A link between autophagy regulatory proteins M-TOR and BECLIN-1 and parameters of lymphogenic metastasis in colorectal cancer
Автор: Vtorushin Sergey V., Rachkovsky Kirill V., Krakhmal Nadezhda V., Stepanov Ivan V., Zavyalova Marina V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Клинические исследования
Статья в выпуске: 4 т.17, 2018 года.
Бесплатный доступ
Currently the impact of autophagy on carcinogenesis remains understudied. On the one hand, autophagy acts as a tumor suppressor, as it activates degradation of oncoproteins, toxic proteins, and damaged cell organelles, that may be aggressive and lead to DNA damage. On the other hand, autophagy may promote tumor cell survival under hypoxia and in the presence of reactive oxygen species, which occurs primarily due to blocking of apoptosis mechanisms, raising the chances for maintaining tumor clone dynamics. Autophagy regulation is a complicated and multi-stage process. The main regulator here is a signaling pathway that activates serine/threonine protein kinase m-TOR (the mammalian target of rapamycin). Data on the impact of autophagic proteins ATg5, LC3A, LC3b, and beclin-1 on malignant cell survival as well as on tumor growth and progression have been reported in literature. However, studies aimed at seeking possible relationships between autophagy and pathogenetic mechanisms of carcinogenesis are of great interest. the aim of the study is to investigate a relationship between the expression parameters of autophagy regulatory proteins m-TOR and beclin-1 and the features of lymphogenic metastasis in colorectal cancer. materials and methods. The study included 105 patients with T1-4N0-3M0 colorectal cancer treated in the Thoracic and Abdominal Department of Cancer Research Institute of Tomsk Research Medical Center from 2012 to 2015. The average age of patients was 59.7±4.3 years. Morphological verification of the diagnosis was performed on the biopsy samples of primary tumor tissue. Staging of colorectal cancer was determined according to the TNM classification of malignant tumors (2002). results. Analysis of the frequency of lymphogenic metastasis depending on the presence or absence of m-Tor and beclin-1 expression in tumor cell cytoplasm revealed a statistically significant link between these variables. Conclusion. The obtained findings clearly exhibit that deceleration or loss of autophagic activity in the tumor is accompanied by implementation of lymphogenic dissemination, which is a predictor of an unfavorable outcome of the disease.
Colorectal cancer, autophagy, lymphogenous metastasis, Beclin-1, m-Tor, prognosis
Короткий адрес: https://sciup.org/140254197
IDR: 140254197 | УДК: 616.34+616.35]-006.6-056.57-091.8-033.2:611-018.98:577.112 | DOI: 10.21294/1814-4861-2018-17-4-41-47
Текст научной статьи A link between autophagy regulatory proteins M-TOR and BECLIN-1 and parameters of lymphogenic metastasis in colorectal cancer
Currently, colorectal cancer is one of the most common cancers worldwide, and every year more and more new cases of this disease are recorded [1]. This cancer is characterized by low five-year survival rate, which is explained by frequent recurrences and high chances for development of lymphogenic and hematogenic metastases [2]. Differences in biological parameters of a primary tumor, variety in the types of carcinoma, differences in metastasis in organs, and resistance to therapy make difficult early detection of a tumor. Shortcomings of existing treatment algorithms dictate the necessity of studying clinical features of colorectal cancer as well as molecular and morphologic parameters of the disease.
According to the literature data, non-surgical treatment options, such as chemotherapy, radiation therapy, hormone therapy and immunotherapy, are the most common treatment strategies for patients with colorectal cancer [3, 4]. Special attention is given to the study of molecular and genetic features of the tumor, that are associated with different options of tumor progression as well as with disease prognosis. Autophagy is one of the processes involved in tumor pathogenesis. Its role in tumor pathogenesis is understudied and data that have been already published in literature are controversial. Autophagy is a catabolic process characterized by degradation of cell organelles. This process is necessary for growth and proliferation of healthy cells and maintenance of intracellular homeostasis [5]. Autophagy is regulated by more than 30 proteins encoded by ATG genes found in yeast fungi as well as by a big number of homologous proteins found in mammals [6].
