Cotton, osmotic potential, protein, rwc, salt stress, variety
Короткий адрес: https://sciup.org/14323704
IDR: 14323704
Текст научной статьи Water status and protein pattern changes towards salt stress in cotton
Currently, a third of all irrigated lands in the world are affected to a greater or lesser degree by salinity and the salinity problem continues to increase (Munns 2005). In Syria, about 50% of irrigated soil suffers from salinity that has been considered as the main limitation of agricultural production. Cotton is planted in the north, east, and central parts of Syria particularly, along the valley of the Euphrates river (and some of its major tributaries). Cotton is an economically important plant grown world-wide as a principal source of staple fiber and vegetable oil. Cotton is one of the major fiber crops in Syria, with a cultivated area amount to 125,000 hectares, and a production of 470,000 tons of seed cotton and lint production is estimated at 160,000 tons. Yarn spinning capacity is estimated at 180,000 tons (USDA 2011). Salinity causes deleterious effects on plant physiology response including, water status changes, ion imbalance, nutritional uptake perturbed, water deficit and destabilization of membrane and protein structure (Aldesuquy et al 2012, Ghogdi et al 2012, Metwali et al 2011, Senadheera et al 2012). Leaf water potential was considered as an effective indices for salt tolerance screening in plant breeding programs. Because, leaf water potential is a thermodynamic expression of water status in plant leaves, which is widely used in plant research in last decades (Aldesuquy et al 2012, Ali et al 2012, Kusvuran 2012, Saleh 2012). Loss of water from turgid leaf tissue in response to transpiration results is not only a significant decline in water potential but also a decline in osmotic potential. Leaf relative water content (RWC) was also considered as an alternative measure of plant water status, reflecting the metabolic activity in plant tissues (Asfaw 2011, Ghogdi et al 2012, Hossain et al 2006, Win et al 2011).
It is well documented that, environmental stresses particularly salinity cause important modification in gene expression. Gene expression is manifested by the appearance of proteins, which are not present before the stimulation. Salinity promotes the synthesis of salt stress - specific proteins, many of these proteins were suggested to protect the cell against the adverse effect of salt stress. Accumulation of these proteins is a common response to salt stress (Kong-ngern et al 2005, Mahmoodzadeh 2009, Meratan et al 2008, Metwali et al 2011, Mohamed 2005).
Thereby, this study aimed to investigate the water status and protein pattern changes in some cultivated cotton varieties grown in Syria under non-saline conditions (control) and salt stress (200 mM NaCl) for 7 weeks.
MATERIALS AND METHODS
Plant materials and growth conditions
Two local varieties have been selected on the basis of their wide range of tolerance towards salinity; Deir-Ezzor22 (DE22) as salt-tolerant and Aleppo118 (A118) as salt-sensitive variety (Saleh 2012). These two varieties were examined compared with two introduced cotton varieties, Niab78 (N78) (known as salt-tolerant) and Deltapine50 (DP50) (known as salt-sensitive) under 0 and 200 mM NaCl for 7 weeks. Seeds of upland cotton ( G. hirsutum . L) Niab78, Deir-Ezzor22, Deltapine50 and Aleppo118 were provided by the General Commission for Scientific Agricultural Research of Syria (GCSAR).
Seeds were soaked in distilled H 2 O for 24 h and then planted in pots filled with a 1/3:2/3 (v/v) mixture of perlite:peat mosse. Germination was carried out in a greenhouse at temperature of 18°C, 12 h photoperiod and relative humidity of 80%. Seedlings were allowed to grow in a greenhouse under controlled conditions (temperature of 25°C, 12 h photoperiod and relative humidity of 80%). Seedlings were irrigated with tap water for one week before the initiation of NaCl treatment. Salt stress application was carried out by adding NaCl (200 mM) to the water. Plants were irrigated twice a week by water with or without salt. All solutions were changed twice a week. The same environmental conditions were maintained during the experiment.
