Yellow Vein Mosaic Virus Resistant Okra Varieties Found Exceptionally Sensitive to Salinity Stress
Автор: S. P. Meera, Anu Augustine
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.17, 2021 года.
Бесплатный доступ
Background: Okra is a nutritious vegetable crop which is highly susceptible to yellow vein mosaic virus (YVMV), a biotic stress factor. Chemical control of YVMV is very difficult; hence a number of tolerant varieties are developed for cultivation. The abiotic stress factors like salinity, which may reduce the yield of YVMV resistant okra, are not yet assessed. Results: Six okra genotypes (Arka Anamika, Anjitha, Manjima, Aruna, Kiran and Susthira) exhibiting resistance to Yellow YVMV were screened for their tolerance to an abiotic stress, salinity. Individual healthy seeds were monitored from 7 to 50 days under saline conditions for seed germination and emergence assays respectively. Parameters like number of geminated seeds and the radicle length were measured and documented at 3rd, 5th and 7th day for the germination assay. Number of seeds emerged from soil by the first 5, 10 and 15 days, the length of the emerged plantlet on the 15th and 30th day and morphological changes of emerged plantlets thereafter, up to 50 days were observed for the emergence assay. The rate of germination, radicle length, percentage of emergence and plantlet length were found to decrease with increasing salinity. Conclusions: All the six YVMV resistant varieties were found to be sensitive to salinity stress irrespective of whether it is germination, emergence or vegetative phase. The resistance to YVMV thus couldn’t contribute any preformed intracellular osmoticum to overcome salt induced cell damage in okra.
Okra, salinity, stress tolerance, Yellow vein mosaic virus resistance
Короткий адрес: https://sciup.org/143173908
IDR: 143173908
Текст научной статьи Yellow Vein Mosaic Virus Resistant Okra Varieties Found Exceptionally Sensitive to Salinity Stress
Okra ( Abelmoschus esculentus L. Moench) is a nutritionally important vegetable grown in tropical and sub-tropical parts of the world for its green non-fibrous fruits or pods (Narendran et al. 2013). Okra yield and fruit quality are affected by shoot and fruit borers aphids, yellow vein mosaic virus and fungi such as Erysiphe cichoracearum , Sphaerotheca fuliginea , and Fusarium oxysporum (Manickavasagam et al. 2015). Okra is highly prone to Yellow vein mosaic virus disease transmitted by white fly ( Bemisiatabaci Gen.) (Shetty et al. 2013). An average of 32% reduction in the number of pods per YVMV infected okra plant was estimated by Sheik et al. (2013). Chemical control of YVMV being difficult, the only practical solution is to develop tolerant/resistant varieties. Okra varieties with biotic stress tolerance have been released through interspecific hybridization, single plant selection mutation, selection among somaclonal variants and genetic engineering.
Coming to the abiotic stress tolerance level of okra, it has been classified as a salt sensitive crop (FAO 1992). Salt stress is unique from other abiotic stresses such as drought or heat in that, toxic accumulation of Na+ and Cl-within the plant cell occurs during salinity (Blomberg and Adler 1993). Only a handful of salinity tolerance screening studies have been performed in okra varieties (Shahid et al. 2011; Haq et al. 2012; Abbas et al. 2014). Though it is evident from these studies that okra is gravely affected by soil salinity, a proper census of salt dependent reduction in okra yield is yet to be carried out. More than 20% of cultivated land worldwide is reported to be affected by the accumulation of salts over long periods of time and thus soil salinity is booming as a major abiotic stress limiting the supply of food crops (Rengasamy 2002).
Crosstalk between abiotic and biotic stress responses are to be considered while looking for a solution for either, in this era of spontaneous climate change, especially for crops which are simultaneously susceptible to pathogen attack and abiotic stresses. A scheme for the interaction interface and overlapping signaling pathways of abiotic and biotic stress at the cellular level was elucidated in detail by Kissoudis et al.
-
(201 4). From literature, it is evident that the plant responses under different stress levels are not linear and that it can be influenced under stress combinations to a significant level (Maggio et al. 2007; Malkinson and Tielborger 2010; Cheng et al. 2013; Muralidharan et al. 2014). Resistance to pathogens can also be differentially affected by various types of abiotic stress and vice versa (Soliman and Kostandi 1998; Koga et al. 2004; Mang et al. 2012; Baker et al. 1998; Kissoudis et al. 2016).
The correlation between YVMV resistance and the salt tolerance becomes crucial as a multitude of defense mechanisms are executed in order to fight a variety of stress factors (Roux et al. 2014; Mickelbart et al. 2015). However until recently no single approach has been suggested to explicate the interactions between abiotic and biotic stress responses in okra. Our research is focused on the germination and emergence response and growth performance of YVMV resistant okra varieties under different levels of salinity stress. The results indicated a significant reduction in growth parameters with gradual increase in the soil salinity. This non linear correlation emphasizes the need to develop okra cultivars with added salt tolerance in addition to resistance to YVMV so as to reach the required good quality yield percentage, for sufficient and healthy okra consumption.
