A case of chronic maxillary sinusitis in a late Neanderthal population of the Altai mountains
Автор: Zubova A.V., Kulkov A.M., Pikhur O.L., Moiseyev V.G., Kolobova K.A., Markin S.V.
Журнал: Archaeology, Ethnology & Anthropology of Eurasia @journal-aeae-en
Рубрика: Anthropology and paleogenetics
Статья в выпуске: 3 т.50, 2022 года.
Бесплатный доступ
We describe a likely case of chronic maxillary sinusitis (CMS) in a Neanderthal skeletal sample from Chagyrskaya Cave, in the Altai Mountains. Signs of CMS were recorded in the Chagyrskaya 57 specimen, which is a fragment of a left maxilla. Alveoli of the upper fi rst molar are partially preserved, and so are the second and third upper molars, with adjacent parts of the walls, and the fl oor of the maxillary sinus. The fragment was found in layer 6b, dating to 53,100–51,100 BP. We analyze the factors that had caused the development of the disease, and assess its etiology. In the 3D-model, generated by computed microtomography, and in the original specimen, porotic changes were registered, situated at the fracture line of the alveoli of M1, lost post-mortem, and near the vestibular roots of both preserved molars. Also, there were isolated bone spicules, 1.0–2.6 mm in size. These signs indicate incipient CMS, evidently caused by chronic periodontal disease combined with a deep alveolar recess of the maxillary sinus. As the periodontal gap expanded, several small nutrient foramina, piercing the bottom of the sinus, merged. As a result, several oro-antral channels formed, whereupon the infection spread into the maxillary sinus. Since the deep alveolar recess is observed in the vast majority of Neanderthal crania with published images or reconstructed maxillary cavities, it can be assumed that Neanderthals were predisposed to odontogenic CMS.
Chronic maxillary sinusitis, Neanderthals, Chagyrskaya Cave, paleopathology, archaeology, Middle Paleolithic
Короткий адрес: https://sciup.org/145146794
IDR: 145146794 | DOI: 10.17746/1563-0110.2022.50.3.131-139
Текст научной статьи A case of chronic maxillary sinusitis in a late Neanderthal population of the Altai mountains
Chronic maxillary sinusitis (CMS) is a persistent longterm inflammation of the mucous membrane of the maxillary sinuses, of an infectious or allergic nature (Arefieva et al., 2014: 11). This is one of the most widespread chronic respiratory diseases in the world today (Slavin, Spector, Bernstein, 2005; Brook, 2009). In most
cases, CMS does not pose a direct threat to life, but its manifestations can cause noticeable physical discomfort. These include obstructed nasal breathing, headaches, and general weakness; and during exacerbations, purulent discharge from the nose, sometimes fever; although it can also be asymptomatic (Arefieva et al., 2014: 26; Sipkin et al., 2013: 83–84).
Unlike many other respiratory diseases, CMS can be relatively easily detected in ancient skeletal remains. The mucosa of the sinus is so tightly related to the periosteum that it actually forms one unit with it; thus, its inflammations rapidly spread to bone tissue. This leads to a chronic inflammation of the walls of the sinus (osteitis), the structure of the bone tissue becomes heterogeneous and exhibits foci of osteosclerosis, osteoporosis, and remodeled bone tissue. These signs can be detected by visual examination of the maxillary sinus, or reconstructed by computed tomography (CT) images (Boocock, Roberts, Manchester, 1995; Sundman, Kjellström, 2013; Biedlingmaier et al., 1996; Erdogan, Fidan, Giritli, 2016; Mafee, Tran, Chapa, 2006; Georgalas et al., 2010; Momeni, Roberts, Chew, 2007; Snidvongs et al., 2014).
In ancient populations, CMS is considered a marker of cumulative stress of a multifactorial nature. A variety of factors stimulating an increase or a decrease in the prevalence of the disease in archaeological samples has been suggested in the literature: anthropogenic air pollution, adverse social conditions, climatic and geographic factors, etc. (Zubova, Ananyeva, Moiseyev et al., 2020; Zubova, Moiseyev, Ananyeva et al., 2022; Lewis, Roberts, Manchester, 1995; Roberts, 2007; Panhuysen, Coenen, Bruintjes, 1997). But those hypotheses are based mainly on the study of modern
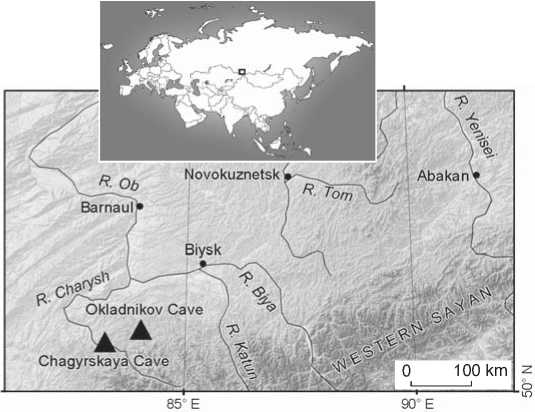
Fig. 1. Location of the caves with the remains of the late Neanderthals in the Altai.
human populations of the last two millennia (Teul et al., 2013; Sundman, Kjellström, 2013; Roberts, 2007; Lewis, Roberts, Manchester, 1995; Panhuysen, Coenen, Bruintjes, 1997), while the prevalence and dominating factors of the epidemiology of CMS in earlier ages and in different species of the genus Homo have not been studied to date.