Water status
The experiment (Five replicates by treatment) was carried out in the greenhouse for 7 weeks. The osmotic potential was measured using a microosmometer (Osmometer) apparatus. While, for relative water content (RWC) measurements: Two leaves from each plant were excised, and their fresh weight was scored immediately. After floating them in deionized water at 4°C overnight, their rehydrated weight was determined. Finally, they were dried in an oven at 70°C overnight and weighed. RWC was calculated using the following formula RWC = (fresh weight - dry weight)/ (rehydrated weight - dry weight) x 100. Whereas, water saturation deficit (WSD) was calculated as described WSD = 100-RWC. Membrane stability index (MSI) was determined by recording the electrical conductivity of leaf ions leaching in double distilled water. Leaf samples (100 mg) were taken in test tubes containing 10 ml of double distilled water in two sets. One set was kept at 40 oC for 30 min and another set at 100 oC in boiling water bath for 15 min and their respective electrical conductivities, C1 and C2 respectively, were measured by a EC meter (HANNA, HI 99301, Hanna Instruments Inc. Woonsocket-RI-USA). Membrane stability index was calculated according to the following formula: MSI = [1 - (C1/C2)] × 100.
Salt tolerance index (STI) of the varieties, expressed as the ratio of total dry weight produced under the NaCl treatments compared to the control treatment x 100. Where, total dry weights (TDW) were obtained after oven drying at 70 °C for 72 h.
Protein extraction
The experiment was stopped 7 weeks after salt application. Leaves samplings of 100 mg for each treatment/variety were ground to a powder in liquid N using a mortar and pestle. Then 0.1 mL SDS sample buffer (Laemmli 1970) was added and the tissues were further ground. After thawing, the homogenates were heated at 95°C for 5 min and then briefly centrifuged at 12 000 X g for 5 min to pellet the cellular debris. The resulting supernatants involved total protein extracts. One-dimensional
SDS-PAGE was run with 5% (w/v) stacking and 15% (w/v) resolving gel bisacrylamide concentrations using the buffer system of Laemmli (1970) in a midle-gel (160 x 180 x 1 mm) electrophoresis apparatus ( Hoefer SE-600 , Groton, USA). Ten micro letters of total protein were loaded per lane. Constant voltage of 50 V was applied for 1 h followed by 150 V until the tracking dye (bromophenol blue) reached the bottom of the gel.
Statistical analysis
All statistical analyses were performed using Statview 4.5 statistical package (Abacus 1996) significance level ( P = 0.05). Data were subjected to analysis of variance (ANOVA) for the determination of differences in means between tested plants of each concentration of NaCl applied. Differences between means were tested for significance by Fisher's PLSD test. Data are expressed as mean of five replicates.
RESULTS AND DISCUSSION
Analysis of variances of data for the different investigated parameters for the four cotton varieties tested in this study was summarize in table 1.
Osmotic potential
Our results showed that potential osmotic (ψ) considerably decreased under saline treatment compared to their respective control for all tested varieties (Fig. 1). This decrease can be attributed to the cellular water loss under salinity (Stoeva and Kaymakanova 2008). Gama et al (2007) reported that the reduced water potential could be explained by the fact that during stress carbon allocation, osmotic adjustment and accumulation of soluble sugars compete with other factors and can affect growth. Analysis of variance indicated that the effect of 200 mM NaCl level on this parameter and among varieties was highly significant (P < 0.001). Moreover, the interaction between variety and salt treatment was also significant (P < 0.001).
Under 200 mM NaCl this index was negatively affected and the reduction was almost by about 63, 89, 96 and 152 % below the control for N78, DE22, DP50 and A118, respectively. Thereby, N78 and DE22 varieties could be considered as salt tolerant varieties compared to the other two tested varieties. This observation was in agreement with Saleh (2012) in cotton as well as for other crops (Aldesuquy et al 2012, Ali et al 2012, Kusvuran 2012).
Relative water content (RWC)
Genotyping variation in RWC was observed for all tested varieties (Fig. 2). Leaf RWC was found to be the highest in N78 and DE22 accessions, whereas DP50 accession was the lowest one under saline conditions (Fig. 2). At 200 mM NaCl, seedlings of N78 and DE22 were able to adjust osmotically, leading to maintenance of relative water content (RWC), in contrast to A118 and DP50, RWC was 31 and 23 % below the control. Whereas, RWC was about 3 and 5 % ( P < 0.01) over the control for DE22 and N78 respectively. Extent of salt-induced effects on relative water content has been used as one of the vital water relation parameters for assessing degree of salt tolerance in plants (Asfaw 2011, Ghogdi et al 2012, Hossain et al 2006, Win et al 2011).