MATERIALS AND METHODS
Plant material collection
Arka Anamika, Anjitha, Manjima, Aruna, Kiran and Susthira were the okra genotypes resistant to Yellow vein mosaic virus selected for the study (Table 3.1). Fresh and good quality seeds were collected from Kerala Agricultural University campuses (Vellayani and Vellanikkara), surface sterilized sequentially with 70% ethanol (2min), mixture of 6% sodium hypochlorite and 0.05% tween 20 (8min) and sterile distilled water ( 2 min, 5 times) and were used for seed germination and seed emergence assays under salinity stress.
Seed germination Assay
Whatman filter paper were spread on sterile petri dishes and moistened with sterile distilled water supplemented with 0mM, 25mM, 50mM, 75mM, 100mM
125mM, 150mM, 175mM and 200mM NaCl individually. Sterile distilled water alone (0mM) served as the experimental control. Normally in a germination test, seeds are randomly selected to include any damaged, discolored or light seeds to avoid any bias. Here, in this salinity screening, in order to cut short the germination hinderers other than salinity, healthy seeds were manually selected. In order to properly monitor the seed responses to germination under salinity stress, the sample size of healthy seeds under each experimental category was limited to 10. Seeds were placed in rows on a wet filter paper, in a culture rack with relatively stable temperature and away from direct sunlight. The number of seeds geminated and radicle length were measured and documented with photographs at 3rd, 5th and 7th day accordingly.
Seed emergence assayPotting mix and electrical conductivity measurement
For the seed emergence assay, 100% organic potting soil from Orgakart was used. Total soluble salts (TSS), pH, Organic carbon, phosphorus and potassium content of the organic potting mix were measured from District Soil Testing Laboratory, Kerala Agriculture Department, Kannur.
Seed emergence and seedling morphology assessment
Twelve seeds per pot were sown in plastic pots filled with organic potting mix placed in shade net under natural conditions. Equal volumes of NaCl at different concentrations [0mM (Control), 50mM, 100mM, 150mM and 200 mM] was applied before sowing of seeds and maintained by supplementing the same concentrations at 5 days intervals. The seedlings were watered enough to maintain the moisture content of potting mix, thus to avoid drought stress.
The growth response to NaCl stress was documented by counting the total number of seeds emerged from the potting mix at 5, 10 and 15 days. The length of the emerged plantlet was measured on the 15th and 30th day of sowing. Morphological changes of plantlets were photographed for up to 50 days.
Statistical analysis
Data was analyzed using paired sample t- test. All statistical analyses were performed at the level of P value<0.05 using SPSS.20 software (SPSS Inc, USA) for windows 7.0.
RESULTS AND DISCUSSION
Germination of YVMV resistant okra seeds declines in response to salinity
Seed germination is the most fundamental and vital growth stage of a plant that determines the yield. A significant reduction in number of germinated seeds was observed under higher salinities for all the six varieties of YVMV resistant okra. On the 3rd day of germination assay, all other varieties except Anjitha and Manjima showed at least a single event of germination for up to 125mM. Kiran exhibited a higher and consistent germination tendency up to 150mM. The same pattern followed till the 5th and 7th day of sowing, with more number of germinated seeds for each experimental group. Even ‘Kiran’, found to be the most salt tolerant YVMV resistant okra strain couldn’t germinate any further at 200mM NaCl. The average lengths of germinated seeds were also found to be decreased with increasing NaCl concentration (Fig. 1- 8; Table 2). The adverse effect of salinity on YVMV resistant okra seed germination may be due to the altered water imbibition rates by seeds with respect to the lower osmotic potential of germination environment (Khan and Weber 2008). Lower water imbibitions can lead to cell toxicity which changes the activities of enzymes of nucleic acid metabolism (Gomes-Filho et al. 2008), alters protein metabolism (Dantas et al. 2007), disturbs hormonal balance (Khan and Rizvi 1994), and reduces the utilization of seed reserves (Othman et al. 2006).
Organic potting mixture ensures single stress factor
The potting mix used in this experiment efficiently hinders other factors like biotic stress, nutrient deficiency and drought stress other than the added salinity stress. This disease and weed free media is made primarily from natural materials, such as peat, compost, and/or manures and organic fertilizers. The high quality compost in the mix provides adequate amounts of phosphate, potash, and trace elements which eliminates the risk of nutrient deficiency. High water holding (sustained release of water and nutrients) capacity of the potting mix prevents wilting out of seedlings. Total soluble salts (TSS), pH, organic carbon content, phosphorus content and potassium content of the organic potting mix were measured as 0.10 mhos/cm, 6, 2.60%, 266.56 kg/ha and 896.0 kg/ha, respectively. The range of TSS value lies within the adequate limit which prevents any extra salt contribution from the growth medium irrespective of the designed salt treatment regime. Higher values for organic carbon, phosphorus and potassium content of the organic potting mix confirmed that the experimental plants were not experiencing any stress related to nutrient deficiency.
YVMV resistance factors fail to complement salinity tolerance in okra
Documentation of seed emergence and plant growth up to 50 days of vegetative phase under NaCl stress clearly indicates that 200mM NaCl caused the plant to wilt and die eventually. Not even single YVMV resistant okra varieties used in the experiment could survive at 200 mM of NaCl. While at lower concentrations of NaCl, okra plants responded differently. There was an evident reduction in emergence and plantlet length with increasing salinity (Fig. 9- 11; Table 3). Soil salinity imposed a reduction in growth rate of plantlets as it reduced the plant’s ability to take up water and may have entered the transpiration stream and eventually injured cells in the transpiring leaves, further reducing growth (Munns 1993; Munns 2005).
