Засуха как форма абиотического стресса и физиологических маркеров стресса от засухи
Автор: Шумилина Ж.С., Кузнецова А.В., Фролов, Гришина Т.В.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.14, 2018 года.
Бесплатный доступ
Засуха является одной из наиболее распространенных форм абиотического стресса и оказывает непосредственное влияние на жизнеспособность и продуктивность растений. С точки зрения физической химии засуха характеризуется снижением водного потенциала среды и воздействием на многие биохимические процессы в клетке растения, что вызывает развитие окислительного стресса. События, вызванные дефицитом воды, приводят к нарушению электронно-транспортных цепей митохондрий и хлоропластов, что приводит к чрезмерному образованию активных форм кислорода и, как следствие, к развитию окислительного стресса. Окислительный стресс, в свою очередь, вызывает изменения на молекулярном и физиологическом уровнях у растений. Именно поэтому многие исследователи используют широкий спектр физиологических параметров для характеристики развития реакции стресса на засуху у растений. В этом обзоре мы попытались обобщить имеющиеся экспериментальные данные многих исследователей последних лет, объяснив основные механизмы развития стрессовой реакции у растений. В этом обзоре мы попытались обобщить имеющиеся экспериментальные данные многих недавних исследований, объяснив основные механизмы реакции на стресс у растений. В частности, мы обратили внимание на те физиологические параметры, изменения которых происходят у большинства видов растений.
Короткий адрес: https://sciup.org/143166698
IDR: 143166698
Текст обзорной статьи Засуха как форма абиотического стресса и физиологических маркеров стресса от засухи
Влияние изменений климата на продуктивность культурных растений стало одним из ведущих направлений современной биологии. Одной из важнейших причин возросшего интереса к данной проблеме явилось заметное изменение интенсивности и частоты многих климатических явлений, а также изменение характеристических погодных параметров, таких как перепад суточных температур и количество образующихся осадков в течение последних лет (Ummenhofer, Meehl, 2017). Согласно имеющимся прогнозам на ближайшие десятилетия, тенденция к снижению количества выпадающих осадков сохранится, что, очевидно, приведет к более частому наступлению засухи (Shanker et al. , 2014).
Засуха как абиотический фактор стресса оказывает непосредственное влияние на работу систем и органов растения, становясь причиной угнетения роста, снижения тургора листьев и водного обмена. Также она вызывает изменение на молекулярном уровне, нарушая работу фотосинтетического аппарата, приводя к замедлению метаболизма, оказывая влияние на паттерн белков и их посттрансляционные модификации (Verslues et al. , 2006). Эти глубинные изменения не случайны и направлены на развитие ответных реакций растений на стресс и адаптации растительного организма к изменяющимся условиям (рис. 1).
Развитие ответа растения на засуху -многостадийный процесс, затрагивающий, как все системы организма, так и каждой клетки в отдельности. И в первую очередь, его основной целью является минимизация потери воды, снижение последствий ее дефицита, а затем формирование устойчивости у растения к данному виду абиотического стресса. Именно поэтому особый интерес в данной области вызывает поиск специфичных белков стресса и метаболитов (Lipiec et al. , 2013), способных в будущем стать ключом не только к более глубокому пониманию молекулярных механизмов развития ответа на засуху, но и обнаружению стратегий, позволяющих повысить засухоустойчивость сельскохозяйственных культур
(Frolov et al., 2017).
Засуха как форма абиотического стресса
Засуха является одной из самых распространенных форм абиотического стресса, оказывающих прямое влияние на жизнеспособность и продуктивность растений. В основе явления засухи лежит осмотический стресс, то есть снижение водного потенциала среды (Ψw) по сравнению с водным потенциалом растения. Таким образом, физиологические проявления засухи сравнимы с такими формами абиотического стресса, как солевой и температурный стресс. В настоящее время, водный потенциал является общепринятым параметром, используемым для количественной оценки содержания воды в почве, а также тканях растений и грибов. Значение водного потенциала среды складывается из осмотического и матричного потенциалов (Ψπ и Ψm, соответственно) и, согласно системе СИ, определяется как величина, характеризующая давление, измеряемое в МПа:
-Ψw = -Ψπ + Ψm
Таким образом, матричный потенциал является общепринятой мерой силы, с которой частицы почвы связывают воду. В то же время, осмотический потенциал представляет собой величину, определяемую концентрацией растворенных веществ, причем меньшие значения Ψπ соответствуют их более высоким концентрациям, и, следовательно, более низкому содержанию воды (Auge, 2001). То есть, чем более отрицательное значение имеет Ψw, тем более сухим является анализируемый образец (Feher, Ford, 1995), в то время как водный потенциал дистиллированной воды близок к нулю. Известно, что даже при небольшом снижении водного потенциала растения начинают испытывать стресс, а при значительном его уменьшении наблюдается некроз тканей (Berdanier, Clark, 2016). Однако величина Ψw, при которой растения лишь в минимальной степени подвергаются действию засухи или гибнут, является специфичной для отдельных видов и сортов культурных растений и может быть определена только экспериментально. Более того, на величину критических для выживания растений значений водного потенциала оказывают значительное влияние такие факторы окружающей среды, как влажность воздуха, освещенность, содержания минеральных питательных веществ в почве, конкуренция, а также возраст и физиологическое состояние самого растения (Grote et al., 2016).
