Why mineral carriers are necessary for microalgae
Автор: Shchemelinina T.N., Anchugova E.M., Kotova O.B., Sun S., Shushkov D.A., Gogonin A.V., Likhanova N.V., Zueva O.M., Korchagina Yu. S.
Журнал: Вестник геонаук @vestnik-geo
Рубрика: Научные статьи
Статья в выпуске: 2 (302), 2020 года.
Бесплатный доступ
Abiotic, biotic and anthropogenic factors inhibit normal life of microorganisms. We have presented that the mineral carrier provides integrity and increases the growth of microalgal cells under stress conditions. Microalgae Chlorella vulgaris, immobilized on an analcime-containing rocks (biogeosorbent), are resistant to increased salinity (34 ‰) and organic pollutants (phenolic water with a concentration of 10 µg/dm3). The advantage of the synergetic complex of the biogeosorbent from its individual components is the reduction of the period of water purification from phenols. Biodestruction of 83 % of phenols in model water occurs in 3 days.
Stress factors, phenolic water, seawater, mineral carrier, analcime-containing rocks, microalgae, biogeosorbent, sorption, biodestruction
Короткий адрес: https://sciup.org/149129416
IDR: 149129416 | УДК: 66.081 | DOI: 10.19110/geov.2020.2.4
Текст научной статьи Why mineral carriers are necessary for microalgae
Characterization of microalgae strain
-
C . vulgaris f. globosa — Chlorophyta unicellular alga. Spherical cells are from 3.3 to 13.3 microns in diameter. The species is characterized by a wide area of distribution in the aquatic and terrestrial environments [2]. Chlorella is an alpha-mesosaprobiont species that can withstand a significant organic pollution.
Characterization of the mineral carrier
The analcime-containing rocks (sample 551) were selected from the “Veslyana” occurrence located on the left bank of the Veslyana River at a distance of about 500 m from the course. The occurrence was found by the employees of Komigeologiya LLC during a complex geological survey with a scale of 1: 2,000,000 (1987—90) and revision works on agromineral raw (2001) and was characterized by minimal overburden and favorable mining and geological conditions. The occurrence is located within the Koinskaya zeolite-bearing area (Knyazhpogost district of the Komi Republic), which is confined to crest of the Sindor swell on the western slope of the Timan Ridge [10].
The specimen of the analcime-containing rocks is represented by Permian brownish-gray argillite. According to X-ray phase analysis the sample is predominated by quartz and analcime. Goethite, hematite and feldspar minerals are minor. Layered silicates are diagnosed by weak reflexes and probably represented by mixed-layer low-ordered il-lite/smectite. The silicate analysis revealed the following components (wt. %): SiO2 — 54.46, TiO2 — 0.92, Al2O3 — 17.68, Fe2O3 — 8.11, FeO — 0.31, MnO — 0.049, CaO — 0.79, MgO — 1.59, K2O — 2.16, Na2O — 4.34, P2O5 — 0.13, LOi — 8.92, CO2 — 0.13 (Total 99.46).
The isotherms of nitrogen adsorption-desorption of analcime-containing rocks are of iV(a) type according to the iUPAC classification [16]. They are characterized by the presence of a hysteresis loop and typical of mesopo-rous sorbents. The adsorption curve shows a sharp rise at low pressures, indicating the presence of micropores in the sample (<2 nm). The rise in the adsorption curve at a relative pressure close to 1 indicates the presence of macropores. The hysteresis loop can be classified as H3 and H4 types, since clay minerals and zeolites are also present in the sample. The pore size distribution curve is characterized by a narrow bimodal distribution of pore radius in the range of 0.8—3 nm with maxima of 0.96 and 2.15 nm. Structural characteristics are presented in Table 1.
The analcime-bearing rocks should be considered as sorption raw of mixed composition, since zeolites are asso- ciated with clay minerals, which are also characterized by high sorption properties.
Microalgae cells were counted using a Goryaev-Thoma counting chamber [14] with Biomed 3 microscope, binocular: LED, quadruple nosepiece, Achromat 4x/10x/40x/100xlenses. The dehydrogenase activity of the aqueous suspension was studied according to the methodology of the All-Russian Scientific Research institute of Water Resources [9].
