ABA biosynthesis defective mutants reduce some free amino acids accumulation under drought stress in tomato leaves in comparison with arabidopsis plants tissues
Автор: AL.asbahi Adnan ali, AL.maqtari Maher ali, Naji Khalid Mohammed
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.8, 2012 года.
Бесплатный доступ
The ability of plants to tolerate drought conditions is crucial for plant survival and crop production worldwide. The present data confirm previous findings reported existence of a strong relation between abscisic acid (ABA) content and amino acid accumulation as response water stress which is one of the most important defense mechanism activated during water stress in many plant species. Therefore, free amino acids were measured to determine any changes in the metabolite pool in relation to ABA content. The ABA defective mutants of Arabidopsis plants were subjected to leaf dehydration for Arabidopsis on Whatman 3 mm filter paper at room temperature while, tomato mutant plants were subjected to drought stresses for tomato plants by withholding water. To understand the signal transduction mechanisms underlying osmotic stress-regulating gene induction and activation of osmoprotectant free amino acid synthesizing genes, we carried out a genetic screen to isolate Arabidopsis mutants defective in ABA biosynthesis under drought stress conditions. The present results revealed an accumulation of specific free amino acid in water stressed tissues in which majority of free amino acids are increased especially those playing an osmoprotectant role such as proline and glycine. Drought stress related Amino acids contents are significantly reduced in the mutants under water stress condition while they are increased significantly in the wild types plants. The exhibited higher accumulation of other amino acids under stressed condition in the mutant plants suggest that, their expressions are regulated in an ABA independent pathways. In addition, free amino acids content changes during water stress condition suggest their contribution in drought toleration as common compatible osmolytes.
Abscisic acid, amino acids, drought resistance, tomato, arabidopsis, hplc
Короткий адрес: https://sciup.org/14323607
IDR: 14323607
Текст научной статьи ABA biosynthesis defective mutants reduce some free amino acids accumulation under drought stress in tomato leaves in comparison with arabidopsis plants tissues
Plants abiotic resistance mechanism is very complex as it is influenced by several environmental factors during developmental stage of plant (Ramachandra-Reddy et al, 2004; Gubler et al., 2005). In case of drought stress tolrtation, plant physiological responses improve water-use efficiency (Sahi C. et al., 2006 and Umezawa et al., 2006) through several mechanisms such as, leaf wilting, reduction in leaf area (Deak and Malamy, 2005; De Smet et al., 2008) reducing of transpiration and photosynthesis activities (Flowers, 2004: Vinocur and Altman, 2005) and accumulation of solutes, such as sugars, amino acids, organic acids and ions – especially potassium (K+) (Wang et al, 2005; Mittler, 2006; Ribaut and Poland, 2004). In addition, drought stress is accompanied with accumulation of free amino acids (Stewart and Larher, 1980) to adjust Nitrogen metabolism which in turn will principally used for synthesis of specific enzymes or proteins involved in drought toleration (Navari-Izzo et al., 1990 + ABA1). Amino acids are not only fundamental protein constituents but also serve as precursors for many essential plant metabolites. Although amino acid biosynthetic pathways in plants have been identified, pathway regulation, catabolism, and downstream metabolite partitioning remain relatively uninvestigated. Beyond their essential function as the building blocks of proteins, amino acids contribute to many aspects of plant biochemistry and physiology. Despite this, there are relatively large gaps in our understanding of the biochemical pathways and regulation of amino acid synthesis in plants. (Jander et al, 2004). Recently, free amino acids are hypothesized to act as osmolytes that protect plant cells against dehydration (Nambara et al., 1998; Joshi and Jander, 2009). The recent progress in gene expression, transcriptional regulation and signal transduction knowledge in plant responses to drought has facilitated gene discovery and enabled genetic engineering using several functional or regulatory genes to activate specific or broad pathways related to drought tolerance in plants. Therefore, plant responses underlying drought tolerance are accompanied by the activation of two main groups of genes (Shinozaki and