This work describes a possible case of CMS in a Neanderthal sample from Chagyrskaya Cave in the Altai Mountains. To the best of our knowledge, there is only one more described case of CMS in Neanderthals detected in the Neanderthal 1 individual (Schultz, 2006). The main goal of the present study is to explore the factors that have led to the development of the recorded chronic inflammation in the maxillary sinus, and to determine the etiology of the disease.
Materials and methods
The bone fragment that is the focus of the present study (Chagyrskaya 57) was found in the Chagyrskaya Cave, in layer 6b. The site (51°26′34.6′′ N; 83°09′18.0′′ E) is located on the left bank of the Charysh River, in the foothills of the Tigirek ridge, in the northwestern Altai (Fig. 1). This karst cavity of northern exposure is located in the low mountains, at an altitude of 353 m above sea level, and 19 m above the river level.
Chagyrskaya Cave is renowned for its collection of Neanderthal remains—the largest one in North Asia. The cave was inhabited during ca 10 thousands years (59–49 ka BP) by a small population that was genetically closer to the late European Neanderthals than to the ancient Altaian groups of the species known from Denisova Cave (Denisova 5) (Mafessoni et al., 2020; Kolobova et al., 2020; Vernot et al., 2021). Evidence of the presence of late Neanderthals in the Altai was also found in Okladnikov Cave. Both archaeological and paleogenetic data suggest that these two caves were exploited by the same population. According to direct dating, the caves were inhabited simultaneously at the final stage of using Chagyrskaya, and at the early stage of populating Okladnikov (Kolobova, Shalagina, Chabai et al., 2019; Skov et al., 2022).
Chagyrskaya Cave was located on the route of seasonal migrations of large herbivores, and is thought to have been a base and hunting camp for Neanderthals. It was used at the end of the summer and at the beginning of the autumn season, when its inhabitants hunted female and young bisons (Kolobova, Chabai, Shalagina et al., 2019; Kolobova et al., 2020). The whole cycle of game utilization, including the extraction of bone marrow and making numerous bone tools, took place in the
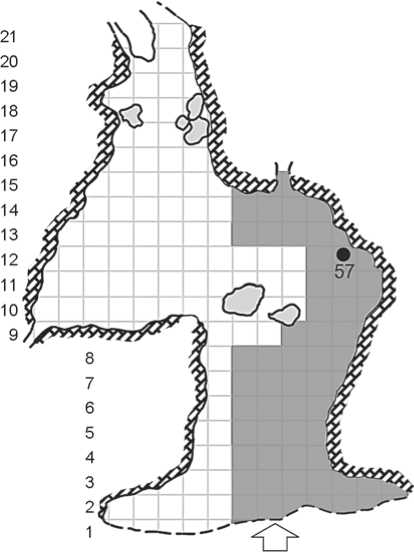
Fig. 2. Scheme of Chagyrskaya Cave indicating the location where specimen 57 was found (the area of the excavation is depicted in gray).
cave. Almost the whole cycle of stone tool manufacture was also recorded at the site. This included production/trimming of high backed bifaces and convergent scrapers (Baumann et al., 2020; Shalagina et al., 2020).
Recent paleogenetic studies have demonstrated that the Chagyrskaya Neanderthals lived in small isolated family groups, which included closely related individuals (i.e. father-daughter, cousins). This fact indirectly suggests that the cave was inhabited for a short period of time. According to the existing genetic models, the Neanderthal groups were patrilocal and exchanged females (Skov et al., 2022). The Neanderthals from Chagyrskaya also had genetic contacts with the Altaian Denisovans, as was shown by detecting a first generation hybrid between the two species (Slon et al., 2018).
Layer 6b is a grayish-brown silty dense porous carbonate silt sediment containing rare angular limestone fragments, bone fragments, lithic artifacts, and river pebbles. The lower boundary is erosive. The layer has a colluvial genesis and includes the remains of material culture moved from the layers 6c/1, 2. From the taphonomical point of view, this layer represents the remains of a hyena’s breeding-den. The Chagyrskaya 57 specimen was found in sq. H-11 of the main chamber of Chagyrskaya Cave (Fig. 2). In this and neighboring squares, 42 more anthropological specimens were detected (Skov et al., 2022). According to the genesis of layer 6b, the Chagyrskaya 57 specimen, as well as the others, was moved from layers 6c/2, 1, from deeper areas of the cave, owing to a colluvial transfer. Direct dating of bone remains produced four AMS-dates beyond the scope of the method (>49,000 and >52,000 BP), and OSL-dates fitting into the range between 53,100 and 51,100 BP (Kolobova et al., 2020).