The surface morphology of samples with immobilized microalgae was studied by TESCAN VEGA 3 LMH scanning electron microscope with X-Max Oxford instruments energy dispersion attachment at an accelerating voltage of 5 kV.
The chemical composition of the rocks was determined by silicate analysis with 12/14 components.
X-ray phase analysis was performed by Shimadzu XRD 6000 diffractometer (CuK a radiation, Ni filter, 30 kV, 30 mA). A powder sample was taken in the range 2—65 2 6 with a speed of 1 deg/min and a scan step of 2 6 0.05°. The phase composition of the clay fraction was determined by X-ray diffraction of oriented and non-oriented samples subjected to standard diagnostic treatments.
The specific surface area, the volume of micro- and mesopores, and the total pore volume of the initial untreated sample were determined by the low-temperature physical sorption of nitrogen using NOVA 1200e Quantachrome analyzer of surface area and pore size at a temperature of —196 °C with preliminary degassing at 350 °C in vacuum within 2 hours. The specific surface area was calculated by BET method, the mesopore volume — by BJH method, the micropore volume by Dubinin-Astakhov method.
The amount of phenols was analyzed by capillary gas chromatography [5].
The microalgae strain was grown on Tamiya medium in Biostat® A MO UniVessel® Glass BB-8822000 2L 230V bioreactor for 3—5 days under conditions of liquidphase fermentation at 350 rpm, temperature 25—27 °С, pH 5.5—6.5, lightening with a lamp 175—150V 50 Hz until a titer of cells in a suspension of 108 cells/cm3 was reached. Tamiya medium (per 1 dm3 of deionized water) of the following composition: KNO 3 — 5 g, KH 2 PO 4 x ЗН 2 О — 1.25 g, MgSO 4 x 7H 2 O — 2.5 g, microelement solutions — 1 cm3 each. The trace element solutions are as follows (per 1 dm3 of deionized water).
-
1. Alkaline solution of EDTA: EDTA — 50 g; KOH — 31 g.
-
2. Acidic iron solution: FeSO 4 x 7H 2 O — 4.98 g, H2SO4— 1 cm3.
-
3. Boric acid solution: H3BO3 – 11.42 g.
-
4. The solution of trace elements: ZnSO 4 x 7H2O — 8.82 g; MnCl2 x 4H 2 O — 1.44 g; MoO3 — 0.71 g; CuSO4 x 5H 2 O — 1.57 g; Co(NO3)2 x 6H2O — 0.49 g.
Then, MA suspension was sprayed onto a mineral carrier (analcime-containing rocks) and dried at a temperature of 25 °C. The ratio of the composition of the biogeo-
Table 1. Specific surface area and porosity of the analcime-containing rocks, size 0.1—0.25 mm
Таблица 1. Удельная площадь поверхности и пористость анальцимсодержащей породы, крупность 0.1—0.25 мм
|
Specific surface area, m2/g Удельная площадь поверхности, м2/г |
Constant С BET |
Total pore volume, cm3/g Общий объем пор, см3/г |
Mesopore volume, cm3/g Объем мезопор, см3/г |
Micropore volume, cm3/g Объем микропор, см3/г |
Average pore radius, nm Средний радиус пор, нм |
|
39.66 |
223.54 |
0.0484 |
0.0365 |
0.017 |
2.44 |
sorbent: mineral carrier is 85—90 %, microalgae strain C. vulgaris — 10—15 %.
RESULTS AND DISCUSSION
Chlorella vulgaris microalgae immobilized on the analcime-containing rocks . An electron microscope study of the biogeosorbent showed the presence of MA cells on the surface of the sample (Fig. 1). During the adsorption immobilization of the cells, which are caused by electrostatic forces, several types of adhesive interaction are simultaneously realized, so, it is difficult to determine the role of each of them individually. According to [4], covalent and ionic interactions have the greatest effect on the binding of C. vulgaris microalgae to the carrier.