The impact of autophagy on carcinogenesis remains unclear [7]. On the one hand, autophagy acts as a suppressor of tumor cell development due to degradation of oncoproteins, toxic proteins, and damaged cell organelles that may be aggressive and lead to DNA damage [8, 9]. On the other hand, autophagy may promote tumor cell survival under hypoxia, in the presence of reactive oxygen species, and under starvation by blocking apoptosis, thus favoring tumor progression [9-11]. The main autophagy regulator is a signaling pathway that activates serine/threonine protein kinase m-TOR (the mammalian target of rapamycin), which is involved in cell metabolism, growth, proliferation, and differentiation [12].
According to E.J. Write et.al., autophagy suppression in the tumor is associated with the oncogenic function of the PI3-K pathway, which triggers the mTORC1 cascade of reactions [13]. According to other authors, tumor cells with activated autophagy are characterized by increased aggressiveness and resistance to chemotherapy. After chemo- and radiation therapy, a rise in the number of autophagosomes in the tumor was observed [8, 14].
Impairment of m-TOR pathway functioning, determined by genetic variations in key genes, was identified in investigations on bladder cancer, breast cancer, lung cancer, and hepatocellular carcinoma [15-18]. According to L. Lei et. al., over the past few years, considerable attention has been given to the impact of the m-TOR pathway on tumor formation [19]. According to the findings of L. Xiao yan L. Zhou, increased proliferative activity in tumor cells in breast cancer is associated with elevated p-mTOR expression [20]. Another study exhibited that p-mTOR expression was associated with a worse prognosis of stomach cancer, however, the survival rate in patients with positive m-TOR expression was significantly higher than that in patients with negative expression of this marker in the tumor [21, 22].
Literature contains data on the impact of autophagy-related proteins ATG5, LC3A, LC3B, and Beclin-1 on cell survival, carcinogenesis, and invasive features of the tumor [23-25]. The Beclin-1 protein plays an essential role in regulating autophagy [26] and is involved in implementation of various signaling pathways. Beclin-1 may act both as an inductor and suppressor of carcinogenesis. Till recently, the gene that encoded Beclin-1 had been viewed as a tumor suppressor gene. However, latest research has not revealed any evidence on a relationship between a loss of this gene or its mutation and development of tumors in different localities [27]. J. Gao et al. reported that transformations in the gene encoding the Beclin-1 protein were identified in only 2.5% of patients with colorectal cancer [28]. In the study of K.J. Schmits et al., a correlation between the level of Beclin-1 expression and low survival rate in patients with stage III and IV colorectal cancer was found [10]. The function of Beclin-1 is associated with many key cell molecules, for instance, with m-TOR and HIFs (a transcription factor that reacts to a decrease in cellular oxygen level) [29].
In recent years, the prognostic significance of Beclin-1 level in stomach cancer [30], epidermoid laryngeal cancer [31], and testicular cancer [32] has been actively studied. In case of monoallelic deletion of the gene encoding Beclin-1, in 40-75% of patients with malignant tumors deceleration of autophagy was observed. Such findings were obtained in investigations on testicular, breast, prostate, and brain cancers [33]. To date, data on the impact of Beclin-1 on tumor progression and disease prognosis remain controversial. According to the findings of Ahn, increased expression of Beclin-1 was observed in 95% of patients with intestinal adenocarcinoma. However, in this study no statistically significant data on the relationship between the level of this marker and clinical and morphological features of the tumor were obtained, thus leading to a hypothesis that Beclin-1 does not play an essential role in tumor suppression [33]. A number of other studies point to a relationship between the level of Beclin-1 expression and the depth of tumor invasion as well as metastasis parameters. Thus, high Beclin-1 expression in patients with colorectal cancer was considered as an indicator of a favorable prognosis and good survival rate [34]. Therefore, the study of autophagy-related proteins is a topical area in modern oncology, since the impact of these proteins on signaling pathways regulating autophagy in healthy and neoplastic cells may become a prospective therapeutic target for cancer.