It has been well documented that water status has a crucial role in higher plants grown under saline conditions, because of many important physiological and morphological processes, such as leaf enlargement, stomatal opening, and associated leaf photosynthesis are directly affected by the reduction of leaf turgor potential which accompanies the loss of water from leaf tissues (Ciçek and Cakirlar 2002).
Water saturation deficit (WSD)
Water saturation deficit (WSD) showed an inverse trend of RWC (Fig. 3). Our data showed that this parameter was decreased by 19 and 8% under saline conditions in N78 and DE22 respectively, while it was increased by 5 and 146% under saline conditions up to their respective control in DP50 and A118 respectively. It is evident that DP50 and A118 showed higher relative WSD (% to the control) than N78 and DE22. Where, this parameter decreased by 8 and 19% below their respective control compared to the saline conditions in DE22 and N78 respectively. Whereas, it increased by 5 and 146% up to control compared to the saline condition in DP50 and A118 respectively. This finding revealed that the DP50 and A118 suffered from more water deficit under salt stress than N78 and DE22. This observation could be reflect that the latter two varieties had developed a better mechanism of salt tolerance enabled them to maintains a well cellular water status. These results were in accordance of Hossain et al (2006) in wheat ( Triticum aestivum L.) who reported that the salt tolerant wheat variety exhibited higher RWC and lower WSD values compared to susceptible one.
Membrane stability index (MSI)
Salt stress caused considerable membrane injuries in the leaves of all tested varieties (Fig. 4). Membrane stability index (MSI) negatively influenced by salinity. This negative impact on DP50 and A118 was pronounced than that on N78 and DE22 varieties (Fig. 4). It was noted, that this estimated parameter was decreased by 18, 21, 31 and 33% in N78, DE22, DP50 and A118 varieties respectively under saline conditions compared to their respective control. Analysis of variance indicated that the effect of 200 mM NaCl level on this parameter and among varieties was highly significant (P < 0.001).
The change in biological membranes stability is a key indicator of cellular damage. Drought and other stresses always results in cellular membrane injures including the increase of membrane permeability (Esfandiari et al 2011, Gomathi and Rakkiyapan 2011, Senadheera et al 2012).
Esfandiari et al (2011) reported that the salt resistance wheat ( Triticum turgidum L.) genotype might be due to increased activity of antioxidant enzymes, low lipid peroxidation, assumingly lower changes in membrane stability index and avoidance of Na+ absorption. Gomathi and Rakkiyapan (2011) reported that salt resistant in sugarcane genotypes associated with lowest lipid peroxidation and highest membrane thermostablity. Recently, Senadheera et al (2012) comparative study showed that salinity tolerance in rice ( Oryza sativa L.) correlated to maintenance lower Na+/K+ in the root and to a higher membrane stability index (MSI).
Salt tolerance index (STI)
As shown in table 2, total dry weight (TDW) was impaired by saline treatment. The decline was estimated to be by 26, 20, 40 and 43% under saline conditions compared to their respective control for N78, DE22, DP50 and A118 respectively. In this respect, the results are in agreement with findings previously obtained by Munns (2002), who reported that, plants show variable capacity to salinity tolerance that could be range from negligible effect to plant death. Considerable differences are found between plant species. For example, after exposure to 200 mM NaCl, a salt-tolerant species such as sugarbeet might have a reduction of only 20% in dry weight, whereas, a moderately tolerant species such as cotton might have a 60% reduction, and a sensitive species such as soybean might die. Moreover, this finding was in recent accordance of Saleh (2011) in cotton. Analysis of variance indicated that the effect of NaCl levels on the previous parameter in all varieties tested was highly significant (P < 0.001).
A STI index was used to identify cotton varieties towards salt stress. It is obvious to notice that, the tested varieties respond differently towards salt stress (Table 2). Regarding the estimated STI, N78 and DE22 exhibited the highest STI values (74.1 and 81.4 respectively), conversely to DP50 and A118 (62.2 and 58.6 respectively). This finding suggest that DP50 and A118 varieties could be considered as salt sensitive while, N78 and DE22 could be considered as salt tolerant one. Analysis of variance indicated that saline treatment effect significantly ( P < 0.001) STI parameter. Other investigations demonstrated genotypic variation in salt tolerance present among these varieties based upon various examined physiological indices (Saleh 2011, 2012). The results indicate that the estimated STI is reliable parameter for cotton ranking variety for their tolerance to salt stress. This result was in agreement of Bagci et al (2007), who reported that, the STI was higher in salt tolerant wheat ( Triticum aestivum L.) genotypes compared to the sensitive one.