Важно отметить, что не только абсолютная величина снижения Ψw среды, но и продолжительность оказывают влияние на растительный организм. Время, в течение которого развивается первичная физиологическая реакция на стресс, у большинства организмов схоже, в то время как период, необходимый для перестройки метаболизма (отражающейся в соответствующих изменениях метаболома и протеома) может существенно различаться. Последний показатель во многом определяется засухоустойчивостью растения и является видоспецифичным (Agarwal et al. , 2006). Засухоустойчивость характеризуется способностью растений выносить различные по продолжительности периоды засухи. При этом, для большинства видов растений, было показано, что кратковременное снижение водного потенциала среды оказывает менее негативное влияние на процессы роста и развития организма, нежели действие засухи в течение длительного времени (Mendivelso et al. , 2014).
С понижением Ψw происходит уменьшение водного потенциала тканей растения. Тканевой водный потенциал (Ψt) является величиной, характеризующей содержание воды в структурах организма, и напрямую зависит от Ψw (Auge, 2001). В норме, водный потенциал различных тканей растения отличается. Так, было показано, что в корнях и нижних листьях тканевой водный потенциал выше, нежели в листьях, расположенных более высоко на стебле (Bittnera et al. , 2012). Помимо этого, Ψt зависит от степени разветвленности побега, а также характера и точки начала ветвления (Bittnera et al. , 2012). То есть, чем более разветвленную структуру имеет побег и чем раньше начинает ветвиться, тем меньше абсолютное снижение Ψt апикальных листьев по отношению к Ψt корня. Этот важный факт необходимо учитывать при изучении влияния засухи на растения.
Окислительный стресс
Дефицит воды провоцирует избыточную генерацию свободных радикалов в растительных клетках. Наиболее распространенной группой метаболитов свободно-радикальной природы, постоянно образующихся в живых клетках, являются активные формы кислорода (АФК) (рис. 2). Эти соединения образуются либо путем перехода электрона в составе атома кислорода на более высокий энергетический уровень (синглетный кислород - 1О 2 ), либо присоединения одного или двух электронов (супероксидный радикал - О 2 •-, гидроксильный радикал - НО•). Перекись водорода также относится к АФК, но не является свободным радикалом (Cooke et al. , 2015).
Отдельные АФК имеют различную способность к диффузии. Например, супероксидный радикал является чрезвычайно химически активным и не распространяется за пределы участка своего формирования, в то время как перекись водорода легко диффундирует через мембраны, что делает её идеальным кандидатом для участия в процессе внутриклеточной сигнализации (Sena, Chandel, 2012). Кроме АФК в клетках организмов встречаются свободные радикалы азота (Ferrari et al. , 2011), серы (Jacob, 2006), углерода (Bild et al. , 2017) и многие другие.
В условиях стресса баланс генерации и утилизации АФК нарушается, что приводит к развитию окислительного стресса, то есть состояния, при котором образование АФК превышает возможности клетки по их детоксикации, что оказывает негативное влияние на работу различных внутриклеточных систем (Lushchak, 2011). В силу своей высокой реактивности, АФК являются чрезвычайно токсичными, так как вызывают повреждение белков, липидов, углеводов и нуклеиновых кислот (Moller et al. , 2007). При этом, в случае развития окислительного стресса, эти повреждения носят преимущественно необратимый характер.
Избыточное производство активных форм кислорода в условиях засухи является результатом нарушения процессов переноса электронов в электрон - транспортных цепях хлоропластов и митохондрий (Skulachev, 2012). При этом, в светлое время суток основным источником генерации АФК в растениях являются хлоропласты и пероксисомы, в то время как митохондрии играют ведущую роль в генерации АФК ночью (Foyer, Noctor, 2003). Так, ориентировочно 1-5% потребляемого митохондриями кислорода расходуется на образование АФК (Moller, 2001). В клетке существует множество систем, способных генерировать активные формы кислорода и азота, но именно хлоропласты и отчасти митохондрии играют ключевую роль в развитии окислительного стресса при засухе у растений.