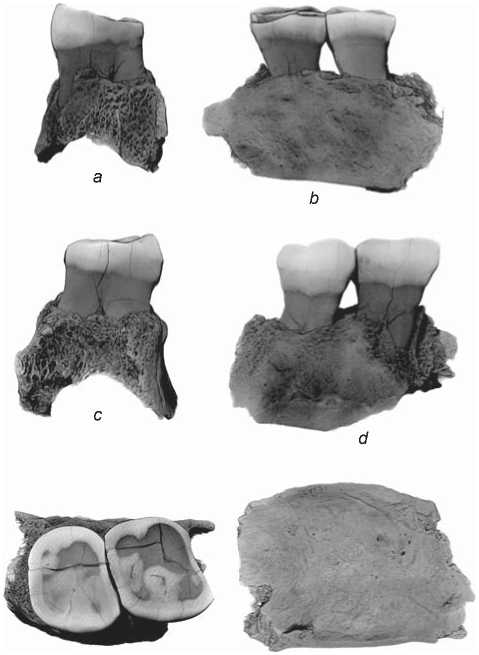
Chagyrskaya 57 (Fig. 3) is a fragment of a left maxilla with the sockets of the roots of the first molar partially preserved; and the second and third molars, and the surrounding structures of the anterior and posterior walls and the floor of the maxillary sinus fully preserved. The length of the fragment is 30.2 mm, width 18.3 mm, height 23.7 mm. The maximum height of the preserved part of the anterior wall of the sinus is 7.1 mm, posterior wall 5.5 mm (taken from the floor of the sinus).
MicroCT scanning of the specimen was carried out in the X-Ray Diffraction Research Center of St. Petersburg
А Б В ГДЕЖЗИКЛМНОП
Entrance
Fig. 3. 3D-model of the Chagyrskaya 57 maxillary fragment. a – mesial norm; b – lingual; c – distal; d – vestibular; e – occlusal; f – view from the side of the floor of the maxillary sinus.
f
e
State University, using Bruker SkyScan-1172 with the following settings: tube voltage 100 kV, amperage 100 μA, aluminum filter 0.5 mm, rotation step 0.25°, resolution 6.64 μm/pixel. Processing of the raw images and creation of a 3D-model of the fragment were carried out in NRecon and CTAn (Bruker-micro CT, Kontich, Belgium), respectively.
The manifestations of CMS were observed both in the 3D-model and the original specimen: osteoporotic and bone-remodeling loci on the floor and internal walls of the sinus. We employed a protocol scoring the manifestations of the disease as four grades (score 0 to 3), depending on the severity of the lesions: 0 – complete absence of pathological changes; 1 – subtle manifestations of osteoporosis: small clusters of pits or bone spicules 1 to 3 mm in length and occupying an area less than 1.5 cm2; 2 – remodeled bone tissue and spicules occupy an area of 1.5 to 2.5 cm2, merge with each other, and form network-like structures; 3 – lesions occupy a half of one of the walls of the sinus or more (Sundman, Kjellstrӧm, 2013: Fig. 2).
Infection of the sinus mucosa can occur in various ways: rhinogenic, hematogenous, or odontogenic (Mukovozov, 1982: 105). An attempt to differentiate between these etiologies was made for the specimen from Chagyrskaya. But this attempt, unfortunately, was seriously limited by the nature of the data available. With hematogenous etiology, the infection penetrates into the sinuses through the circulatory system, which is observed in severe infectious diseases such as typhoid, influenza, or scarlet fever. As these diseases do not produce specific skeletal markers, it is virtually impossible to diagnose a hematogenous infection in bone specimens.
Rhinogenic sinusitis develops with respiratory infections and certain types of allergies. It can be differentiated by the presence of signs of inflammation in the nasal cavity and ostiomeatal complex, bilateral damage to the sinuses, and the spread of inflammation not only to the maxillary, but also to other paranasal sinuses (Ibid.: 110). For the Chagyrskaya 57 individual, only a single fragment of the maxillae is present, making the determination of uni- vs. bilateral localization of the inflammation, as well as the description of the ostiomeatal complex, impossible.
Odontogenic forms of the disease develop as a result of the penetration of the microorganisms of the oral cavity into the maxillary sinus through the channels forming as a result of resorbtion of the alveolar bone owing to a long course of chronic periodontitis, chronic periodontitis, or osteomyelitis (Buskina, Gerber, 2000; Abrahams, Glassberg, 1996). Unlike those of a rhinogenic or a hematogenous etiology, such forms of the disease can be easily detected in skeletal specimens. In order to diagnose these, the presence of a chronic disease of the dentition must be confirmed, and the channels of oro-antral communication through which the infection penetrated into the sinus should be detected.
In order to determine the possible odontogenic nature of CMS in the Chagyrskaya 57 specimen, a protocol for scoring pathological manifestations of the dentition was employed. The protocol included the fixation of deposits of supragingival calculus, ante-mortem dental trauma, signs of initial and secondary caries, enamel hypoplasia, and markers of chronic periodontitis in the original specimen. Manifestations of the periodontal disease were described following Ogden (2007), whose protocol permits differentiation of chronic inflammations and normal age changes of periodontal tissue or dental roots related to the compensatory reaction to lowering of the dental crowns due to attrition. The 3D-model of the specimen was used to determine the presence or absence of: the oro-antral fistula; hypercementosis on the teeth-roots; expansion of the periodontal space; and changes in the structure of the compact of alveolar cells and in the sequesters of bone tissue marking inflammatory processes of various etiologies.