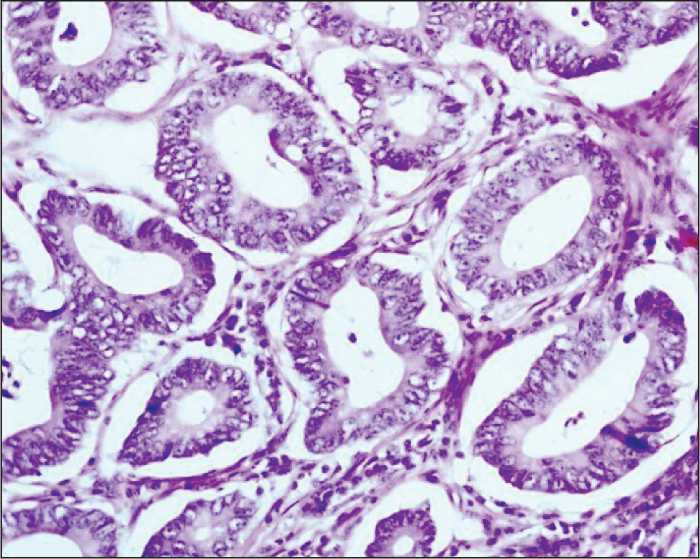
Figure 1. Well-differentiated adenocarcinoma with the minimal stromal component. Hematoxylin and eosin staining (200× magnification)
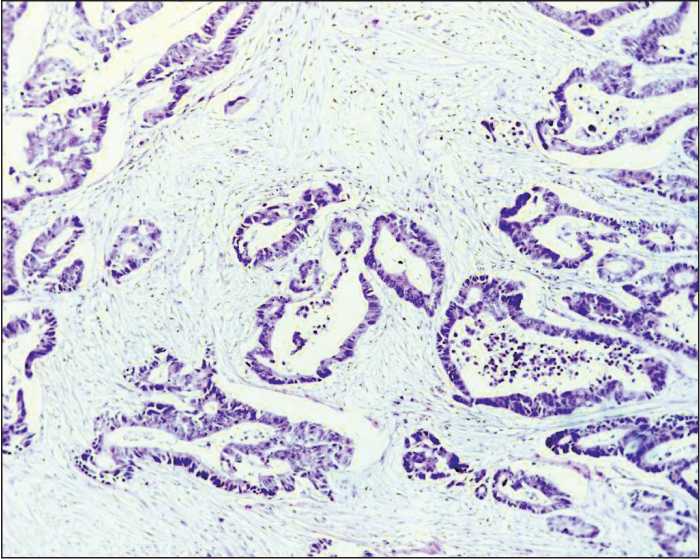
Figure 2. Moderately differentiated adenocarcinoma with pronounced stroma.