Генерация активных форм кислорода в хлоропластах
У высших растений и водорослей фотосинтез происходит в хлоропластах, которые содержат множество тиллакоидов. Именно в мембране тиллакоидов расположена электрон-транспортная цепь, позволяющая осуществлять процесс фотосинтеза (Pfannschmidt, 2003), в ходе которого идет активная генерация атомарного кислорода. Данный процесс происходит при участии белковых комплексов. Одной из их главных функций является передача свободных электронов, кроме того, сам фотосинтез включает в себя этап генерации супероксидного радикала. Именно поэтому хлоропласты являются основной структурной единицей клетки, ответственной за производство АФК у растений (Bratt et al. , 2016).
Абиотические стрессоры различной природы, в том числе засуха, способствуют увеличению продукции активных форм кислорода в хлоропластах. Большой вклад в данный процесс вносит фотосистема II. Ввиду того, что при длительном дефиците воды, работа фотосистемы II, а именно H 2 O-пластохиноноксидоредуктазы нарушается, а при этом генерация свободных электронов продолжается, что приводит к усилению продукции АФК в хлоропластах в соответствии с механизмом, представленным на рис.3 (Chen et al. , 2016).
Так, в норме, в процессе фотосинтеза свободные электроны передаются от светособирающих комплексов к фотосистеме I, которая, в свою очередь, восстанавливает ферроредоксин. Далее, эти эквиваленты расходуются на восстановление NADP+ до НАДФH ферментом ферроредоксин-НАДФ- редуктазой (рис. 3). Однако в условиях снижения водного потенциала происходит перегрузка электрон - транспортной цепи и часть электронов ферроредоксина и железосерных кластеров фотосистемы I расходуется на частичное восстановление кислорода в ходе реакции Мелера (Mehler), сопровождающееся генерацией супероксидного радикала (Heber, 2002). В совокупности, данные процессы способствуют развитию окислительного стресса.
Генерация активных форм кислорода в митохондриях
Митохондрии растений выполняют функцию энергетических фабрик и являются основными источниками такого вида АФК, как перекись водорода (Huang et al. , 2008). Известно, что образование небольшого количества активных форм кислорода является нормой при процессе аэробного дыхания (Rhoads et al. , 2006). Однако, в условиях засухи, продукция АФК в митохондриях значительно увеличивается. При этом основными источниками АФК в условиях стресса являются НАДН-дегидрогеназный и цитохром-bc1 комплексы митохондриальной электрон-транспортной цепи (рис. 4).
Также, помимо белков дыхательной цепи в генерации активных форм кислорода принимают участие ферменты - антиоксиданты. Так, было показано, что супероксиддисмутаза повышает уровень перекиси водорода в тканях растений (Wang et al. , 2016).
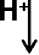
Также необходимо отметить вклад реакции Фентона, то есть восстановление пероксида водорода до гидроксильного радикала (•ОН), в присутствии ионов переходных металлов переменной валентности (преимущественно Fе2+ или Cu+), в развитие окислительного стресса (Glasauer, Chandel, 2014).
Антиоксидантные системы растений
В условиях засухи растения подвергаются окислительному стрессу и стремятся противостоять разрушающему воздействию АФК и других свободных радикалов путем запуска антиоксидантных систем. В настоящее время известно несколько стратегий, направленных на сохранения жизнеспособности растения в условиях окислительного стресса. Все они вовлечены в активацию или синтез de novo ферментативных и неферментативных антиоксидантов, то есть веществ, способствующих детоксикации свободных радикалов и перекиси водорода (Hassan et al., 2017). Основными антиоксидантными ферментами, обладающими высоким сродством к определенным АФК, являются различные изоформы супероксиддисмутазы (СОД) и каталазы. СОД представляет собой группу металлоферментов, катализирующих реакцию диспропорционирования супероксидного радикала с образованием перекиси водорода и кислорода (Wang et al., 2013). Каталаза, в свою очередь, восстанавливает перекись водорода до воды (Mhamdi et al., 2010). Таким образом, СОД и каталаза образует первую линию специфической антиоксидантной защиты клетки.