Results
The floor of the maxillary sinus of the Chagyrskaya 57 individual exhibits porotic changes located at the fracture line of the socket of M1 (lost post-mortem) and in the area of the vestibular roots of both preserved molars. The lesions spread towards the central part and the deepest point of the floor (Fig. 4). The minimum area affected by osteoporosis is 1.12 cm2, which corresponds to score 1 of the severity of CMS manifestations, but does not reach the threshold of 1.52 cm necessary for the registration of score 2. It can be suggested that the area affected by osteoporosis could have been larger if the first molar had been preserved, but the morphology of the observed bone changes confirms a weak development of the disease. Besides osteoporosis, the preserved part of the sinus displays only isolated bone spicules (1.0 to 2.6 mm), localized closer to the posterior wall and not forming continuous structures.
Of all of the dental pathologies that could have been potential sources of pathogens, only chronic periodontal disease of moderate severity (grades 3–4 according to the Ogden scale (2007)) was observed in Chagyrskaya 57. The lesion suggesting the presence of periodontal disease was resorbtion of the maxillary alveolar margin. Another manifestation of the disease was widening of the periodontal fissure between M2 and M3 (Fig. 5). The distance between the mesio-lingual root of the second molar and the wall of the alveolus is 0.45 mm, while the same distance from the disto-lingual root is 0.28 mm. The lesions are stronger pronounced in M3, where the

Fig. 4. Pathological changes of the floor of the maxillary sinus.
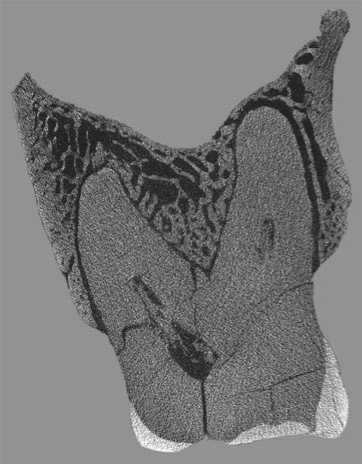
Fig. 5. Widening of the periodontal gap of the second molar.
periodontal gap reaches 0.6 mm near the mesio-lingual root, and 0.36 mm near the vestibular root.
None of the apical parts of all of the alveoli display the loci of inflammatory destruction of bone tissue that are typically observed during the growth of granulation tissue or the formation of cystogranuloma. In addition, subtle deposits of dental calculus were observed in the vestibular walls of both molars in the area of the maximum crown width.
Several channels connecting the sinus and molar alveoli were detected in the CT images, despite the absence of apical inflammations (Fig. 6). Two of the channels were observed in the area of the mesio-vestibular root of M2: one was rounded, 0.6 mm in diameter; the second was 0.2 mm in width and 0.4 mm in length. One more channel (0.3 mm in diameter) was found in the socket of the disto-vestibular root. Very small penetrating openings were also detected in the floor of the socket of the lingual root of the third molar. The emergence of these channels is related to the anatomy of the maxillary sinus of the Chagyrskaya 57 individual. Despite the small size of the fragment from Chagyrskaya, it was possible to observe enlargement of the alveolar recess, accompanied by an alveolar pocket. The deepest point of the floor of the sinus is lower than the apexes of the molar roots (Fig. 7); thus, their sockets are separated from the sinus only by a narrow layer of compact tissue pierced by small nutrient foramina. The thickness of this layer at the points where the roots approach the cavity of the sinus varies from 0.1 to 0.3 mm. As the periodontal gap was widening, some of the foramina merged forming oro-antral channels through which the infection spread from the oral cavity to the sinus.
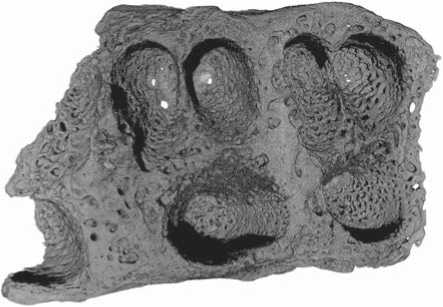
Fig. 6. Oro-antral channels in the alveoli of the upper permanent molars.
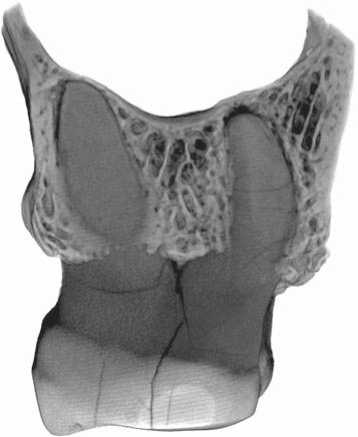
Fig. 7. Position of the floor of the maxillary sinus with respect to the apexes of the roots of the upper molars.