Hematoxylin and eosin staining (100× magnification)
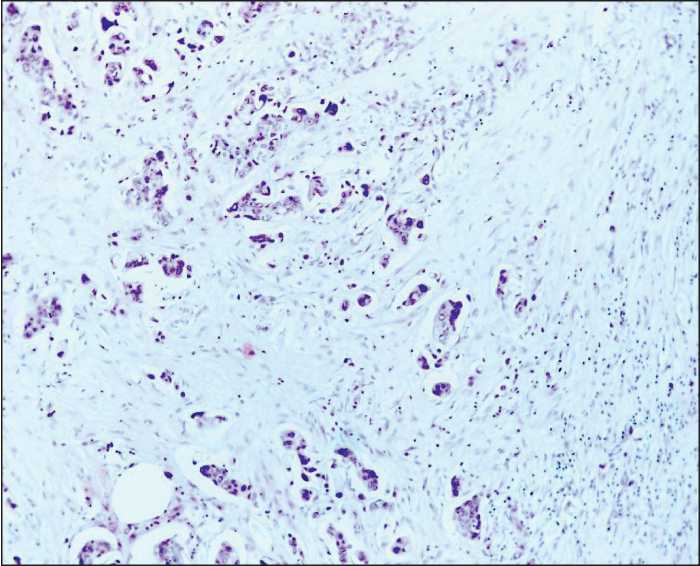
Figure 3. Poorly differentiated adenocarcinoma. Discrete tumor cells, small tumor cell clusters. Hematoxylin and eosin staining (100× magnification)
Material and Methods
The study included 105 patients with T1-4N0-3M0 colorectal cancer treated in the Thoracic and Abdominal Oncology Unit at the Research Institute of Oncology of Tomsk Research Medical Center from 2012 to 2015. The average age of patients was 59.7±4.3 years. Morphological verification of the diagnosis was performed on the biopsy samples of primary tumor tissue. Staging of colorectal cancer was determined according to the TNM classification of malignant tumors (2002). Stage I cancer was detected in 8 patients (7.6%), stage IIA in 16 patients (15.2%), stage IIB in 43 patients (41%), stage IIIA in 33 patients (31.5%), stage IIIB in 4 patients (3.8%), and stage IIIC in 1 case (0.9%). Tumor localization in the colon was the following: the cecum in 16 patients (15%), ascending colon in 8 patients (7%), hepatic flexure in 3 patients (3%), transverse colon in 2 patients (2%), splenic flexure in 23 patients (22%), descending colon in 6 patients (5%), sigmoid colon in 31 patients (30%), and rectosigmoid junction in 16 patients (16%). All patients underwent hemicolectomy or colon resection. In the post-operative period, 48 patients received different adjuvant treatments according to clinical recommendations. The patients had been followed up for 3 years. To evaluate the disease activity, patients’ medical records were analyzed. The surgical specimens were sent to further morphological examination. Fixation, processing to a paraffin block, preparation of slices, and staining were performed in compliance with standard instructions. Morphological examination of the specimens was conducted using the light microscope (“Axioscope A1” Carl Zeiss). Digital images of the stained histology and immunohistochemistry slides were acquired using AxioCam MRc5 camera with AxioVision 4.6.3 (Carl Zeiss) software for digital image processing. When investigating the surgical specimens, we performed gross examination of the primary tumor: we evaluated the size and form of the tumor, the depth of tumor invasion, and the status of resection margins. To assess the status of regional lymph nodes, all lymph nodes were investigated with respect to the presence or absence of metastases. The diagnosis of colorectal cancer was made according to the WHO Histological classification of Gastrointestinal Tumors (WHO, 2013). In 92 patients (87.6%), the tumor histotype corresponded to colon adenocarcinoma with various degrees of differentiation (Figures 1-3). In 13 patients (12.4%), other histological subtypes were detected (mucinous cancer, cancer with neuroendocrine differentiation). Histological examination of the tumor tissue was conducted with assessment of the following parameters: the degree of differentiation, depth of colon wall invasion, presence of perineural or lymphovascular invasion, presence of tumor necrosis, presence and intensity of inflammatory infiltration, and degree of stroma representation in the tumor.