Influence of stress factors. The biogeosorbent was tested for resistance to relatively high temperatures, to conditions of increased salinity and to the influence of organic pollutants. To do this, the biogeosorbent samples (Table 2) were thermostated at high temperatures, added into model phenolic wa- ter and seawater taken in the coastal zone of Vladivostok. The control sample was not exposed to stress factors.
Samples No. 1, 2, 0.1 g each, were added into the sterile Tamiya nutrient medium and the control cultivation was carried out, periodically selecting samples to count MA cells and determine dehydrogenase activity (Table 3). For samples of biogeosorbent No. 3 and 4, 50 cm3 of sea or phenolic water was poured into flasks 100 cm3 and 0.1 g of biogeosorbent was added. Experimental conditions: room temperature, lighting with a grow lamp, aeration at 180 rpm.
Being the most informative parameters for assessing cell viability, metabolic and synthetic activity [13], the biomass yield and dehydrogenase activity were estimated during the experiment (on days 3, 7, 14 and 30).
The biomass yield (the ratio of newly synthesized substance of growing cells to the amount of substrate consumed — the source of matter and energy for cell growth) is a characteristic of the efficiency of the conversion of the substrate to biomass. The conversion process is derived from metabolism (plurality of biochemical reactions in

Fig. 1. SEM images of Chlorella vulgaris microalgae immobilized on the analcime-containing rocks
Рис. 1. СЭМ-изображения микроводорослей Chlorella vulgaris , иммобилизованных на анальцимсодержащей породе
Table 2. Experimental conditions
Таблица 2. Условия эксперимента
|
Biogeosorbent sample No. Номер образца биогеосорбента |
Conditions of sample processing Условия обработки образцов |
Exposure time, days Время экспозиции, сутки |
|
1 |
Control / Контроль |
– |
|
2 |
Temperature 80 °С / Температура 80 °С |
1 |
|
3 |
Seawater, salinity 34 ‰ Морская вода, соленость 34 ‰ |
30 |
|
4 |
Model phenolic water 10 μg/dm3 Модельная фенольная вода с концентрацией 10 мкг/дм3 |
30 |
Table 3. Titer of cells of Chlorella vulgaris microalgae, cells/cm3 Таблица 3. Титр клеток микроводорослей Chlorella vulgaris, кл/см3
|
Время экспозиции, сутки Exposition time, day |
Biogeosorbent sample No. / № образца биогеосорбента |
|||
|
1 |
2 |
3 |
4 |
|
|
3 |
2.4 х 10 5 |
— |
single cells detected обнаружены единичные клетки |
7.2 х 10 6 |
|
7 |
1.9 х 10 6 |
— |
то же id. |
2.3 х 10 6 |
|
14 |
1.4 х 10 7 |
— |
» |
3.7 х 10 6 |
|
30 |
1.3 х 10 7 |
— |
1.8 х 104 |
1.1 х 10 7 |
Note: Designations 1—4 are taken from Table 2. / Ïðèìå÷àíèå: Обозначения 1—4 — из табл. 2.
cells) [6]. The preferable medium for MA cultivation and their maximum yield is Tamiya medium, since it is a nutrient solution of a high concentration of mineral salts. This medium was used to cultivate a control sample of biogeosorbent and to test the survival of samples No. 2 and 3.
in the Tamiya nutrient medium, a gradual accumulation of the biomass of C. vulgaris occurred in the control sample. The maximum yield was distinguished on day 14 (Table 3). Dehydrogenase activity directly correlated with an increase in the number of cells (Fig. 3).
Temperature . No living cells were found in the sample of the nutrient medium in which the biogeosorbent thermostated at a temperature of 80 °C was placed. No dehydrogenase activity was detected (Table 3, Fig. 2).
Salinity of water. The most important abiotic factor in the habitat of aquatic organisms, including unicellular algae, is the total salinity of seawater. in the first 14 days of culturing a sample of biogeosorbent No. 4 in seawater with a salinity of 34 ‰, single MA cells were detected, then the biomass increased by day 30 (Table 3). Dehydrogenation processes corresponded to the dynamics of the accumulation of MA cells (Fig. 2).