Yamaguchi-Shinozaki, 2007; Zhang et al., 2004). One group is involved in accumulation of compatible metabolites such as the phytohormone abscisic acid (ABA), amino acids or osmotically active compounds, while the other is involved in the perception of drought stress and in the transmission of the stress signal (Bruce, 2002, Colmer, 2006 ) (AA-ABA 2). ABA plays important role in many plant cellular plant cellular processes including seed development, dormancy, germination, vegetative growth, adaptation to water deficit, through combinatorial signaling pathways (Shinozaki et al, 2003 + ABA1) along with its previously known functions in regulation of plant development, stomatal opening, low temperature and salinity adaptive responses. Moreover, ABA serves as a stress signal for production of some metabolites such as, osmolytes (Munns and Sharp, 1993; Verslues and Zhu, 2005 + AA-ABA3). It is clear that there is cellular collaboration for protection against drought that can be achieved accumulation of osmoprotective compound and their elicitors. Therefore, the current study aimed to determine the levels of free amino acids in response to a water deficit in ABA defective tomato and Arabidopsis various mutants at the different growth stages. This will increase our understanding of the regulatory networks controlling the drought stress response. Achievement of the previous objective in the present investigation was done by determination of free amino acids levels in response to a water deficit in different ABA defective mutants of Tomato and Arabidopsis plants at different growth stages. The availability of ABA-deficient mutants in Arabidopsis and tomato has provided invaluable opportunities to investigate the role of ABA in plant stress responses through osmoprotectant free amino acids. (AA-ABA 1). The various mutants have been used to analyse the role of ABA in stress tolerance, however, there is limited information available on the correlation between stress induced amino acids and ABA accumulation in plants.
MATERIALS AND METHODS
Plant Materials:
The selected plants used for drought stress experiments in the current study were the ABA-biosynthetic mutants and wild-types strains of tomato (Lycopersicon esculentum) and Arabidopsis plants. Three tomato wilty mutants, notabilis ( not ), flacca ( flc ) and sitiens ( sit ) defective in ABA biosynthesis were used for the drought experiment along with wild type control tomato plants and four Arabidopsis thaliana ABA-biosynthetic mutants were ( aba2-2 , aba3-1 , aao3-1 and nced3-4 ). The control used in this experiment was wild-type (ColO).
Seeds sterilization and Vernalization:
Mutant and wild-type seeds of both plants were surface sterilized with a 1 min wash of 70% ethanol, followed by 50% bleach and 0.01% Tween-80 for 10 min. Then, Tomato and Arabidopsis seeds were rinsed four times with sterilized water, and were suspended in 0.1% sterile phytoagar (Gibco BRL Life Technologies). Seeds were cold-stratified at 4°C for 3 days.
Seeds germination and plant growth
Arabidopsis plant seeds were germinated in Cornell Mix with Osmocoat fertilizer (Landry et al., 1995) in 20 x 40-cm nursery flats in Conviron growth chambers. Photosynthetic photon flux density was 200 µ mol m–2 s–1, the photoperiod was 16:8 day: night, the temperature was 23°C, and the relative humidity was 50%.
Tomato seeds were germinated in soil pots fertilized with 20:20:20 (N:phosphate: potassium) fertilizer (J.R. Peters) under greenhouse conditions under 14 h of light and with photosynthetically active radiation between 400 and 500 mmol photons m–2 at the soil surface. Greenhouse temperatures ranged from C during the day/night.
They were selected to harvest leaves, flowers and siliques for amino acid analysis.
Reduced amino acid contents in drought stressed tomato mutant ‘ sitiens’ ( sit ) were rescued by exogenous ABA feeding.
Drought tolerance Experiments:
For the drought stress experiments performed on tomato plants, wild types and mutants were grown in the same flats, and irrigated to six-week-old plants was withheld for 6 to 10 days, until complete wilting.
For dehydration experiments performed on Arabidopsis plants, tissues were detached from the 3-weeks-old plants and dehydrated on paper towels for 18 h under ambient humidity. Plant tissue was weighed before and after the dehydration treatments.
In dehydration experiments of stressed tomato mutant ‘ sitiens’ ( sit ) rescued by exogenous ABA feeding tomato plants, leaves of ABA-defective mutants fed by ABA and ABA-defective mutants fed by water leafs were detached from 6-week-old plants and allowed to feed with ABA (25 uM) for 24 hours.