According to Saleh (2011) investigation, DE22 variety relatively performed better under salinity compared to the other tested varieties, particularly the A118 variety. Overall, most of the studies investigating salt stress tolerance focus on the development of genotypes with lower Na+ accumulation (exclusion of Na+) and higher K+/Na+ ratio in plant tissues (Saleh 2011, Senadheera et al
Protein pattern
To understand whether some of protein may be involved in salt stress response in four cotton varieties, we investigated their expression pattern in response to salt stress. Salt stress induced noticeable changes in protein pattern from the four cotton varieties tested (Fig. 5). Comparing the protein profiles between control plants and those salt treated using SDS-PAGE showed that protein changes found under salt treatment compared to their respective control (Fig. 5). The expression of (~19, ~21 and 26 kDa) and (~21 kDa) protein was highly increased by salt treatment for N78 and DE22 respectively, indicating that it could be play a role in salt stress response. On the other hand, newly synthesis protein of ~30 kDa was recorded for both DE22 and N78 varieties under saline treatment compared to their respective control. Whereas, for the two other tested varieties (DP50 and A118) decreases of some polypeptide bands (~19, 26 and 34 kDa) were observed under saline condition compared to their respective control; reflecting their sensitivity to salt stress.
Other researches revealed the decrease of some polypeptide bands under saline condition compared to their respective control e.g. 20 kDa (Amini et al 2007), 23 and 55 kDa (Kong-ngern et al 2005) and 21.1 kDa (Sousa et al 2004). Depressed protein synthesis and acceleration and its degradation in plants in response to salt stress have been reported by a number of workers (Kong-ngern et al 2005, Mohamed 2005, Meratan et al 2008, Mahmoodzadeh 2009, Metwali et al 2011).
Mahmoodzadeh (2009) revealed an increase in bands intensity for 18, 27, 30, 32 and 50 kDa protein was observed in salt Canola treated plants. Metwali et al (2011) stated that SDS-PAGE analysis revealed that plant grown under salinity showed induction or suppression in the synthesis of few polypeptides. Where, 27, 17 & 99 kDa were inhibited, 27 & 87 kDa were not expressed, 63 & 87 kDa were expressed and newly synthesis protein of 51 kDa were recorded in Wheat ( Triticum aestivum L) cultivars. Consequently, the present data clearly support the correlation between water status and protein pattern changes with salinity tolerance.
In conclusion, the current study showed that: I) Water status is highly sensitive to salinity and is dominant in determining the plant responses to salt stress; II) High salinity tolerance recorded for N78 and DE22 varieties was attributed to their greatest potential osmotic values and the better physiological mechanisms associated with their less affected water relation and STI indices; III) Protein pattern changes was correlated with salt tolerance performance. Thereby, cotton varieties that could be considered as salt tolerant will help in boosting crop production in salt affected regions.
Table 1: Analysis of variances (mean squares) of data for the four cotton varieties after 7 weeks growth at 0 and 200 mM NaCl
|
S.O.V |
df |
Ψ |
RWC |
WSD |
MSI |
TDW |
STI |
|
V |
3 |
297.667* |
398.499ns |
398.499ns |
378.366* |
1.694ns |
276.368ns |
|
T |
1 |
4040.1* |
261.786ns |
261.786ns |
3543.033* |
21.978* |
9541.517* |
|
V x T |
3 |
201.367* |
465.624ns |
465.624ns |
45.437ns |
0.62ns |
276.368ns |
* : significant at 0.05 level, ns: Non-significant, V: variety, T: treatment
Table 2: Salt tolerance index (STI) of four cotton varieties after 7 weeks growth at 0 and 200 mM NaCl
|
Variety |
Control |
200 mM |
||
|
TDW |
STI |
TDW |
STI |
|
|
N78 |
4.744 aX |
100 aX |
3.528 bX |
74.068 bX |
|
DE22 |
4.778 aX |
100 aX |
3.818 bX |
81.400 bX |
|
DP50 |
4.202 aX |
100 aX |
2.536 bY |
62.399 bY |
|
A118 |
4.834 aX |
100 aX |
2.746 bY |
58.575 bY |
|
LSD 0.05 (T) = 0.692 |
LSD 0.05 (T) = 12.832 |
|||
|
LSD 0.05 (V) = 0.978 |
LSD 0.05 (V) = 17.370 |
|||
Mean with the same letters in each column (a-b) and each row (X-Y) don’t differ significantly at P = 0.05. (T): treatment; (V): variety.