Discussion and conclusions
The bone lesions observed in the studied specimen point toward CMS of odontogenic etiology of an initial to middle stage of severity. Chronic periodontal disease, accompanied by the presence of an alveolar pocket of the maxillary sinus, was the likely cause of developing of CMS. This is the second published case of this pathology in Neanderthals. The first one was detected in the Neanderthal 1 individual (Schultz, 2006), where signs of inflammation accompanied by the formation of tumor-like objects in the zygomatic cavity were observed (Schultz, Schmidt-Schultz, 2015: 976–977). When the latter study was published, the prevalence of CMS was typically explained by anthropogenic air pollution and adverse social conditions; in particular, living in a cave implying constant inhalation of smoke from a fire was suggested as the main cause of the disease (Ibid.). But as has been demonstrated recently, the influence of the factors mentioned above on the prevalence of CMS cannot be confirmed statistically for archaeological samples (Zubova et al., 2022). Thus, the link between the maxillary sinus pathology of Neanderthal 1 and the low air quality in the cave is not as clear. The state of preservation of that specimen does not permit an assessment of the possible influence of odontogenic infection on the development of CMS.
Our analysis of the Chagyrskaya 57 specimen has shown that the pathology of the sinus had, most likely, an odontogenic origin. Two groups of factors were crucial for the emergence of the disease. The first is the anatomical features of the maxillary sinuses, namely the excessive development of the alveolar recess. Such morphology is considered one of the main factors predisposing to the development of odontogenic sinusitis in modern humans (Glazyev, Piskunov, 2017: 38); though, according to clinical data, it is observed only in 17 % of patients (Emelyanova, 2017: 16). The second group of factors includes the causes of chronic periodontal disease. These are mainly genetic predisposition, the presence of concomitant medical diseases, and poor oral hygiene causing the accumulation of pathogens that destroy connective tissue and cause bone loss (Clarke, Carey, 1985; Jenkins, Kinane, 1989).
At present, we are not able to carry out an analysis that would determine the statistical significance of the factors listed above across the whole Neanderthal species: the two published cases are clearly insufficient for this purpose. However, the literature on the subject suggests that an anatomical predisposition to CMS could be one of the features of the pathological status of Homo neanderthalensis. There is a consensus that a large size of the maxillary sinuses is typical of Neanderthals (Tattersall, 2002: 55; Buck et al., 2019: Pl. S3). The number of publications of photo images of CT reconstructions of Neanderthal’s maxillary sinuses is as low as 6–7 specimens, even in the studies specifically aimed at describing the morphology of this structure (see, e.g., (Zollikofer et al., 2008; Buck et al., 2019)). But in all the cases when the quality of an image permits a thorough assessment, a deep intrusion of the alveolar recess into the alveolar process of the maxilla, similar to that in Chagyrskaya 57, can be observed. This is true for Guattari 1 (Buck et al., 2019: Fig. 8), La Chapelle-aux-Saints, La Ferrassie 1 (Ibid.: Pl. S3), Forbes’ Quarry 1 (Rae, Koppe, Stringer, 2011: Fig. 1; Zollikofer et al., 2008), Spy 1 (Schwartz, Tattersall, 1996: Fig. 2), Artenac 1 (Mann et al., 2007: Fig. 1b). Thus, it can be reasonably suggested that at least these individuals were susceptible to the development of CMS. Such a predisposition is most clearly pronounced in the individual from Artenac 1, displaying, according to the published image, porotic changes and numerous vascular impressions in the floor of the maxillary sinus (Ibid.). At the moment, we will refrain from detecting the presence of CMS in that specimen, but it can be assumed that further research will confirm the diagnosis. The abundance of dental pathologies in Neanderthals (Spikins et al., 2019; Sergi, Ascenzi, Bonucci, 1972; Condemi et al., 2012; Topic, Rascic-Konjhodzic, Sajko, 2012; Lozano et al., 2013; López-Valverde et al., 2012; Dean et al., 2013) provides additional arguments, and further increases the likelihood of high prevalence of odontogenic CMS in that species.
Owing to the absence of necessary data, it is difficult to say at present how important was the biological stress associated with CMS for the adaptive strategies of Neanderthals. The two available cases had entirely different consequences for the affected individuals, and thus, exerted different adaptive pressures on the populations. The lingering inflammation of the maxillary sinus in the Neanderthal 1 specimen, which was probably recurrent and accompanied by suppuration, is thought be an indirect cause of the death of the individual owing to the suppression of his immune system (Schultz, Schmidt-Schultz, 2015: 977). But there is no reason to suggest a marked decrease in the viability of the Chagyrskaya 57 individual, since CMS in this case was significantly weaker and likely proceeded with minimal manifestations, or asymptomatically.
An additional complication of discussing this issue is the absence of an objective possibility of identifying evidence of special care for individuals with chronic diseases, including CMS, in the Middle Paleolithic populations. Many cases of severe injuries and diseases were described in Neanderthals. Healing of such morbid conditions implied, theoretically, a serious contribution of fellow tribesmen to the care of the sick. There is evidence of possible medicinal use of some inedible plants containing anti-inflammatory and pain-relieving substances (Hardy et al., 2012). Also, the presence of natural antibiotics from mold fungi that develop on plant debris was detected in the dental calculus of Neanderthals (Weyrich et al., 2017). However, it is still practically impossible to determine how much the special care helped the recovery of the patient in each specific case, and how much healing was due to the individual’s strength of body and its immunity. The presence of traces of medicinal plants in the dental calculus of some individuals also cannot be directly linked with their specific diseases. The plants might have been used accidentally or for religious or magical purposes, while their healing properties remained unknown to the Neanderthals.