Неферментативные антиоксиданты не обладают специфичностью к определенным активным формам кисорода и способны обезвреживать любые виды свободных радикалов. К ключевым антиоксидантам неферментативной природы относятся: аскорбиновая кислота, глутатион, каротиноbды, флавоноиды и токоферолы (Ahmad et al., 2010). Так, при изучении влияния засухи на пшеницу было показано, что аскорбат и глутатион позволяют растению поддерживать уровень пероксида водорода в тканях на постоянном уровне (Szechynska et al., 2007). Кроме того, обнаружены металлопротеины, связывающие ионы металлов переменной валентности и препятствующие их вовлечению в реакцию Фентона и связанное с этим усиление генерации АФК (Fry et al., 2002). Перечисленные антиоксиданты способны легко нейтрализовать небольшие количества избыточных активных форм кислорода. Однако при развитии сильного окислительного стресса они не способны полностью предотвращать окислительное повреждение липидов, белков и нуклеиновых кислот. Физиологический ответ растительного организма на действие засухи
Как любой другой вид стресса, засуха становится причиной развития изменений у растений на физиологическом уровне. Несмотря на обширное видовое разнообразие, у большинства растений проявляются схожие физиологические изменения в условиях дефицита воды. Именно поэтому, большинство исследователей в данной области используют в работе классический модельный объект: Arabidopsis thalianа L., так как он обладает хорошо изученным, полностью секвенированным и небольшим по размеру геномом (около 157 млн. пар нуклеотидов), а также неприхотлив в выращивании (He et al. , 2017). Однако в последние годы все больше внимание уделяется изучению ответа на стресс у культурных растений, ведь интенсивность проявления тех или иных физиологических изменений напрямую коррелирует с уровнем интенсивности засухи, который является видоспецицефичным. В настоящее время, в качестве модельных объектов чаще всего выбирают широко распространенные культурные растения, такие как ячмень (Gonzalez et al. , 2008), пшеница (Moinuddin et al. , 2005), кукуруза (Chimenti et al. , 2006), соя (Taheri et al. , 2012), подсолнечник (Rauf, Sadaqat, 2008), рапс (Niknam et al. , 2003) и горох (Naim-Feil et al. , 2017).
Изменение корневой системы
Важнейшей структурой, непосредственно вовлеченной в ответ на снижение Ψw, является корневая система растения. Всасывание и проведение питательных веществ, микроэлементов и воды является основной ее функцией, которая угнетается в условиях засухи. Потому реакция корневой системы на засуху вызывает особый интерес у исследователей (Comas et al., 2013). Такая реакция носит, в значительной степени, адаптивный характер. Длина корней, их количество и разветвленность изменяется в зависимости от степени выраженности дефицита воды. Известно, что устойчивые к засухе растения обладают более развитой корневой системой, что необходимо для увеличения площади всасывающей поверхности при недостатке влаги (Liu et al., 2008). Интересно, что кратковременная засуха способствует увеличению длины корней, в то время как длительный дефицит воды отрицательно влияет на скорость развития корневой системы (Luo et al., 2016). Очевидно, усиленный рост корней на ювенильных стадиях развития растений имеет важное значение и позволяет организму приспособиться к изменяющимся условиям среды на начальных этапах постэмбрионального онтогенеза, когда запасные питательные вещества семени дают возможность осуществлять рост и пролиферацию клеток. В тоже время показано, что усиление роста корней сопровождается торможением развития побега (Wu, Cosgrove, 2000). Это, в свою очередь, обусловлено меньшей чувствительностью клеток корней к дефициту воды, по сравнению с клетками побега. Причиной этому, возможно, является более сильная транспирация листьев побега по сравнению с корнем.
Изменение устьичной проводимости
Еще одним направлением адаптации растения к дефициту воды является минимизация ее потери, которая проявляется изменением подвижности листа, утолщением кутикулы и закрытием устьиц. Было показано, что до 90% испарения воды с поверхности растений происходит через устьичные щели (Wang et al. , 2009). Устьица листа представляют собой структуры, через которые происходит газообмен между тканями листа и окружающей средой. Показано, что высокий уровень газообмена необходим для эффективного осуществления фотосинтеза, при этом, потери воды ограничиваются благодаря эффективному контролю уровня транспирации путем изменения устьичной проводимости (Yang et al. , 2011). В свете этого, закрытие устьиц при понижении водного потенциала является важнейшим механизмом, направленным на сохранение воды в условиях засухи. Действительно, даже при небольшом снижении Ψw устьица растения закрываются (Fang, Xiong, 2015).