It was proven that different levels of abiotic stress have a significantly different impact on disease susceptibility (Soliman and Kostandi 1998; Desprez-Loustau et al. 2006). Salinity stress imposes its damaging potential through both osmotic effects and ion toxicity resulting from accumulation of ions, in particular Na+ and Cl-. Whereas, pathogen attack has been shown to reduce photosynthesis and water use efficiency thus inducing abnormal stomata opening patterns, which were critical for plant tolerance to abiotic stress (Bilgin et al. 2010; Grimmer et al. 2012).
Osmotic protection via solute (Proline, glycine betaine, sucrose, raffinose, mannitol, sorbitol and cyclic polyhydric alcohols) accumulation is one of the major lines of defense in salt tolerant varieties in addition to the stress-induced activation of signaling cascade (Nounjana et al. 2012). Similarly, disease resistant plant lines protect plants from pathogens by pre-formed structures and chemicals, and by infection-induced responses of the immune system. Studies on the biochemical basis of resistance to YVMV revealed that generations of okra that were highly resistant to YVMV contained higher moisture, phenols, flavanols orthodihydroxy phenol and total chlorophyll content in addition to enhanced peroxidase activity than susceptible cultivars (Sharma et al. 1981). Hence the internal environment of YVMV resistant strains of okra is expected to be rich in pre-formed biomolecules to reduce cell damage despite substantial pathogen levels. In the present study, we consider the osmotic adjustment of plant cell interior in both salinity and YVMV stress as a common defense mechanism to assume that the YVMV resistance can impart salinity tolerance in the selected varieties of okra. Contrary to this assumption, the study suggested lack of positive correlation between YVMV resistance and salinity tolerance in okra.
Table 1: Okra varieties resistant to YVMV
|
Name |
Source |
Reaction to stresses |
|
Arka Anamika |
Indian institute of horticulture research, Bangalore |
Resistant to YVMV |
|
Anjitha |
Department of Plant Breeding and Genetics, College of Agriculture, Vellayani |
Resistant to YVMV and tolerant to shoot and fruit borer |
|
Manjima |
Department of Plant Breeding and Genetics, College of Agriculture, Vellayani |
Resistant to YVMV and moderately tolerant to shoot and fruit borer |
|
Aruna |
Kerala Agricultural University, Vellanikkara |
Resistant to YVMV |
|
Kiran |
Department of Plant Breeding and Genetics, College of Agriculture, Vellayani |
Resistant to YVMV and tolerant to shoot and fruit borer |
|
Susthira |
Kerala Agricultural University, Vellanikkara |
Highly resistant to YVMV and susceptible to shoot and fruit borer |
Атка An amik a