Summing up, on the basis of our analysis of the Chagyrskaya 57 specimen and published data it is possible to hypothesize that Neanderthals were anatomically predisposed to the development of odontogenic chronic CMS. Such a predisposition could have been related to the higher, as compared to modern humans, prevalence of enlargement of the alveolar recess of the maxillary sinus. The assessment of the influence of CMS on the level of biological stress in the Neanderthal populations awaits further research relying on a more representative sample.
Acknowledgement
This study was supported by the Russian Science Foundation, Project No. 21-18-00376.
Список литературы A case of chronic maxillary sinusitis in a late Neanderthal population of the Altai mountains
- Abrahams J.J., Glassberg R.M. 1996 Dental disease: A frequently unrecognized cause of maxillary sinus abnormalities? American Journal of Roentgenology, vol. 166: 1219-1223.
- Arefieva N.A., Vishnyakov V.V., Ivanchenko O.A., Karpishchenko S.A., Kiselev A.B., Kozlov V.S., Kozlov R.S., Kosyakov S.Y., Kochetkov P.A., Lopatin A.S., Nakatis Y.A., Otvagin I.V., Piskunov G.Z., Polyakov D.P., Turovsky A.B. 2014 Khronicheskiy rinosinusit: Patogenez, diagnostika i printsipy lecheniya (klinicheskiye rekomendatsii). Moscow: Prakt. meditsina.
- Baumann M., Plisson H., Rendu W., Maury S., Kolobova K., Krivoshapkin A. 2020 Neandertalian bone industry at Chagyrskaya cave (Altai, Russia). Quaternary International, vol. 559: 68-88.
- Biedlingmaier J.F., Whelan P., Zoarski G., Rothman M. 1996 Histopathology and CT analysis of partially resected middle turbinates. Laryngoscope, vol. 106: 102-104.
- Boocock P., Roberts C.A., Manchester K. 1995 Maxillary sinusitis in medieval Chichester, England. American Journal of Physical Anthropology, vol. 98: 483-495.
- Brook I. 2009 Sinusitis. Periodontology 2000, vol. 49: 126-139.
- Buck L., Stringer C.B., MacLarnon A., Rae T.C. 2019 Variation in paranasal pneumatisation between Mid-Late Pleistocene hominins. Bullétins et Mémoires de la Société d’Anthropologie de Paris. URL: https://doi.org/10.3166/bmsap-2019-0056
- Buskina A.V., Gerber V.K. 2000 K vorposu o klinicheskoy klassifi katsii khronicheskogo odontogennogo gaimorita. Vestnik otorinolaringologii, No. 2: 20-22.
- Clarke N., Carey S. 1985 The aetiology of chronic periodontal disease: An alternative perspective. Journal of American Dental Association, No. 110: 689-691.
- Condemi S., Tardivo D., Bruno F., Ricci S., Giunti P., Longo L. 2012 A case of osteolithic lesion on an Italian Neanderthal jaw. Comptes Rendus Palevol, vol. 11: 79-83.
- Dean M.C., Rosas A., Estalrrich A., García-Tabernero A., Huguet R., Lalueza-Fox C., Bastir M., de la Rasilla M. 2013 Longstanding dental pathology in Neanderthals from El Sidrón (Asturias, Spain) with a probable familial basis. Journal of Human Evolution, vol. 64 (6): 678-686.
- Emelyanova A.N. 2012 Klinicheskoye znacheniye variantov anatomicheskogo stroyeniya verkhnechelyustnoy i lobnoy pazukh: Cand. Sc. (Medicine) Dissertation. Kursk.
- Erdogan E., Fidan V., Giritli E. 2016 Radiologic imaging in chronic sinusitis. Different Aspects of Rhinosinusitis. URL: https://smjournals.com/ebooks/differentaspects-rhinosinusitis/chapters/DARS-16-05.pdf
- Georgalas C., Videler W., Freling N., Fokkens W. 2010 Global Osteitis Scoring Scale and chronic rhinosinusitis: A marker of revision surgery. Clinical Otolaryngology, vol. 35: 455-461.
- Glazyev I.E., Piskunov I.S. 2017 Anatomicheskiye predposylki razvitiya odontogennogo verkhnechelyustnogo sinusita. Rossiyskaya rinologiya, No. 3: 35-41.
- Hardy K., Buckley S., Collins M.J., Estalrrich A., Brothwell D., Copeland L., García-Tabernero A., GarcíaVargas S., de la Rasilla M., Lalueza-Fox C., Huguet R., Bastir M., Santamaría D., Madella M., Wilson J., Fernández Cortés A., Rosas A. 2012 Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften, Bd. 99: 617-626.
- Jenkins W.M., Kinane D.F. 1989 The ‘high risk’ group in periodontitis. British Dental Journal, No. 167: 168-171.
- Kolobova K.A., Chabai V.P., Shalagina A.V., Krajcarz M.T., Krajcarz M., Rendu W., Vasiliev S.K., Markin S.V., Krivoshapkin A.I. 2019 Exploitation of the natural environment by Neanderthals from Chagyrskaya Cave (Altai). Quartär, vol. 66: 7-31.