Various expression parameters of the tumor in colorectal cancer were investigated on the paraffin sections using the immunohistochemical assay according to the standard procedure. The following antibodies were used in the investigation: Anti-Beclin 1 antibody ab 62472 Abcam (polyclonal, 1:100), and Rabbit Anti-human m-Tor Antibody (polyclonal, 1:100). Application of antibodies was preceded by control reactions with external controls according to the manufacturer’s instructions. The expression of Beclin-1 (cytoplasmic expression in the Golgi complex) and m-Tor (membrane and cytoplasmic staining) was evaluated as the percentage of positively-stained cells in the tumor detected per 10 fields of vision at 400× magnification. Along with quantitative assessment of the studied marker
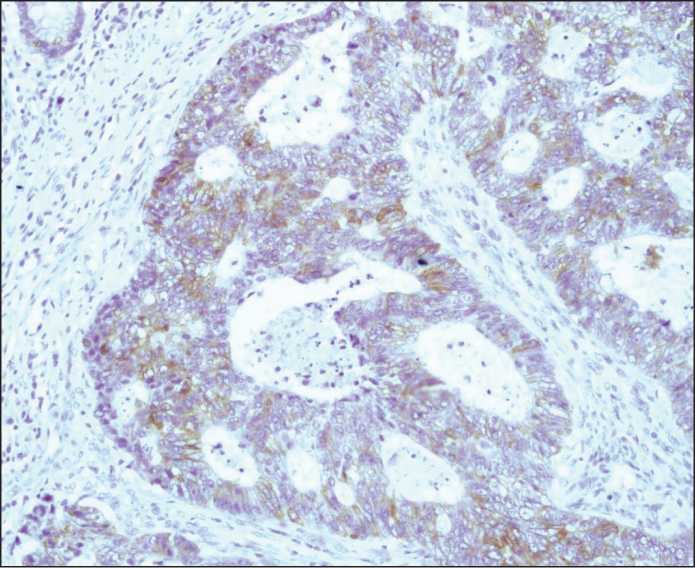
Figure 4. Moderately pronounced heterogeneous cytoplasmic expression of m-Tor in colon adenocarcinoma. The immunohistochemical reaction.
Diaminobenzidine and hematoxylin staining (200× magnification)
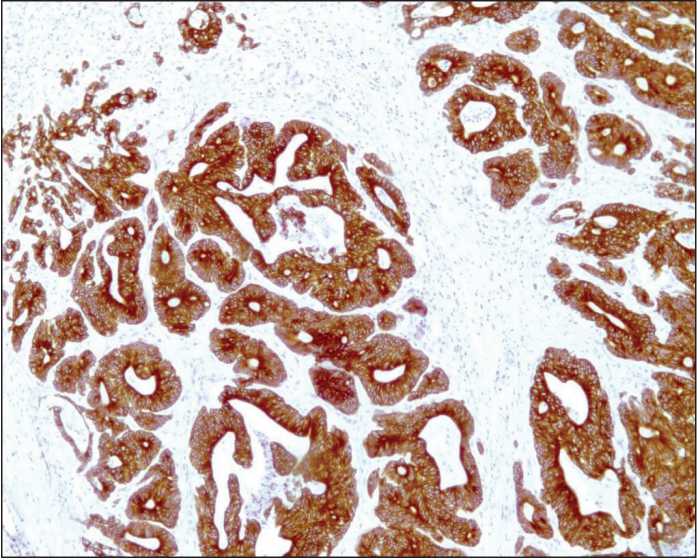
Figure 5. Strongly apparent homogeneous cytoplasmic expression of m-Tor in colon adenocarcinoma.
The immunohistochemical reaction. Diaminobenzidine and hematoxylin staining (100× magnification)
Table 1
Distribution of the patients according to the number of lymph nodes affected by metastases
Up to 4 lymph nodes (N1) 39 (88 %)
More than 4 lymph nodes (N2) 5 (12 %)
Table 2
A link between the presence of m-Tor expression and the state of regional lymph nodes in the patients with colorectal cancer
|
m-TOR expression in the tumor cells |
Presence of metastases in the regional lymph nodes (abs. num., %) |
|||
|
N0 |
N+ |
|||
|
Negative expression |
1 (1.7 %) |
5 (10.8) |
χ2=4.4 |
|
|
Positive expression |
58 (98.3 %) |
41 (89.2 %) |
p=0.04 |
Table 3 |
Percentage of beclin-1 and m-Tor expression depending on the presence of lymphogenic metastases
Presence of metastases
Percentage of m-TOR expression Percentage of Beclin-1 expression
N0
79.6±25.1
24.5±26.1
N+
67.3±33.9
19.2±20.9
F=4.5 р=0.03
F=1.2 р=0.2
expression in the tumor, heterogeneity of staining was investigated at 150× magnification. In cases of positive marker expression in the tumor cells with equal staining intensity, the expression was estimated as homogeneous. Cases where the tumor had regions with both positive and negative marker expression as well as regions with different staining intensity were considered as cases with heterogeneous expression distribution (Figures 4-5).