Figure 1. Germination assay of Arka Anamika on the 3rd, 5th Figure 2. Germination assay of Anjitha on the 3rd, 5th and and 7th day of sowing at NaCl concentrations 0, 25, 50, 75, 7thday of sowing at NaCl concentrations 0, 25, 50, 75,
100, 125, 150, 175 and 200mM 100, 125, 150, 175 and 200mM
Anjitha

Manjima

Figure 3. Germination assay of Manjima on the 3rd, 5th and Figure 4. Germination assay of Aruna on the 3rd, 5th and 7th day of sowing at NaCl concentrations 0, 25, 50, 75, 100, 7th day of sowing at NaCl concentrations 0, 25, 50, 75 125, 150, 175 and 200mM 100, 125, 150, 175 and 200mM
Aruna

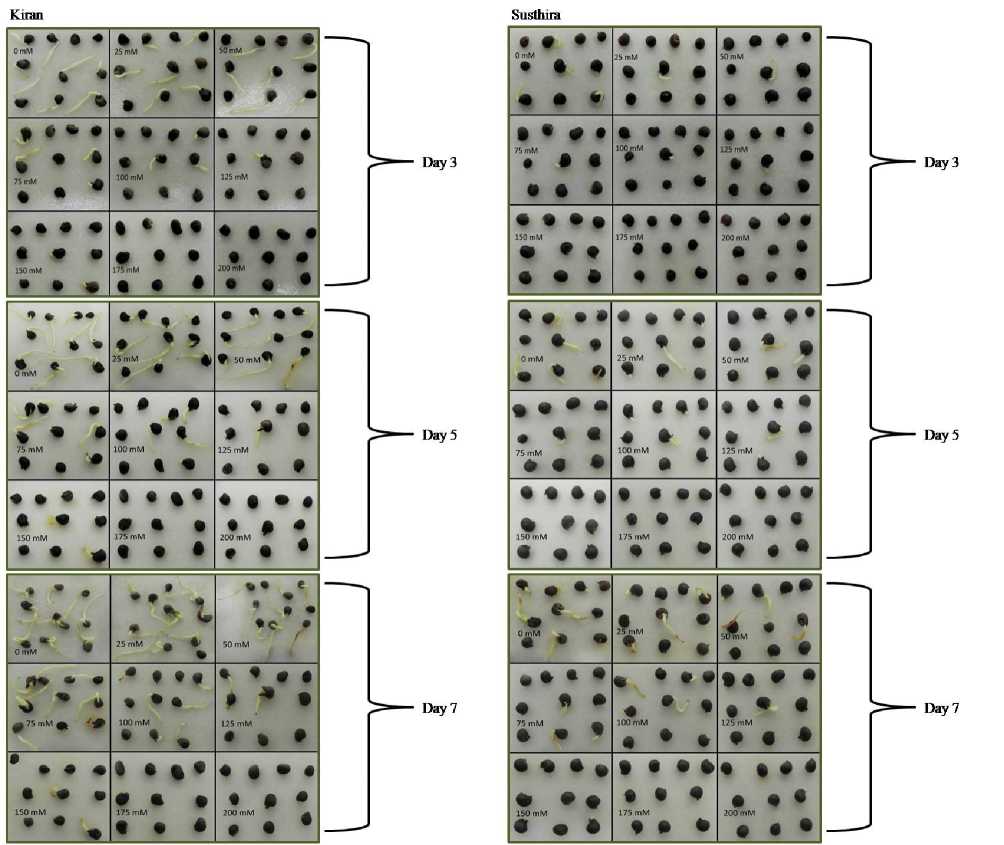
Figure 5. Germination assay of Kiran on the 3rd, 5th and 7th day of sowing at NaCl concentrations 0, 25, 50, 75, 100, 125, 150, 175 and 200mM
Figure 6. Germination assay of Susthira on the 3rd, 5th and 7th day of sowing at NaCl concentrations 0, 25, 50, 75, 100 125, 150, 175 and 200mM
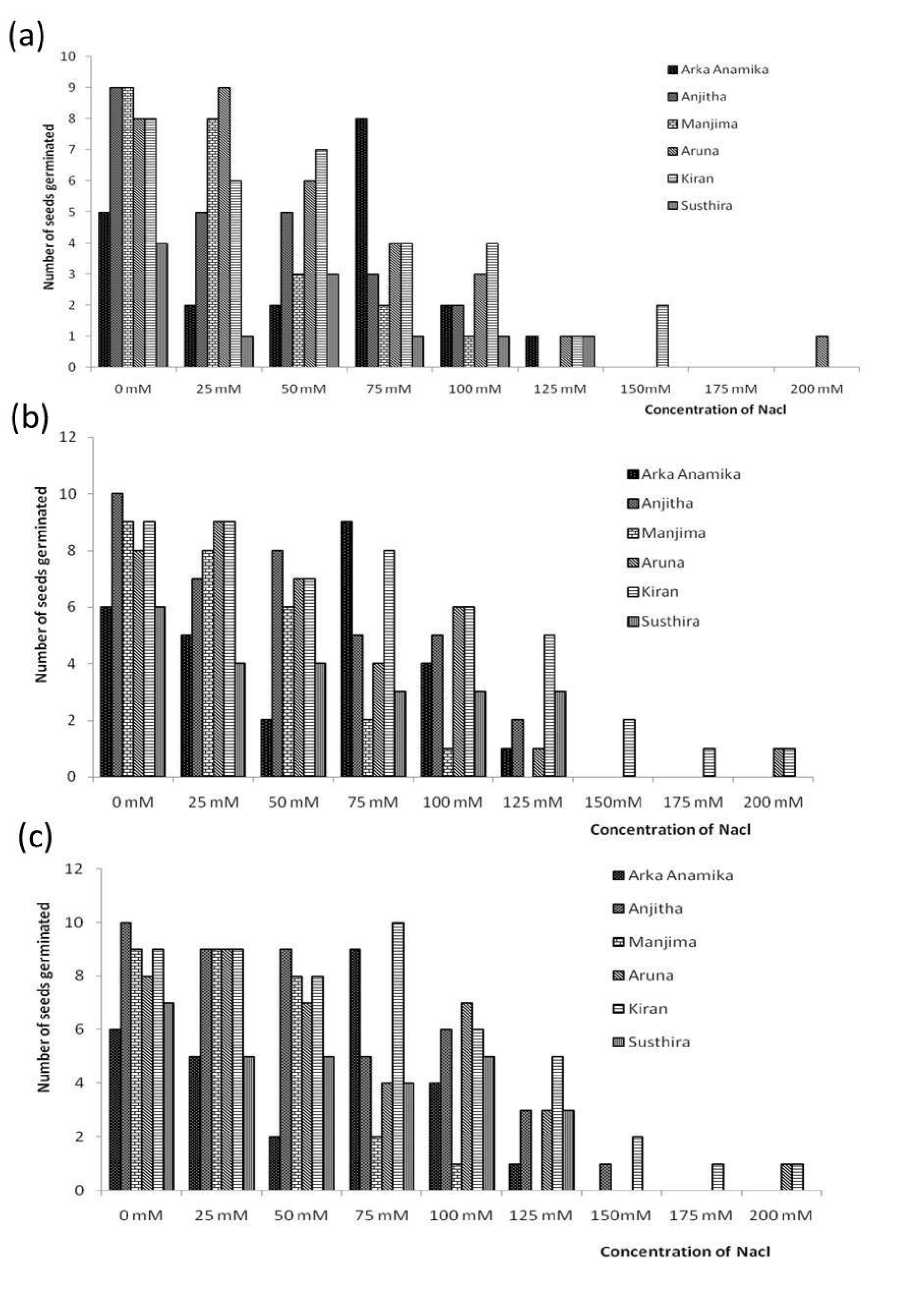
Figure 7. Number of seeds germinated on (a) 3rd, (b) 5th and (c) 7thday of sowing in seed germination assay
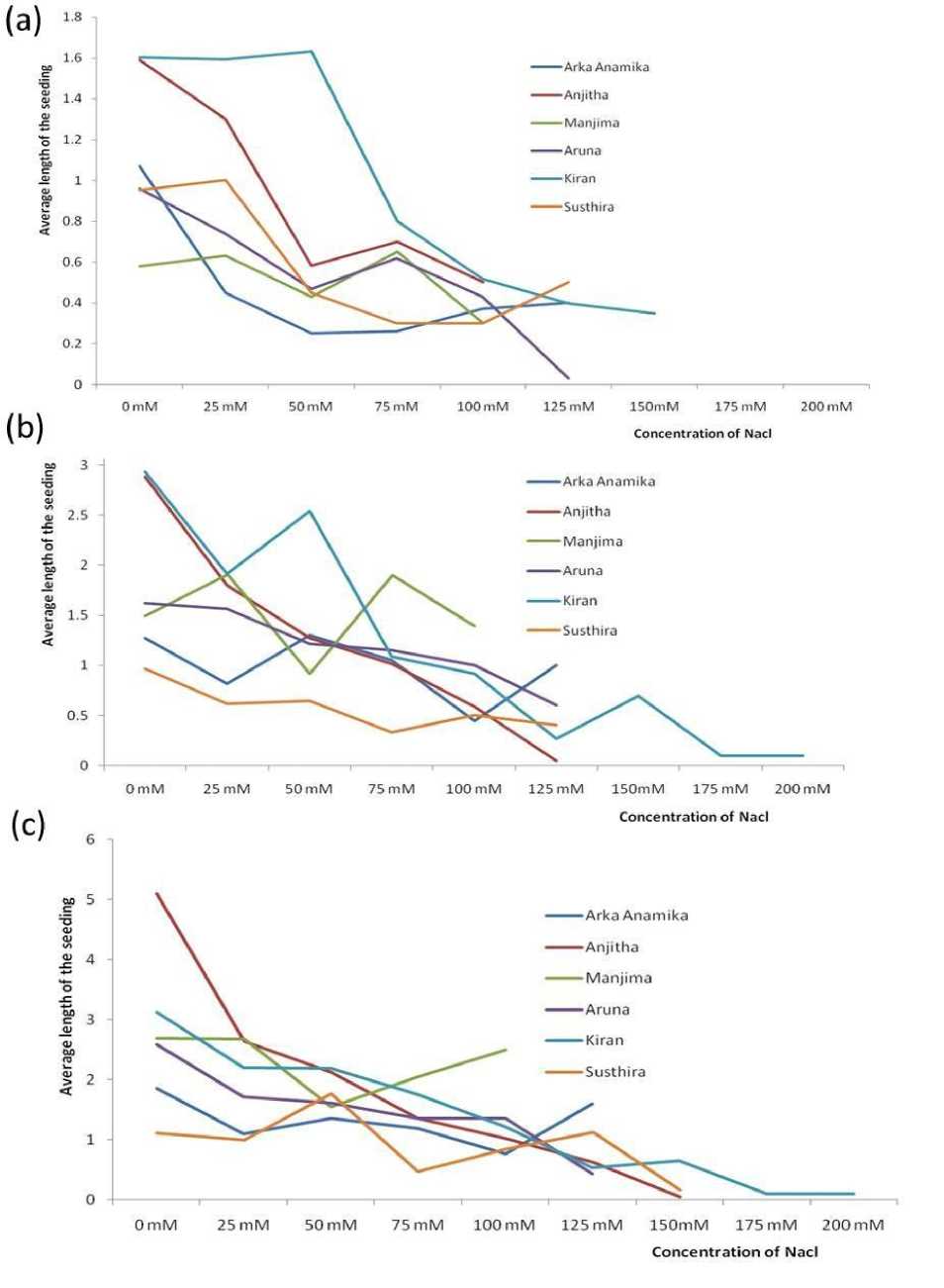
Figure 8. Average length of radicle on (a) 3rd, (b) 5th and (c) 7th day of sowing in seed germination assay
Table 2: Average length of radicle and standard deviation (Sd) in various concentration of NaCl for six different varieties of YVMV resistant okra (Arka Anamika, Anjitha, Manjima, Aruna, Kiran and Susthira) every 3rd, 4th and 7th day of sowing in seed germination assay.