Относительное содержание воды листа
Относительное содержание воды в листе также изменяется в условиях засухи и напрямую зависит от Ψw, уровня транспирации и непосредственно связано с активностью тканей растения (Siddique et al. , 2001). В условиях благоприятного водного режима, тканевый водный потенциал молодого листа выше, чем зрелого. Однако, в условиях дефицита воды, Ψt листьев всех возрастов снижается, потому данный параметр часто используется в качестве показателя устойчивости растений к дегидратации тканей. Снижение относительного содержания воды в тканях в условиях засухи показано для многих растений (Nayyar, Gupta, 2006). Кроме того обнаружено, что интенсивность и продолжительность засухи влияет на скорость снижения относительного содержания воды в тканях листа. При этом, чем ниже Ψw и длительность воздействия осмотического стресса, тем меньше содержание воды в тканях листа (Deeba et al. , 2012).
Влияние засухи на активность фотосинтетического аппарата
Дефицит воды, связанный с действием засухи оказывает непосредственное влияние на фотосинтетический аппарат клетки, воздействуя на функции всех его компонентов (Allen, Ort, 2001). Это, в свою очередь, приводит к перестройке метаболизма растения и, следовательно, влияет на рост и урожайность растения в целом (Chandra, 2003). Уже на первых этапах развития засухи дефицит воды приводит к закрытию устьиц, что влечет за собой снижение уровня потребления CO2, являющегося ключевым участником процесса фотосинтеза. В результате нарушения газообмена снижается активность фотосистем I и II, что негативно влияет на скорость фотосинтеза (Chaves et al. , 2009). Таким образом, замедление фотосинтеза также является ранней реакцией растения на дефицит воды. Установлено, что интенсивность транспирации и активность фотосинтеза согласованно снижались в условиях засухи в листьях хлопчатника (Deeba et al. , 2012) и кукурузы (Anjum et al. , 2011).
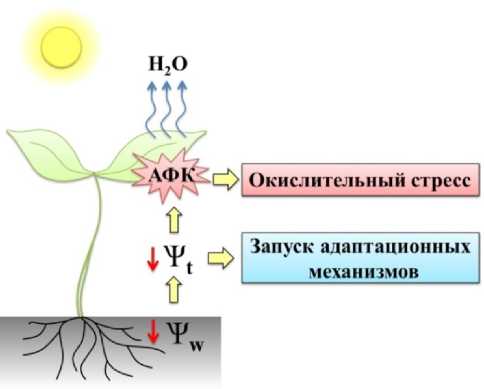
Figure 1. Схема развития ответа растения на засуху: Ψw - водный потенциал среды, Ψt - тканевой водный потенциал, АФК – активные формы кислорода.
Синглетный Супероксидный Пероксид кислород - радикал - ион
Ю2 «----о2 —-—» о2- ——* О22"
Молекулярный кислород |-|+
но2е
Пергидроксил радикал
Н2О2
Перекись водорода
Гидроксилный радикал
Figure 2. Схема генерации активных форм кислорода.
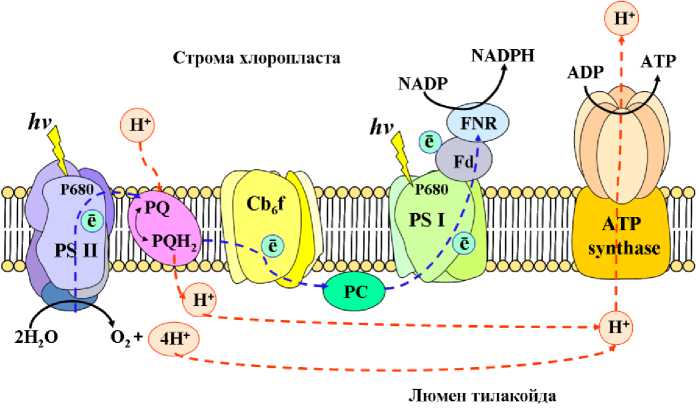
Figure 3. Схема электрон-транспортной цепи хлоропластов: PSII-фотосистема II, PSI- фотосистема I, PQ/PQH2- восстановленная и окисленная формы пластохиноа, Cb6f -цитохром b6f , Fd -ферредоксин, FNR - ферредоксин-НАДФ-редуктаза, PC - пластоцианин.
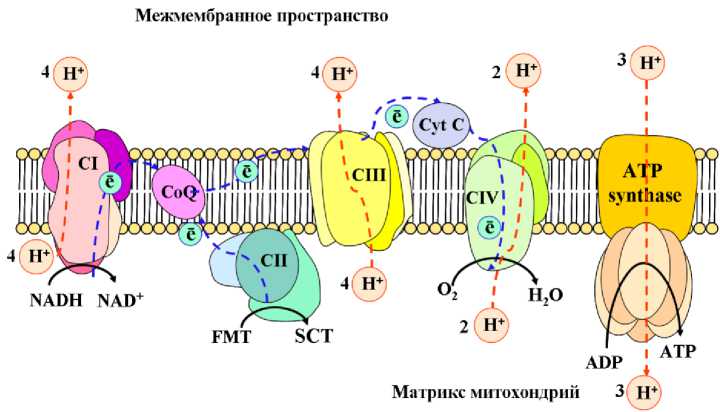
Figure 4. Схема электрон-транспортной цепи митохондрий: СI – НАДН-дегидрогеназный комплекс, СII - сукцинатдегидрогеназа, FMT - фумарат, SСТ - сукцитат, СIII - комплекс цитохромов bc1 , СIV - цитохром c оксидаза, СytC - цитохром С.