- Kolobova K., Roberts R., Chabai V., Jacobs Z., Krajcarz M., Shalagina A., Krivoshapkin A., Li B., Uthmeier T., Markin S., Morley M., O’Gorman K., Rudaya N., Talamo S., Viola B., Derevianko A. 2020 Archaeological evidence for two separate dispersals of Neanderthals into Southern Siberia. PNAS, vol. 117 (6): 2879-2885.
- Kolobova K.A., Shalagina A.V., Chabai V.P., Markin S.V., Krivoshapkin A.I. 2019 Signifi cation des technologies bifaciales au Paléolithique moyen des montagnes de l’Altaï. L’Anthropologie, vol. 123 (2): 276-288.
- Lewis M.E., Roberts C.A., Manchester K. 1995 Comparative study of the prevalence of maxillary sinusitis in Later Medieval urban and rural populations in Northern England. American Journal of Physical Anthropology, vol. 98: 497-506.
- López-Valverde A., López-Cristiá M., Prados-Frutos J.C., Gómez de Diego R., de Vicente J., Cutando A. 2012 Oral pathology in the Iberian Neanderthals. African Journal of Biotechnology, vol. 11 (23): 6359-6363.
- Lozano M., Subirà M.E., Aparicio J., Lorenzo C., Gómez-Merino G. 2013 Toothpicking and periodontal disease in a Neanderthal specimen from Cova Foradà site (Valencia, Spain). PLoS ONE, vol. 8 (10). URL: https://doi.org/10.1371/journal.pone.0076852
- Mafee M.F., Tran B.H., Chapa A.R. 2006 Imaging of rhinosinusitis and its complications: Plain film, CT, and MRI. Clinical Reviews in Allergy and Immunology, vol. 30: 165-186.
- Mafessoni F., Grote S., de Filippo C., Slon V., Kolobova K.A., Viola B., Markin S.V., Chintalapati M., Peyregne S., Skov L., Skoglund P., Krivoshapkin A.I., Derevianko A.P., Meyer M., Kelso J., Peter B., Prüfer K., Pääbo S. 2020 A high-coverage Neandertal genome from Chagyrskaya Cave. PNAS, vol. 117 (26): 15133-15136.
- Mann A., Vandermeersch B., Delagnes A., Tournepiche J.-F. 2007 Human fossil remains from the Mousterian levels of Artenac (Charente). Comptes Rendus Palevol, vol. 6: 581-589.
- Momeni A.K., Roberts C.C., Chew F.S. 2008 Imaging of chronic and exotic sinonasal disease: Review. American Journal of Roentgenology, vol. 189 (suppl. 6): 35-45.
- Mukovozov I.N. 1982 Differentsialnaya diagnostika khirurgicheskikh zabolevaniy chelyustno-litsevoy oblasti. Leningrad: Meditsina.
- Ogden A. 2007 Advances in the palaeopathology of teeth and jaws. In Advances in Human Palaeopathology, R. Pinhasi, S. Mays (eds.). Сhichester: John Wiley & Sons, Ltd, pp. 283-307.
- Panhuysen R., Coenen V., Bruintjes T. 1997 Chronic maxillary sinusitis in medieval Maastricht, the Netherlands. International Journal of Osteoarchaeology, vol. 7: 610-614.
- Rae T.C., Koppe T., Stringer C.B. 2011 The Neanderthal face is not cold adapted. Journal of Human Evolution, vol. 60: 234-239.
- Roberts C.A. 2007 A bioarcheological study of maxillary sinusitis. American Journal of Physical Anthropology, vol. 133: 792-807.
- Schultz M. 2006 Results of the anatomical-palaeopathological investigations on the Neanderthal skeleton from the Kleine Feldhofer Grotte (1856) including the new discoveries from 1997/2000. Rheinische Ausgrabungen, vol. 58: 277-318.
- Schultz M., Schmidt-Schultz T.H. 2015 Paleopathology: Vestiges of pathological conditions in fossil human bone. In Handbook of Paleoanthropology. Berlin, Heidelberg: Springer, pp. 969-981.
- Schwartz J.H., Tattersall I. 1996 Signifi cance of some previously unrecognized apomorphies in the nasal region of Homo neanderthalensis. PNAS, vol. 93: 10852-10854.
- Sergi S., Ascenzi A., Bonucci E. 1972 Torus palatinus in the Neanderthal Circeo I skull. A histologic, microradiographic and electron microscopic investigation. American Journal of Physical Anthropology, vol. 36: 189-197.
- Shalagina A.V., Kharevich V.M., Mori S., Bomann M., Krivoshapkin A.I., Kolobova K.A. 2020 Rekonstruktsiya tekhnologicheskikh tsepochek proizvodstva bifasialnykh orudiy v industrii Chagyrskoy peshchery. Sibirskiye istoricheskiye issledovaniya, No. 3: 130-151.
- Sipkin A.M., Nikitin A.A., Lapshin V.P., Nikitin D.A., Chukumov R.M., Kryazhinova I.A. 2013 Verkhnechelyustnoy sinusit: Sovremenniy vzglyad na diagnostiku, lecheniye i reabilitatsiyu. Almanakh klinicheskoy meditsiny, No. 28: 82-87.