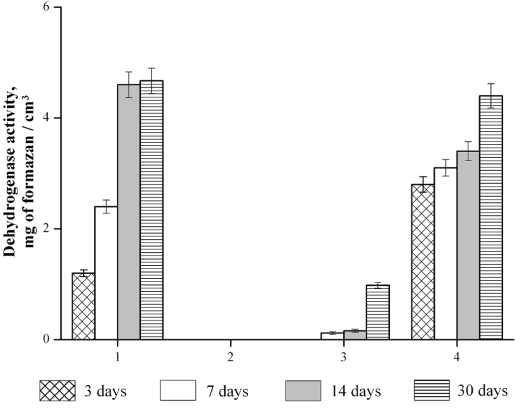
Fig. 2. Dehydrogenase activity of biomass. Designations 1—4 — from Table 2
Рис. 2. Дегидрогеназная активность биомассы. Обозначения 1—4 — из табл. 2
Organic pollutant. Phenol is especially dangerous because it is easily soluble in water. To accelerate biooxidation of organic pollutants the biochemical, biological adsorption and other methods are used [7].
We noted a high tolerance of MA to phenolic water with a mass concentration of 10 μg/dm3. When the biogeosorbent was introduced into phenolic water, MA desorption occurred, followed by cell cultivation in the medium. By the end of 30 days, the cell titer in this variant was similar to the titer of the cells of the control sample (Table 3). Dehydrogenase activity was increasing during biomass accumulation (Fig. 2).
Phenol destruction. The dynamics of the phenol content after the biogeosorbent (AMA) had been applied was studied. For comparison, a mineral carrier — analcimecontaining rocks without microalgae (A), suspended microalgae (MA) — was tested. Model phenolic water with- out additives was taken as a control: zero control (K0) — analyzed for phenol content at the beginning of the experiment, and experimental control (EC) — analyzed for phenol content after 3 and 45 days.
After 3 days from the start of the experiment, adsorption and biooxidation processes were noted in all variants (Fig. 3). The maximum treatment of phenols regarding zero control was distinguished with exposure time. in suspended MA treatments, phenols were dropped by 74 % in 3 days and 90 % in 45 days. Analcime-containing rocks showed high sorption activity to phenols — 78 and 97 % of the zero control for 3 and 45 days, respectively.
A synergetic complex of microorganisms C. vulgaris and analcime-containing rocks contributed to the intensifying of sorption and destruction of phenolic compounds compared to similar processes that occur when only analcite-containing rock or a MA suspension are introduced into the water (Fig. 3). Algorithm of the biogeosorbent functioning: sorption of phenols by a biogeosorbent → active absorption by microalgae → accumulation → biodeostruction. Moreover, when phenolic water is treated with the biogeosorbent, partial desorption of C. vulgaris micro-algal cells from the carrier occurred, and the phenol biodegradation processes were carried out simultaneously by immobilized and suspended cells. in the joint process of biological and sorption treatment of the model water by the biogeosorbent, the efficiency of phenols reduction was 84 and 98 % of the zero control for 3 and 45 days, respectively.
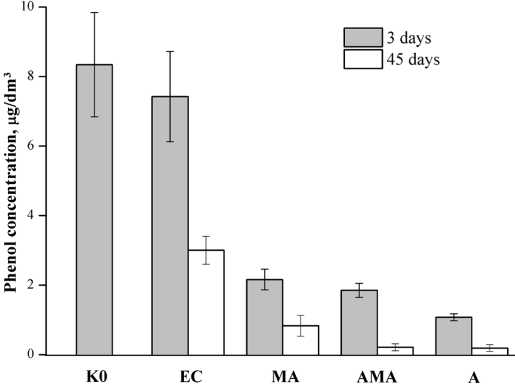
Fig. 3. Efficiency of phenol reduction in water relative to the zero control (K0) and experimental control (EC), mg/dm3: MA — suspended microalgae, A — analcime-containing rocks, AMA — analcime-containing rock with immobilized microalgae
Рис. 3. Эффективность очистки воды от фенолов относительно нулевого контроля (К0) и контроля экспериментального (КЭ), мг/дм3: МВ — микроводросли, А — анальцим-содержащая порода, АМВ — анальцимсодержащая порода с иммобилизированными микроводорослями in the control variant phenol content decreased due to physical weathering, so it is more advantageously to compare it with EC. Reducing the amount of phenols for 3 and 45 days was: MA variant — 70 and 72 %, А — 25 and 93 %, АМA — 82 and 93 %, respectively (Fig. 3).