К причинам снижения активности фотосинтеза также относят изменение содержания хлорофиллов а и b под влиянием засухи. Уменьшение или отсутствие в изменении уровня хлорофилла в условиях сниженного водного потенциала было давно отмечено исследователями, причем данный параметр также зависит от интенсивности и продолжительности стрессового воздействия (Zhang, Kirkham, 1996). Под влиянием засухи происходит аутоокисление хлорофиллов, а синтез новых фотосинтетических пигментов замедляется (Manivannan et al. , 2007), что и вызывает снижение активности фотосинтеза. Также показано, что фотосинтетический аппарат ювенильных и молодых листьев проявляет большую устойчивость к засухе, по сравнению с более зрелыми листьями (Chastain et al. , 2016).
CONCLUSUSION
Таким образом, в настоящее время показано, что окислительный стресс, возникающий под влиянием засухи в растительных клетках, служит триггером запуска защитных систем и развитию изменений на физиологическом уровне. Данные изменения могут быть использованы как маркеры стресса у растений, столкнувшихся с дефицитом воды, что помогает исследователю более точно охарактеризовать физиологическое состояние экспериментальных растений. Некоторые подходы, применяемые исследователями описаны нами ранее в обзоре Осмоловской с соавторами «Modeling of Drought in the Experiment and Assessment of its Effects on Plants» (Osmolovskaya et al., 2017).
ACKNOWLEDGEMENT
Работа выполнена в Санкт-Петербургском государственном университете при финансовой поддержке Российского научного фонда (проект № 17-16-01042).
Список литературы Засуха как форма абиотического стресса и физиологических маркеров стресса от засухи
- Agarwal P.K., Agarwal P., Reddy M.K., Sopory S.K. (2006) Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep., 25(12), 1263-1274
- Ahmad P., Jaleel C.A., Salem M.A., Nabi G., Sharma S. (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol., 30(3), 161-167
- Allen D.J., Ort D.R. (2001) Impact of chilling temperatures onphotosynthesis in warm climate plants. Tren. Plant Sci., 6(1), 36-42
- Anjum S.A., Wang L.C., Farooq M., Hussain M., Xue L.L., Zou C.M. (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci., 197(9), 177-185
- Auge R.M. (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycor., 11(1), 3-42
- Berdanier A.B., Clark J.S. (2016) Multiyear drought-induced morbidity preceding tree death in southeastern. US forests. Ecol. Appl., 26(1), 17-23
- Bild W., Ciobica A., Padurariu M., Bild V. (2013) The interdependence of the reactive species of oxygen, nitrogen, and carbon. J. Physiol. Biochem., 69(7), 147-154
- Bittnera M., Janotta D., Ritterb P., Köcherc F., Beesed E. (2012) Functional-structural water flow model reveals differences between diffuse-and ring-porous tree species. Agric. and For. Meteorol., 158(15), 80-89
- Bratt A., Rosenwasser S., Meyer A., Fluhr R. (2016) Organelle redox autonomy during environmental stress. Plant Cell and Environ., 39(9), 1909-1919
- Chandra S. (2003) Effects of leaf age on transpiration and energyexchange of Ficus glomerata, a multipurpose tree species of central Himalayas. Physiol. Mol. Biol. Plan., 9(7), 255-260
- Chastain D.R., Snider J.L., Choinski J.S., Collins G.D., Perry C.D., Whitaker J., Grey T.L. (2016) Leaf ontogeny strongly influences photosynthetic tolerance to drought and high temperature in Gossypium hirsutum. J. Plant Physiol., 199(3), 18-28
- Chaves M.M., Flexas J., Pinheiro C. (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot., 103(11), 551-560
- Chen Y.E., Liu W.J., Su Y.Q., Cui J.M., Zhang Z.W., Yuan M., Zhang H.Y., Yuan S. (2016) Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Phys. Plantar., 158(2), 225-235
- Chimenti C.A., Marcantonio M., Hall A.J. (2006) Divergent selection for osmotic adjustment results in improved drought tolerance in maize (Zea mays L.) in both early growth and flowering phases. Field Crops Res., 95(3), 305-315
- Comas L.H., Becker S.R., Cruz V.M.V., Byrne P.F., Dierig D.A. (2013) Root traits contributing to plant productivity under drought. Front. Plant Sci., 4(1), 399-442