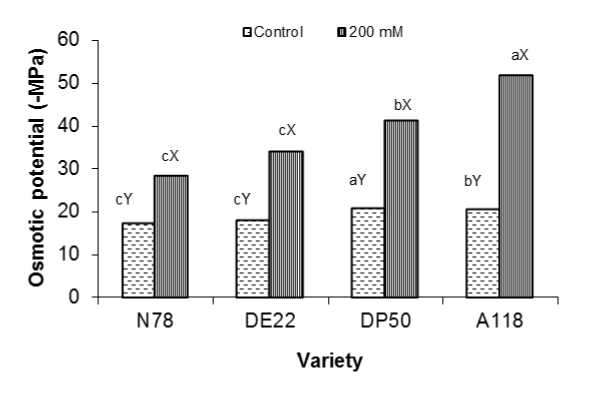
Figure 1: Potential osmotic (-MPa) of four cotton varieties after 7 weeks growth at 0 and 200 mM NaCl. Mean with the same letters among varieties (a-c) and (X-Z) within the same variety don’t differ significantly at P = 0.05
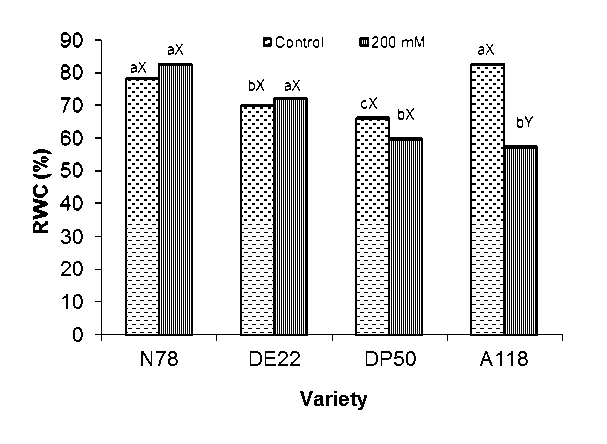
Figure 2: Relative water content RWC (%) of four cotton varieties after 7 weeks growth at 0 and 200 mM NaCl. Mean with the same letters among varieties (a-c) and (X-Y) within the same variety don’t differ significantly at P = 0.05
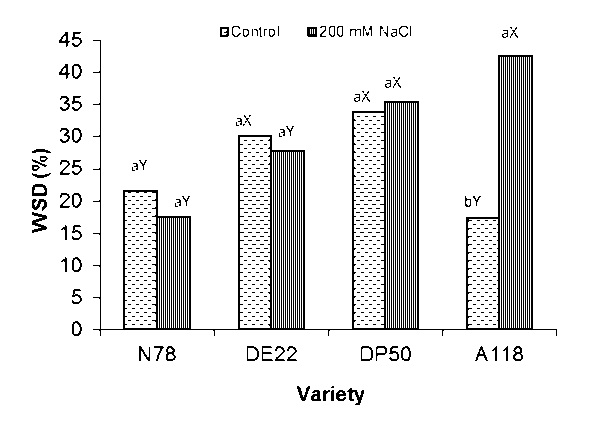
Figure 3: Water saturation deficit WSD (%) of four cotton varieties after 7 weeks growth at 0 and 200 mM NaCl. Mean with the same letters among varieties (a-b) and (X-Y) within the same variety don’t differ significantly at P = 0.05
□ Control Ш200 mM NaCI
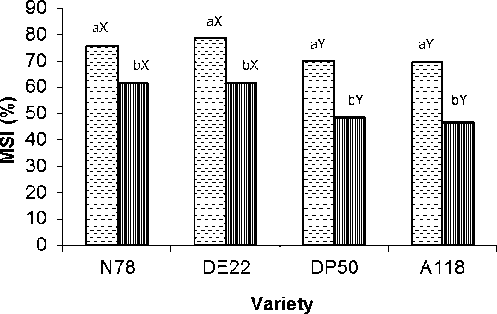
Figure 4: Membrane stability index (MSI) (%) of four cotton varieties after 7 weeks growth at 0 and 200 mM NaCl Mean with the same letters among varieties (a-b) and (X-Y) within the same variety don’t differ significantly at P = 0.05
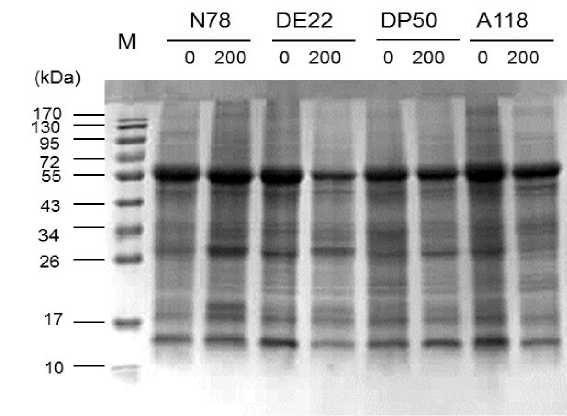
Figure 5: SDS-PAGE profiles of leaf proteins from four cotton varieties after 7 weeks growth at 0 and 200 mM NaCl
ACKNOWLEDGMENTS
We thank Dr. I. Othman (Director General of AECS) and Dr. N. Mir A li (Head of Molecular Biology and Biotechnology Department in AECS) for their support, and also the Plant Biotechnology group for technical assistance.
REFRENCES
Abacus Concept (1996) Statview 4.5 Statistical Program Abacus Concepts Corporation, Berkeley, CA, USA.
Aldesuquy, H.S., Baka, Z.A., El-Shehaby, O.A. and Ghanem, H.E. (2012). Efficacy of seawater salinity on osmotic adjustment and solutes allocation in wheat ( Triticum aestivum ) flag leaf during grain filling. Int. J. Plant Physiol. Biochem ., 4 (3) , 33-45.