|
Okra variety |
DS* |
Radicle length± sd (cm) of NaCl treatment (mM) |
||||||||
|
0 |
25 |
50 |
75 |
100 |
125 |
150 |
175 |
200 |
||
|
ArkaAnamika |
3 |
1.07±0.47 |
0.45±0.07 |
0.25±0.07 |
0.26±0.31 |
0.37±0.18 |
0.4±0 |
- |
- |
- |
|
5 |
1.27±0.6 |
0.82±0.28 |
1.3±0.14 |
1.05±0.32 |
0.45±0.4 |
1±0 |
- |
- |
- |
|
|
7 |
1.85±0.52 |
1.1±0.44 |
1.35±0.21 |
1.19±0.43 |
0.77±0.62 |
1.6±0 |
- |
- |
- |
|
|
Anjitha |
3 |
1.59±0.46 |
1.3±0.53 |
0.58±0.36 |
0.7±0.35 |
0.5±0.14 |
- |
- |
- |
- |
|
5 |
2.88±1.04 |
1.8±1.19 |
1.27±0.96 |
1.02±0.79 |
0.59±0.61 |
0.05±0 |
- |
- |
- |
|
|
7 |
5.1±1.29 |
2.64±1.3 |
2.13±0.48 |
1.36±1.11 |
1.03±0.43 |
0.63±0.38 |
0.05±0 |
- |
- |
|
|
Manjima |
3 |
0.58±0.42 |
0.63±0.36 |
0.43±0.35 |
0.65±0.21 |
0.3±0 |
- |
- |
- |
- |
|
5 |
1.5±0.6 |
1.91±0.44 |
0.92±0.58 |
1.9±0.14 |
1.4±0 |
- |
- |
- |
- |
|
|
7 |
2.69±1.61 |
2.68±0.91 |
1.55±0.61 |
2.05±0.07 |
2.5±0 |
- |
- |
- |
- |
|
|
Aruna |
3 |
0.96±0.48 |
0.74±0.47 |
0.47±0.39 |
0.62±0.45 |
0.43±0.32 |
0.03±0 |
- |
- |
- |
|
5 |
1.62±0.83 |
1.56±0.53 |
1.21±0.36 |
1.15±0.3 |
1±0.41 |
0.6±0 |
- |
- |
0.5±0 |
|
|
7 |
2.59±1.54 |
1.72±1.02 |
1.61±0.68 |
1.35±0.41 |
1.36±0.44 |
0.43±0.42 |
- |
- |
0.7±0 |
|
|
Kiran |
3 |
1.6±0.58 |
1.59±0.58 |
1.63±0.67 |
0.8±0.5 |
0.52±0.33 |
0.4±0 |
0.35±0.07 |
- |
- |
|
5 |
2.93±1.12 |
1.91±1.05 |
2.54±0.87 |
1.09±0.98 |
0.92±0.79 |
0.27±0.36 |
0.7±0 |
0.1±0 |
0.1±0 |
|
|
7 |
3.13±1.48 |
2.21±1.17 |
2.19±0.76 |
1.75±0.72 |
1.22±0.64 |
0.54±0.47 |
0.65±0.07 |
0.1±0 |
0.1±0 |
|
|
Susthira |
3 |
0.95±0.1 |
1±0 |
0.45±0.65 |
0.3±0 |
0.3±0 |
0.5±0 |
- |
- |
- |
|
5 |
0.97±0.41 |
0.62±0.79 |
0.65±0.68 |
0.33±0.4 |
0.5±0.36 |
0.4±0 |
- |
- |
- |
|
|
7 |
1.11±0.64 |
1±0.77 |
1.76±1.4 |
0.47±0.68 |
0.84±0.58 |
1.13±0.47 |
0.17±0.06 |
- |
- |
|
DS* Days from sowing
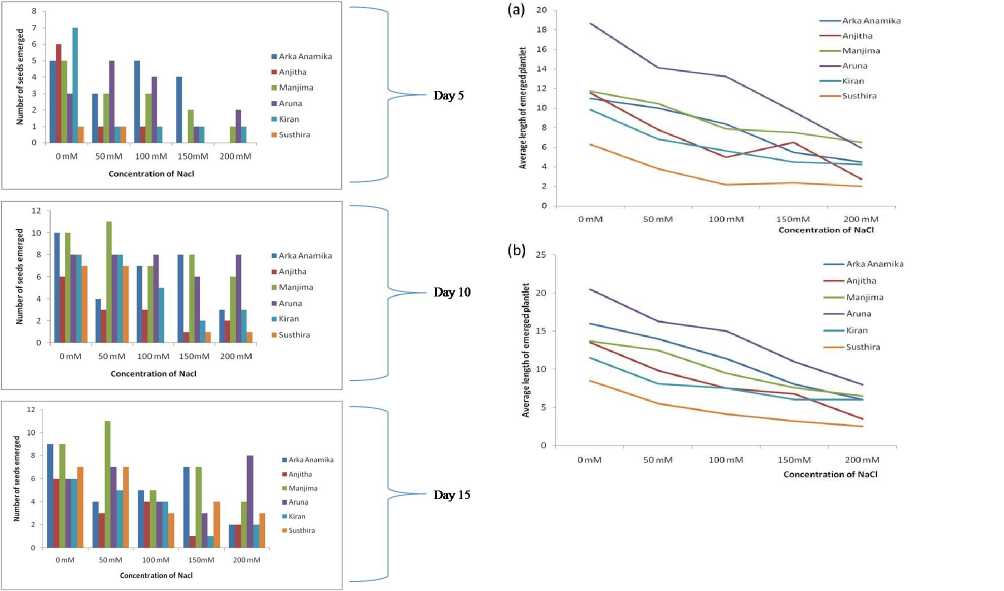
Figure 9. Number of seeds emerged on the 5th, 10th and 15th day of sowing at NaCl concentrations 0, 50, 100, 150 and 200mM in seed emergence assay
Figure 10. Average length of emerged plantlet observed on (a) 15th day and (b) 30th day of sowing in seed emergence assay.