- Skov L., Peyrégne S., Popli D., Lasi L.M.N., Devièse T., Slon V., Zavala E.I., Hajdinjak M., Sümer A.P., Grote S., Bossom A.M., López D.H., Nickel B., Nagel S., Richter J., Essel E., Gansauge M., Schmidt A., Korlevic P., Comeskey D., Derevianko A.P., Kharevich A., Markin S.V., Talamo S., Douka K., Krajcarz M.T., Roberts R.G., Higham T., Viola B., Krivoshapkin A.I., Kolobova K.A., Kelso J., Meyer M., Pääbo S., Peter B.M. 2022 Genetic insights into the social organization of Neanderthals. Nature. (In press).
- Slavin R.G., Spector S.L., Bernstein I.L. 2005 The diagnosis and management of sinusitis: A practice parameter update. Journal of Allergy and Clinical Immunology, vol. 116: 13-47.
- Slon V., Mafessoni F., Vernot B., Filippo C. de, Grote S., Viola B., Hajdinjak M., Peyrégne S., Nagel S., Brown S., Douka K., Higham T., Kozlikin M.B., Shunkov M.V., Derevianko A.P., Kelso J., Meyer M., Prüfer K., Pääbo S. 2018 The genome of the offspring of a Neanderthal mother and a Denisovan father. Nature, vol. 561: 113-116.
- Snidvongs K., Earls P., Dalgorf D., Sacks R., Pratt E., Harvey R.J. 2014 Osteitis is a misnomer: A histopathology study in primary chronic rhinosinusitis. International Forum of Allergy and Rhinology, vol. 4 (5): 390-396.
- Spikins P., Needham A., Wright B., Dytham C., Gatta M., Hitchens G. 2019 Living to fight another day: The ecological and evolutionary significance of Neanderthal healthcare. Quaternary Science Review, vol. 217: 98-118.
- Sundman E.A., Kjellström A. 2013 Chronic maxillary sinusitis in medieval Sigtuna, Sweden: A study of sinus health and effects on bone preservation. International Journal of Osteoarchaeology, vol. 23 (4): 447-458.
- Tattersall I. 2002 The case for saltational events in human evolution. The Speciation of Modern Homo sapiens: Proceedings of the British Academy, vol. 106: 49-59.
- Teul I., Lorkowski J., Lorkiewicz W., Nowakowski D. 2013 Sinusitis in people living in the medieval ages. In Neurobiology of Respiration: Advances in Experimental Medicine and Biology. Dordrecht: Springer, pp. 133-138.
- Topić B., Raščić-Konjhodžić H., Sajko M.Č. 2012 Periodontal disease and dental caries from Krapina Neanderthal to contemporary man - skeletal studies. Acta Medica Academica, vol. 41 (2): 119-130.
- Vernot B., Zavala E.I., Gómez-Olivencia A., Jacobs Z., Slon V., Mafessoni F., Romagné F., Pearson A., Petr M., Sala N., Pablos A., Aranburu A., Castro J.M.B., de Carbonell E., Li B., Krajcarz M.T., Krivoshapkin A.I., Kolobova K.A., Kozlikin M.B., Shunkov M.V., Derevianko A.P., Viola B., Grote S., Essel E., Herráez D.L., Nagel S., Nickel B., Richter J., Schmidt A., Peter B., Kelso J., Roberts R.G., Arsuaga J.-L., Meyer M. 2021 Unearthing Neanderthal population history using nuclear and mitochondrial DNA from cave sediments. Science, vol. 372 (6542).
- Weyrich L.S., Duchene S., Soubrier J., Arriola L., Llamas B., Breen J., Morris A.G., Alt K.W., Caramelli D., Dresely V., Farrell M., Farrer A.G., Francken M., Gully N., Haak W., Hardy K., Harvati K., Held P., Holmes E.C., Kaidonis J., Lalueza-Fox C., de la Rasilla M., Rosas A., Semal P., Soltysiak A., Townsend G., Usai D., Wahl J., Huson D.H., Dobney K., Coope A. 2017 Neanderthal behavior, diet, and disease inferred from ancient DNA in dental calculus. Nature, vol. 544: 357-361.
- Zollikofer C.P.E., Ponce de Leon M., Schmitz R.W., Stringer C.B. 2008 New insights into Mid-Late Pleistocene fossil hominin paranasal sinus morphology. Anatomical Record, vol. 291: 1506-1516.
- Zubova A.V., Ananyeva N.I., Moiseyev V.G., Stulov I.K., Dmitrenko L.M., Obodovskiy A.V., Potrakhov N.N., Kulkov A.M., Andreev E.V. 2020 The use of computed tomography for the study of chronic maxillary sinusitis: Based on crania from the Pucará De Tilcara Fortress, Argentina. Archaeology, Ethnology and Anthropology of Eurasia, vol. 48 (3): 143-153.
- Zubova A.V., Moiseyev V.G., Ananyeva N.I., Stulov I.K., Andreev E.V. 2022 Chronic maxillary sinusitis recorded in archaeological samples: Geographical distribution and predisposing factors. Archaeology, Ethnology and Anthropology of Eurasia, vol. 50 (1): 147-157.