Ali, A., Basra, S.M.A., Iqbal, J., Hussain, S., Subhani, M.N., Sarwar, M. and Ahmed, M. (2012). Augmenting the salt tolerance in wheat ( Triticum aestivum ) through exogenously applied silicon. Afr. J. Biotech ., 11 (3) , 642-649.
Amini, F., Ehsanpour, A.A., Hoang, Q. T. and Shin, J.Sh. (2007). Protein pattern changes in tomato under in vitro salt stress. Russ. J. Plant. Physiol ., 54 (4) , 464-471.
Asfaw, K.G. (2011.) Effects of salinity on seedling biomass production and relative water content of twenty sorghum ( Sorghum biolor L. Moench) accessions. Asian J. Agric. Sci ., 3 (3) , 242-249.
Bagci, S.A., Ekiz, H. and Yilmaz, A. (2007). Salt tolerance of sixteen wheat genotypes during seedling growth. Turk. J. Agric. Fore ., 31 , 363372.
Çiçek, N. and Çakirlar, H. (2002). The effect of salinity on some physiological parameters in two maize cultivars. Bulgar. J. Plant Physiol ., 28 ,
66-74.
Esfandiari E., Enayati V. and Abbasi A. (2011). Biochemical and physiological changes in response to salinity in two durum wheat ( Triticum turgidum L.) genotypes. Not. Bot. Hort. Agrobot. Cluj ., 39 , 165-170.
Gama, P.B., Inanaga, S., Tanaka, K. and Nakazawa, R. (2007). Physiological response of common bean ( Phaseolus Vulg . L.) seedlings to salinity stress. Afr. J. Biotech ., 6 , 79-88.
Ghogdi, E.A., Izadi-Darbandi, A. and Borzouei, A. (2012). Effects of salinity on some physiological traits in wheat ( Triticum aestivum L.) cultivars. Indian J. Sci. Technol ., 5 (1) , 1901-1906.
Gomathi, R. and Rakkiyapan, P. (2011). Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int. J. Plant Physiol. Biochem ., 3 , 67-74.
Hossain, A.A., Halim, M.A., Hossain, F. and Maher Niger, M.A. (2006). Effect of NaCl salinity on some physiological characters of wheat ( Triticum aestivum L.). Bangladesh J. Bot ., 35 (1) , 9-15.