Table 3. Average length of emerged plantlet and standard deviation (Sd) in various concentration of NaCl for six different varieties of YVMV resistant okra (Arka Anamika, Anjitha, Manjima, Aruna, Kiran and Susthira) on 15th and 30thday of sowing.
|
Okra variety |
DS* |
Length of emerged plantlet ± sd (cm) of NaCl treatment |
||||
|
0mM |
50mM |
100mM |
150mM |
200mM |
||
|
ArkaAnamika |
15 |
11±3.8 |
10±1.4 |
8.4±2.07 |
5.5±0.91 |
4.5±0 |
|
30 |
16±3.6 |
14±1.2 |
11.4±1.9 |
8±0.8 |
6±0 |
|
|
Anjitha |
15 |
11±3.06 |
7.83±2.02 |
0.5±2.12 |
6.5±0 |
2.75±0.35 |
|
30 |
13.5±2.9 |
9.8±2 |
7.5±2.1 |
6.8±0 |
35±0.28 |
|
|
Manjima |
15 |
11.72±2.59 |
10.45±3.76 |
7.9±2.63 |
7.5±2.45 |
6.5±1.47 |
|
30 |
13.7±2.3 |
12.5±3.5 |
9.5±2.4 |
7.6±2.2 |
6.5±1.38 |
|
|
Aruna |
15 |
18.68±3.39 |
14.14±3.08 |
13.25±1.26 |
9.67±3.06 |
5.94±4.06 |
|
30 |
20.5±3.37 |
16.3±3.1 |
15±1.18 |
11±2.96 |
8±3.58 |
|
|
Kiran |
15 |
9.83±1.75 |
6.8±1.20 |
5.63±1.1 |
4.5±0 |
4.25±0.35 |
|
30 |
11.5±1.68 |
8.1±1.19 |
7.5±1.12 |
6±0 |
6±0.33 |
|
|
Susthira |
15 |
6.29±1.29 |
3.79±1.99 |
2.17±1.25 |
2.38±0.48 |
2±0 |
|
30 |
8.5±1.3 |
5.5±1.76 |
4.1±1.21 |
3.2±0.36 |
2.5±0 |
|
DS* Days from sowing

Figure 11. Morphological response showing mass reduction in plant growth with increasing salinity for six different varieties of YVMV resistant okra (a) Arka Anamika, (b) Anjitha, (c)Manjima, (d) Aruna, (e) Kiran and (f) Susthira at the end of 50th day.
CONCLUSION
It was concluded that there is no evidence of positive correlation between the YVMV resistance and salinity tolerance in okra. The preformed pathogen defense strategies of YVMV resistance, ultimately fails to impart tolerance to salinity. Pathogen induced aspects of YVMV resistance is not monitored in this experiment which can be focused in future perspectives of the present scenario. The study suggests the need to focus on salinity tolerance, while developing disease tolerant varieties of okra so as to obtain quality and quantity of yield.
ACKNOWLEDGMENT
This work was supported by the University Grants Commission [grant number 2121330906]; and Kerala State Council for Science Technology and Environment [grant number 1320/2014/KSCSTE].
CONFLICTS OF INTEREST
All authors have declared that they do not have any conflict of interest for publishing this research.
AUTHOR CONTRIBUTIONS
Список литературы Yellow Vein Mosaic Virus Resistant Okra Varieties Found Exceptionally Sensitive to Salinity Stress
- Abbas T., Pervez M.A., Ayyub C.M., Shaheen M.R., Tahseen S., Shahid M.A., Bilal R.M. and Manan A. (2014) Evaluation of different okra genotypes for salt tolerance. Int. J. Pl. An.and Env. Sci .,4(3), 23-30.
- Baker S.J., Newton A.C., Crabb D., Guy D.C., Jefferies R.A., Mackerron D.K.L., Thomas W.T.B. and Gurr S.J. (1998) Temporary partial breakdown of mloresistance in spring barley by sudden relief of soil water-stress under field conditions: the effects of genetic background and mlo allele. Plant. Pathol., 47, 401-410.
- Bilgin D.D., Zavala J.A., Zhu J., Clough S.J., Ort D.R. and Delucia E.H. (2010) Biotic stress globally down regulates photosynthesis genes. Plant. Cell. Environ., 33, 1597-1613.
- Blomberg A. and Adler L. (1993) Tolerance of fungi to NaCl. In: Jennings, DH (ed), Stress tolerance of fungi. New York, Marcel Dekker, pp. 209-232.
- Cheng C., Gao X., Feng B., Sheen J., Shan L. and He P. (2013) Plant immune response to pathogens differs with changing temperatures. Nat. Commun., 4, 2530.
- Dantas B.F., De Sa R.L and Aragao C.A. (2007) Germination, initial growth and cotyledon protein content of bean cultivars under salinity stress. Rev. Bras. de Sementes., 29, 106-110.
- Desprez-Loustau M.L., Marcais B., Nageleisen L.M., Piou D. and Vannini A. (2006) Interactive effects of drought and pathogens in forest trees. Ann. For. Sci., 63, 597-612.
- FAO (1992) Irrigation and Drainage paper. 48, USA. Gomes-Filho E., Machado Lima C.R.F., Costa J.H., da Silva A.C., daGuia Silva Lima M., de Lacerda C.F. and Prisco J.T. (2008) Cowpea ribonuclease: properties and effect of NaCl-salinity on its activation during seed germination and seedling establishment. Plant. Cell. Rep., 27, 147-157.