Thus, the analcime-containing rocks are a “transport base” for C. vulgaris microalgal cells, preserving them un- 27
der stressful conditions. introducing it into a nutritious environment, microalgal cells re-activate their vital activities. C. vulgaris microalgae are tolerant to increased salinity (34 ‰) and saturation with organic pollutants (phenolic water with a concentration of 10 μg/dm3).
Any agent used ( C. vulgaris microalgae, analcimecontaining rocks, the biogeosorbent) is effective in remediation of phenols, but a distinctive feature of the biogeosorbent and its advantage is the reduction in the cleaning period, when used, and absence of secondary wastes due to biodegradation of phenols in the sorbent.
Conclusions
Thus, the algorithm of biogeosorbent functioning is as follows: sorption of phenols by the biogeosorbent → active absorption by microalgae → accumulation → biodeostruction.
By comparing of the mineral composition and sorption-structural characteristics of analcime-containing rocks and sorbents with microalgae immobilized, phenol destructive properties of biogeosorbents were estimated. Chlorella vulgaris microalgae, immobilized on analcime-containing rocks was found to be tolerant to high salinity (34 ‰) and organic pollutants (phenolic water with a concentration of 10 μg/dm3). The mineral carrier provides preserving the cell viability and increases the growth of microalgae cells.
The advantage of the synergetic complex of the biogeosorbent over its individual components is the reduction of the period of phenol treatment in water. Biodegradation of 83 % of phenols in model water occurs in 3 days.
The authors are grateful to the Geonauka CCU and the eco-analytical laboratory of the Institute of Biology, Komi SC UB RAS for their assistance in the analytical work.
The reported study was funded by RFBR and NSFC according to the research project RFBR ¹ 20-55-53019 and NSFC ¹ 4191101331, 41672039.
This work was partially supported by State Task No. AAAA-A17-117121270025-1 “Development of biocat-alytic systems based on enzymes, microorganisms and plant cells, their immobilized forms and associations for processing plant materials, obtaining biologically active substances, biofuels, remediation of contaminated soils and of wastewater treatment”.
Список литературы Why mineral carriers are necessary for microalgae
- Aizdaycher N.A., Stonik I.V. Vliyanie solenosti morskoi vody na vidy roda Attheya West (Bacillariophyta) iz yaponskogo morya (Rossiya) (The influence of sea water salinity on species of the genus Attheya West (Bacillariophyta) from the Sea of Japan (Russia)). Algology, 2013, V. 23, No. 1, pp. 37-43
- Andreeva V. M. Rod Chlorella: morfologiya, sistematika, printsipy klassifikatsii (Genus Chlorella: morphology, systematics, principles of classification). Science, 1975, 110 p.
- Vasilieva S. G., Lobakova E. S., Lukyanov A. A., Solovchenko A. E. Primenenie immobilizovannyh mikrovodoroslei v biotehnologii (Application of immobilized microalgae in biotechnology). Tomsk State University Journal, Ser. 16, Biology, 2016, No. 3, pp. 65-72.
- Immobilizatsiya kletok na nositele ili na poverhnosti nositelya (Immobilization of cells on a carrier or on a carrier surface). URL: https://www.kazedu.kz/referat/174283/1
- Metodika izmerenii massovoi kontsentratsii fenola metodom kapillyarnoi gazovoi hromatografii (Methodology for measuring the mass concentration of phenol by capillary gas chromatography). No. 88-17641-006-2013 (FR.1.31.2013.15054), 2013 Edition.