Kong-ngern, K., Daduang, S., Wongkham, C., Bunnag, S., Kosittrakun, M. and Theerakulpisut, P. (2005). Protein profiles in response to salt stress in leaf sheaths of rice seedlings. ScienceAsia ., 31 , 403-408.
Kusvuran, S. (2012). Effects of drought and salt stresses on growth, stomatal conductance, leaf water and osmotic potentials of melon genotypes ( Cucumis melo L.). Afr. J. Agric. Res ., 7 (5) , 775-781.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature . 227 (259) , 680-685.
Mahmoodzadeh, H. (2009). Protein profiles in response to salt stress in seeds of Brassica napus . Res. J. Environ. Sci ., 3 (2) , 225-231.
Meratan A.A., Ghaffari S.M. and Niknam, V. (2008). Effects of salinity on growth, proteins and antioxidant enzymes in three Acanthophyllum species of different ploidy levels. JSUT . 33 (4) , 1-8.
Metwali, E.M.R., Eid, M.H. and Bayoumi, T.Y. (2011). Agronomical traits and biochemical genetics markers associated with salt tolerance in wheat cultivars ( Triticum aestivum L). Aust. J. Basic Appl. Sci ., 5 (5) , 174-183.
Mohamed, A.A. (2005). Two-dimensional electrophoresis of soluble proteins and profile of some isozymes isolated from maize plant in response to NaCl. Res. J. Agric. Biol. Sci ., 1 (1) , 38-44.
Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell. Environ ., 25, 239250.
Munns, R. (2005). Genes and salt tolerance: Bringing them together. New Phytol ., 167 , 645663.
Saleh, B. (2011). Effect of salt stress (NaCl) on biomass and K+/Na+ ratio in cotton. J. Stress Physiol. Biochem ., 7 (4) , 5-14.
Saleh, B. (2012). Effect of salt stress on growth and chlorophyll content of some cultivated cotton varieties grown in Syria. Comm. Soil Sci. Plant Anal ., 43 (15) , 1976 -1983.
Senadheera, P., Tirimanne, S. and Maathuis, F.J.M. (2012). Long term salinity stress reveals cultivar specific differences in root oxidative stress response. Rice Science ., 19 , 36-43.
Stoeva, N. and Kaymakanova, K. (2008). Effect of salt stress on the growth and photosynthesis rate of bean plants ( Phaseolus vulgaris L.). J. Central Europ. Agric ., 9 , 385-392.
Sousa, M.F., Campos, F.A.P., Prisco, J.T., Eneas-Filho, J. and Gomes-Filho, E. (2004). Growth and protein pattern in cowpea seedlings subjected to salinity. Biol. Plant ., 47 , 341-346.
USDA, (2011). Syria cotton and products annual cotton report, GAIN Report (.
Wang, W., Vinocur, B., Shoseyov, O. and Altman, A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci ., 9 , 244-252.
Win, K.T., Oo, A.Z., Hirasawa, T., Ookawa, T. and Yutaka, H. (2011). Genetic analysis of Myanmar Vigna species in responses to salt stress at the seedling stage. Afr. J. Biotech ., 10 (9) , 16151624.
Список литературы Water status and protein pattern changes towards salt stress in cotton
- Abacus Concept (1996) Statview 4.5 Statistical Program Abacus Concepts Corporation, Berkeley, CA, USA.
- Aldesuquy, H.S., Baka, Z.A., El-Shehaby, O.A. and Ghanem, H.E. (2012). Efficacy of seawater salinity on osmotic adjustment and solutes allocation in wheat (Triticum aestivum) flag leaf during grain filling. Int. J. Plant Physiol. Biochem., 4 (3), 33-45.
- Ali, A., Basra, S.M.A., Iqbal, J., Hussain, S., Subhani, M.N., Sarwar, M. and Ahmed, M. (2012). Augmenting the salt tolerance in wheat (Triticum aestivum) through exogenously applied silicon. Afr. J. Biotech., 11 (3), 642-649.
- Amini, F., Ehsanpour, A.A., Hoang, Q. T. and Shin, J.Sh. (2007). Protein pattern changes in tomato under in vitro salt stress. Russ. J. Plant. Physiol., 54 (4), 464-471.
- Asfaw, K.G. (2011.) Effects of salinity on seedling biomass production and relative water content of twenty sorghum (Sorghum biolor L. Moench) accessions. Asian J. Agric. Sci., 3 (3), 242-249.