- Grimmer M.K., John Foulkes M. and Paveley N.D. (2012) Foliar patho-genesis and plant water relations: a review. J. Exp. Bot., 63, 4321-4331.
- Haq I., Khan A.A., Khan I.A. and Azmat M.A. (2012) Comprehensive screening and selection of okra (Abelmoschus esculentus) germplasm for salinity tolerance at the seedling stage and during plant ontogeny. J. Zhejiang Univ. Sci. B (Biomed and Biotechnol)., 13(7), 533-544.
- Khan M.A. and Rizvi Y. (1994) Effect of salinity, temperature and growth regulators on the germination and early seedling growth of Atriplex griffithii var. Stocksii. Can. J. Bot., 72, 475-479.
- Khan M.A. and Weber D.J. (2008) Ecophysiology of high salinity tolerant plants (tasks for vegetation science). Springer, Amsterdam.
- Kissoudis C., van de Wiel C., Visser R.G.F. and Van Der Linden G. (2014) Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant. Sci., 5, 207.
- Kissoudis C., Sunarti S., van de Wiel C., Visser R.G., van der Linden C.G. and Bai Y.J. (2016) Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. Exp. Bot. 67(17), 5119-32.
- Koga H., Dohi K. and Mori M. (2004) Abscisic acid and low temperatures suppress the whole plant-specific resistance reaction of rice plants to the infection of Magnaporthe grisea. Physiol. Mol. Plant. Pathol., 65, 3-9.
- Maggio A., Raimondi G., Martino A. and De Pascale S. (2007) Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exper. Bot., 59, 276–282.
- Malkinson D. and Tielborger K. (2010) What does the stress-gradient hypothesis predict? Resolving the discrepancies. Oikos., 119, 1546-1552.
- Mang H.G., Qian W.Q., Zhu Y., Qian J., Kang H.G., Klessig D.F. and Hua J. (2012) Abscisic acid deficiency antagonizes high-temperature inhibition of disease resistance through enhancing nuclear accumulation of resistance proteins SNC1 and RPS4 in Arabidopsis. The Plant Cell., 24, 1271-1284.
- Manickavasagam M., Subramanyam K., Ishwarya R., Elayaraja D. and Ganapathi A. (2015) Assessment of factors influencing the tissue culture-independent Agrobacterium-mediated in planta genetic transformation of okra [Abelmoschus esculentus (L.) Moench]. Plant Cell Tiss. Organ Cult., 123, 309-320.
- Mickelbart M.V., Hasegawa P.M. and Bailey-Serres J. (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet., 16, 237-251.
- Munns R. (1993) Physiological processes limiting plant growth in saline soil: some dogmas and hypotheses. Plant Cell Environ., 16, 15-24.
- Munns R. (2005) Genes and salt tolerance: bringing them together. New Phytol., 167, 645-663.
- Muralidharan S., Box M.S., Sedivy E.L., Wigge P.A., Weigel D. and Rowan B.A. (2014) Different mechanisms for Arabidopsis thaliana hybrid necrosis cases inferred from temperature responses. Plant Biol., 16, 1033-1041.
- Narendran M., Deole S.G., Harkude S., Shirale D., Nanote A., Bihani P. and Zehr U.B. (2013) Efficient genetic transformation of okra [Abelmoschus esculentus (L.) Moench] and generation of insectresistant transgenic plants expressing the cry1Ac gene. Plant Cell Rep., 32, 1191-1198.
- Nounjana N., Nghiab P.T. and Theerakulpisuta P. (2012) Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol., 169, 596-604.
- Othman Y., Al-Karaki G., Al-Tawaha A.R. and Al-Horani A. (2006) Variation in germination and ion uptake in barley genotypes under salinity conditions. World J. Agric. Sci., 2, 11-15.
- Rengasamy P. (2002) Transient salinity and subsoil constraints to dry land farming in Australian sodic soils: an overview. Aust. J. Ex. Agric., 42, 351-61.
- Roux F., Voisin D., Badet T., Balagué C., Barlet X., Huard-Chauveau C., Roby D. and Raffaele S. (2014) Resistance to phyto pathogens e tutti quanti: placing plant quantitative disease resistance on the map. Mol. Plant. Pathol., 15, 427-432.
- Shahid M.A., Pervez M.A., Balal R.M., Ahmad R., Ayyub C.M., Abbas T. and Akhtar N. (2011) Salt stress effects on some morphological and physiological characteristics of okra (Abelmoschus esculentus L.). Soil Environ., 30, 66-73.
- Sharma B.R., Kumar V. and Bayay K.L. (1981) Biochemical basis of resistance to yellow vein mosaic virus in okra. Genetics Agraria., 35, 121–30.
- Sheikh M.A., Khan S.Z. and Mahmood I. (2013) Effect of bhendi yellow vein mosaic virus on yield components of okra plants. J. Plant Pathol., 95 (2), 391-393.
- Shetty A.A., Singh J.P. and Singh D. (2013) Resistance to yellow vein mosaic virus in okra: a review. Biol. Agric. and Hortic. 29(3), 159-164.
- Soliman M.F. and Kostandi S.F. (1998) Effect of saline environment on yield and smut disease severity of different corn genotypes (Zea mays L.). J. Phytopathol., 146, 185-189.