- Cooke J., Dryden M., Patton T., Brennan J., Barrett J. (2015) The antimicrobial activity of prototype modified honeys that generate reactive oxygen species (ROS) hydrogen peroxide. BMC Research Not., 8(20), 2-5
- Deeba F., Pandey A.K., Ranjan S., Mishra A., Singh R., Sharma Y.K., Shirke P.A. (2012) Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem., 53(1), 6-18
- Fang Y., Xiong L. (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cel. and Molec. Life Sci., 72(4), 673-689
- Feher J.J., Ford G.D. (1995) A simple student laboratory on osmotic flow, osmotic pressure, and the reflection coefficient. The American J. of Phys., 268(6), 10-20
- Ferrari C.K., Souto P.C., Franca E.L., Honorio-Franca A.C. (2011) Oxidative and nitrosative stress on phagocytes’ function: from effective defense to immunity evasion mechanisms. Arch. Immunol. Ther. Exp., 59(2), 441-448
- Foyer C.H., Noctor G. (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant., 119(1), 355-364
- Frolov A., Bilova T., Paudel G., Berger R., Balcke G.U., Birkemeyer C., Wessjohann L.A. (2017). Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol in fusion drought model. J. Plant Physiol., 208, 70-83
- Fry S.C., Miller J.G., Dumville J.C. (2002) A proposed role for copper ions in cell wall loosening. Plant Soil., 247(1), 57-67
- Glasauer A., Chandel N.S. (2014) Targeting antioxidants for cancer therapy. Biochem. Pharmacol., 92(1), 90-101
- Gonzalez A., Martın I., Ayerbe L. (2008) Yield and osmotic adjustment capacity of barley under terminal water-stress conditions. J. of Agr. and Crop Sci., 194, 81-91
- Grote R., Gessler, A., Hommel R., Poschenrieder W., Priesack E. (2016) Importance of tree height and social position for drought-related stress on tree growth and mortality. Trees -Struc. and Func., 30(5), 1467-1482
- Hassan W., Noreen H., Rehman S., Gul S., Amjad Kamal M., Kamdem J.P., Zaman B. (2017) Oxidative stress and antioxidant potential of one hundred medicinal plants. Current Top. in Medic. Chem., 17(12), 1336-1370
- He H., Yan J., Yu X., Liang Y., Fang L., Vibe H., Zhang A. (2017) Biochemical and biophysical research communications the NADPH-oxidase AtRbohI plays a positive role in drought-stress response in Arabidopsis thaliana. Biochem. and Biophys. Res. Com., 491(3), 834-839
- Heber U. (2002) The Mehler reaction in relation to cyclic electron transport in C3 plants. Photosyn. Res., 73(2), 223-231
- Huang D., Wu W., Abrams S.R., Cutler A.J. (2008) The relationship of drought-related gene expression in Arabidopsis thaliana to hormonal and environmental factors. Journal of Exper. Bot., 59(5), 2991-3007
- Jacob C. (2006) A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep., 23(6), 851-863
- Lipiec J., Doussan C., Nosalewicz A. Kondracka K. (2013) Effect of drought and heat stresses on plant growth and yield: a review. Int. Agrophys., 27, 463-477
- Liu X., Hua X., Guo J., Qi D., Wang L., Liu Z., Jin Z., Chen S., Liu G. (2008) Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotec. Lett., 30(10), 1275-1280
- Luo Z., Guan H., Zhang X., Zhang C., Liu, N., Li G. (2016) Responses of plant water use to a severe summer drought for two subtropical tree species in the central southern China. J.of Hydr., 8, 1-9
- Lushchak V.I. (2011) Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol., 101, 13-30
- Manivannan P., Jaleel C.A., Sankar B., Kishorekumar A., Somasundaram R., Lakshmanan G.M, Panneerselvam R. (2007) Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B: Biointerf., 59(10), 141-149
- Mendivelso H.A., Camarero J.J., Gutiуrrez E., Zuidema P.A. (2014) Agricultural and forest meteorology time-dependent effects of climate and drought on tree growth in a neotropical dry forest. Agricultural and Forest Meteor., 188, 13-23
- Mhamdi A., Queval G., Chaouch S., Vanderauwera S., Breusegem F., Noctor G. (2010) Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. of Experim. Bot., 61(15), 4197-4220