The obtained data were processed using “Statistica 10” software. The findings were analyzed with descriptive statistics and dispersion analysis. To calculate statistical significance of the differences, the non-parametric Chi-square test and Spearman’s correlation coefficient were used. The differences between the studied variables were considered statistically significant at p<0.05.
Results
Since the presence of lymphogenic metastases is one of the key prognostic criteria for the course of colorectal cancer, we analyzed the frequency of metastases depending on the parameters of autophagy marker expression.
Table 1 represents the data on the distribution of the patients depending on the number of lymph nodes affected by metastases. In the study, most patients had less than 4 metastatic lymph nodes; it corresponded to N1 parameter in the TNM classification.
Analysis of the frequency of lymphogenic metastasis depending on the presence or absence of m-Tor expression in tumor cell cytoplasm revealed a statistically significant link between these variables. We found that metastases in regional lymph nodes were more often observed under negative m-Tor expression in tumor cells (Table 2).
Moreover, we identified a relationship between the percentage of the tumor cells with positive m-Tor expression and the presence of lymphogenic metastases. In patients with metastatic regional lymph nodes, lower m-Tor expression in tumor cells was detected (Table 3).
Following the obtained findings, we found that the presence or absence of Beclin-1 expression in tumor cells had no statistically significant link with the number of metastatic lymph nodes. What is more, we did not reveal a statistically significant relationship between the percentage of Beclin-1 expression and the parameters of lymphogenic metastasis (F=1.2, р=0.2) (Table 3).
Conclusion
The present study allowed us to identify the link between the expression features of m-Tor protein and the parameters of lymphogenic metastasis in patients with colorectal cancer. Thus, the presence of metastatic lymph nodes was more frequently registered under negative expression of the marker in tumor cells. We revealed that a statistically significant decrease in m-Tor expression in tumor cells occurred in the presence of metastases in regional lymph nodes. The obtained findings clearly exhibit that deceleration or loss of autophagic activity in the tumor is accompanied by implementation of lymphogenic dissemination, which is a predictor of an unfavorable outcome of the disease.
The expression characteristics of Beclin-1 appeared to have no relation to the parameters of lymphogenic metastasis. It may be explained by less involvement of this molecular and biologic marker in carcinogenesis and tumor progression in colorectal cancer.
Список литературы A link between autophagy regulatory proteins M-TOR and BECLIN-1 and parameters of lymphogenic metastasis in colorectal cancer
- Haggar F.A., Boushey R.P. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009 Nov; 22(4): 191-7. DOI: 10.1055/s-0029-1242458
- Birgisson H., Wallin U., Holmberg L., Glimelius B. Survival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survival. BMC Cancer. 2011 Oct 11; 11: 438. DOI: 10.1186/1471-2407-11-438
- Meyerhardt J.A., Mayer R.J. Systemic therapy for colorectal cancer. N. Engl. J. Med. 2005; 352(5): 476-487. DOI: 10.1056/NEJMra040958
- Brenner H., Kloor M., Pox C.P. Colorectal cancer. Lancet. 2014 Apr 26; 383(9927): 1490-1502. DOI: 10.1016/S0140-6736(13)61649-9
- Nagelkerke A., Sweep F.C., Geurts-Moespot A., Bussink J., Span P.N. Therapeutic targeting of autophagy in cancer. Part I: molecular pathways controlling autophagy. Semin Cancer Biol. 2015 Apr; 31: 89-98. DOI: 10.1016/j.semcancer.2014.05.004