- Bagci, S.A., Ekiz, H. and Yilmaz, A. (2007). Salt tolerance of sixteen wheat genotypes during seedling growth. Turk. J. Agric. Fore., 31, 363-372.
- Çiçek, N. and Çakirlar, H. (2002). The effect of salinity on some physiological parameters in two maize cultivars. Bulgar. J. Plant Physiol., 28, 66-74.
- Esfandiari E., Enayati V. and Abbasi A. (2011). Biochemical and physiological changes in response to salinity in two durum wheat (Triticum turgidum L.) genotypes. Not. Bot. Hort. Agrobot. Cluj., 39, 165-170.
- Gama, P.B., Inanaga, S., Tanaka, K. and Nakazawa, R. (2007). Physiological response of common bean (Phaseolus Vulg. L.) seedlings to salinity stress. Afr. J. Biotech., 6, 79-88.
- Ghogdi, E.A., Izadi-Darbandi, A. and Borzouei, A. (2012). Effects of salinity on some physiological traits in wheat (Triticum aestivum L.) cultivars. Indian J. Sci. Technol., 5 (1), 1901-1906.
- Gomathi, R. and Rakkiyapan, P. (2011). Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. Int. J. Plant Physiol. Biochem., 3, 67-74.
- Hossain, A.A., Halim, M.A., Hossain, F. and Maher Niger, M.A. (2006). Effect of NaCl salinity on some physiological characters of wheat (Triticum aestivum L.). Bangladesh J. Bot., 35 (1), 9-15.
- Kong-ngern, K., Daduang, S., Wongkham, C., Bunnag, S., Kosittrakun, M. and Theerakulpisut, P. (2005). Protein profiles in response to salt stress in leaf sheaths of rice seedlings. ScienceAsia., 31, 403-408.
- Kusvuran, S. (2012). Effects of drought and salt stresses on growth, stomatal conductance, leaf water and osmotic potentials of melon genotypes (Cucumis melo L.). Afr. J. Agric. Res., 7 (5), 775-781.
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227 (259), 680-685.
- Mahmoodzadeh, H. (2009). Protein profiles in response to salt stress in seeds of Brassica napus. Res. J. Environ. Sci., 3 (2), 225-231.
- Meratan A.A., Ghaffari S.M. and Niknam, V. (2008). Effects of salinity on growth, proteins and antioxidant enzymes in three Acanthophyllum species of different ploidy levels. JSUT. 33 (4), 1-8.
- Metwali, E.M.R., Eid, M.H. and Bayoumi, T.Y. (2011). Agronomical traits and biochemical genetics markers associated with salt tolerance in wheat cultivars (Triticum aestivum L). Aust. J. Basic Appl. Sci., 5 (5), 174-183.
- Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell. Environ., 25, 239-250.
- Munns, R. (2005). Genes and salt tolerance: Bringing them together. New Phytol., 167, 645-663.
- Saleh, B. (2011). Effect of salt stress (NaCl) on biomass and K+/Na+ ratio in cotton. J. Stress Physiol. Biochem., 7 (4), 5-14.
- Saleh, B. (2012). Effect of salt stress on growth and chlorophyll content of some cultivated cotton varieties grown in Syria. Comm. Soil Sci. Plant Anal., 43 (15), 1976 -1983.
- Senadheera, P., Tirimanne, S. and Maathuis, F.J.M. (2012). Long term salinity stress reveals cultivar specific differences in root oxidative stress response. Rice Science., 19, 36-43.
- Stoeva, N. and Kaymakanova, K. (2008). Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). J. Central Europ. Agric., 9, 385-392.
- Sousa, M.F., Campos, F.A.P., Prisco, J.T., Eneas-Filho, J. and Gomes-Filho, E. (2004). Growth and protein pattern in cowpea seedlings subjected to salinity. Biol. Plant., 47, 341-346.
- USDA, (2011). Syria cotton and products annual cotton report, GAIN Report, (www.fas.usda.gov).
- Wang, W., Vinocur, B., Shoseyov, O. and Altman, A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci., 9, 244-252.
- Win, K.T., Oo, A.Z., Hirasawa, T., Ookawa, T. and Yutaka, H. (2011). Genetic analysis of Myanmar Vigna species in responses to salt stress at the seedling stage. Afr. J. Biotech., 10 (9), 1615-1624.