- Moinuddin M., Fischer K.D., Sayre M.P., Reynolds M.P. (2005) Osmotic adjustment in wheat in relation to grain yield under water deficit environments. Agron. J., 97(1), 1962-1971
- Moller I.M. (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Mol. Biol., 52(9), 561-591
- Moller I.M., Jensen P.E., Hansson A. (2007) Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol., 58(9), 459-481
- Naim-Feil E., Toren M., Aubert G., Rubinstein M., Rosen A., Eshed R., Sherman A., Ophir R., Saranga Y. (2017) Drought response and genetic diversity in Pisum fulvum, a wild relative of domesticated pea. Crop Sci., 57(3), 1145-1159
- Nayyar H., Gupta D. (2006) Differential sensitivity of C3 and C4 plants towater deficit stress: association with oxidative stress andantioxidants. Environ. Exp. Bot., 58(1), 106-113
- Niknam S.R., Ma Q., Turner D.W. (2003) Osmotic adjustment and seed yield of Brassica napus and B. juncea genotypes in a water-limited environment in south-western Australia. Anim. Produc. Sci., 43(3), 1127-1135
- Osmolovskaya N.G., Shumilina J.S., Grishina T.V., Didio A.V., Lukasheva E.M., Bilova T.E., Frolov A.A. (2017) Modeling of Drought in the Experiment and Assessment of its Effects on Plants. J. Stress Physiol. Biochem., 13(4), 110-120
- Pfannschmidt T. (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci., 8(1), 33-41
- Rauf S., Sadaqat H.A. (2008) Identification of physiological traits and genotypes combined to high achene yield in sunflower (Helianthus annuus L.) under contrasting water regimes. Austr. J. of Crop Sci., 1(1), 23-30
- Rhoads D.M., Umbach A.L., Subbaiah C.C., Siedow J.N. (2006) Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol., 141(3), 357-366
- Sena L.A., Chandel N.S. (2012) Physiological roles of mitochondrial reactive oxygen species. Mol. Cel., 48(2), 158-167
- Shanker A.K., Maheswari M., Yadav S.K., Desai S., Bhanu D., Attal N.B., Venkateswarlu B. (2014) Drought stress responses in crops. Funct. and Integ. Gen., 14(1), 11-22
- Siddique B., Hamid A., Islam M. (2001) Drought stress effects onwater relations of wheat. Bot. Bull. Acad. Sin., 41(1), 35-39
- Skulachev V.P. (2012) Mitochondria-targeted antioxidants as promising drugs for treatment of age-related brain diseases. J. Alzheimers Dis., 28(3), 283-289
- zechynska M., Skrzypek E., Dabrowska G., Biesaga-Koscielniak J., Filek M., Wedzony M. (2007) The role of oxidative stress induced by growth regulators in the regeneration process of wheat. Acta Physiol. Plant., 29(7), 327-337
- Taheri N., Zarghami R., Oveysi M., Tarighaleslami M. (2012) The effect of source limitations on yield and yield components of soybean (Glycine max L.) under drought stress. World Appl. Sciences J., 18(6), 788-795
- Ummenhofer C.C., Meehl G.A. (2017) Extreme weather and climate events with ecological relevance. Philosoph. Trans. of the R. Soc., 372, 1-12
- Verslues P.E., Agarwal M., Katiyar-Agarwal S., Zhu J., Zhu J.-K. (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J., 45(4), 523-539
- Wang F.L., Zhou J., Pan L., Li G., Zaidi Z., Cheng F. (2016) Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol. and Biochem., 109(1), 248-261
- Wang J., Griffiths R., Ying J., Mc Court P., Huang Y. (2009) Development of drought-tolerant (Brassica napus L.) through genetic modulation of ABAmediated stomata responses. Crop Sci., 49(1), 1539-1554
- Wang L., Liang W., Xing J., Tan F., Chen Y., Huang L., Chen, W. (2013) Dynamics of chloroplast proteome in salt-stressed mangrove Kandelia candel (L.) druce. J. of Proteome Res., 12(11), 5124-5136
- Wu Y., Cosgrove D.J. (2000) Adaptation of roots to low water potentialsby changes in cell wall extensibility and cell wall proteins. J. Exp. Bot., 51(3), 1543-1553
- Yang J., Ordiz M., Jaworski J.G., Beachy R.N. (2011) Induced accumulation of cuticular waxes enhances drought tolerance in Arabidopsis by changes in development of stomata. Plant Phys. and Biochem., 49(12), 1448-1455
- Zhang J, Kirkham M.B. (1996) Antioxidant response to drought insunflower and sorghum seedlings. New Phytol., 132(8), 361-373