Arabidopsis and Tomato sampled tissues were collected for free amino acid extractions and HPLC evaluation analysis.
Amino Acid Analysis by HPLC:
Tissues were sampled concurrently for wildtype and transgenic plants from four desiccated plants in each experiment.
For free amino acid analysis, plant tissue was pre-weighed and was frozen in liquid nitrogen along with 3-mm steel balls (Abbott Ball Company) and was homogenized using a Harbil 5G-HD paint shaker (Fluid Management). Amino acids levels were analyzed as described previously (Joshi et al., 2006) using an AccQ-fluor reagent kit (Waters). Aliquots (10 µL) were used for HPLC analysis using a Waters 2790 separation module. Separation was performed on 3.9 x150 mm AccQ-Tag column (Waters) at 38°C, and amino acids were detected using a Waters 2487 dual-wavelength absorbance detector at 280 nm and a Waters 2475 photodiode array detector with excitation at 280 nm and emission at 395 nm. Eluent A (sodium acetate and trimethylamine, pH 5.04; Waters) and solution B (60% acetonitrile:40% water) were used at a 1 mL min–1 flow rate with the following gradient: 0 to 0.01 min, 100% A; 0.01 to 5.0 min, linear gradient to 97% A, 3% B; 5 to 12 min, to 95% A, 5% B; 12 to 15 min, 92% A, 8% B; 15 to 45 min, 65% A, 35% B; 45 to 49 min, to 65% A, 35% B; 49 to 50 min, to 100% B; 50 to 60 min, to 100% B, 61 to 68 min, 100% A. Standard curves were prepared using amino acids purchased from Sigma-Aldrich. Amino acid concentrations were calculated by comparing peak areas to those of standard curves and normalized using L-nor leucine as an internal control.
RESULTS
The detached leafs from both plant species, Arabidopsis and tomato wild-type and mutants defective in ABA biosynthesis used for the drought experiment have shown that free amino acids extracts of fresh and drought stressed and dehydrated tissues detected by using HPLC, as per methods described by (Joshi and Jander , 2009) were subjected to quantification done by comparing peak areas to those of standard curves and normalized using L-nor-leucine as an internal control shown in (Fig. 1).
In case of amino acid contents found in tomato mutant leafs rescued by exogenous ABA feeding before being subjected to drought stress experiment, we found significant differences observed between amino acids content in ABA fed and water fed controls leaf tissues of the tomato mutant ‘ sitiens’ ( sit ) as it is appeared in (Fig. 2).
These ABA induced amino acids include Tyr, Ile, Arg, Leu, Val, His, Ser and Thr which were found in higher concentration in drought stressed tomato mutant ‘ sitiens’ ( sit ) rescued by exogenous ABA feeding in comparison with water fed control with the rate average more than 60%. Significantly reduced branched chain amino acid accumulation in ABA-biosynthetic tomato mutants ‘not and ‘sit’ suggests direct role of ABA in stress induced amino acid synthesis.
Generally, ABA-defective mutants are more droughts susceptible as compared to the wild types control. This was clearly found in of species, tomato and Arabidopsis , examined in this study. This is underlined by the relatively low production of leave biomass and expression level of some ABA-dependent amino acids under water deficit conditions. Failure to accumulate many stress-induced amino acids in flowers and siliques of Arabidopsis plants defective in ABA biosynthesis implies ABA-mediated regulation of amino acid synthesis in reproductive tissues.
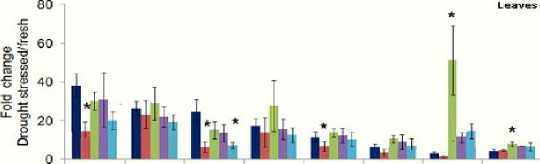
The levels of free amino acids in 3-week old tomato leaves ( Lycopersicon esculentum L.) notabilis ( not ), flacca ( flc ) and sitiens ( sit ) mutants were determined as appeared in (Fig. 3) in which the levels of 10 different amino acids were changed in response to a water deficit. The flacca ( flc ) tomato mutant displays a wilty phenotype as a result of abscisic acid deficiency did not show any significant change in amino acid level comparing to wild type tomato. However, amino acid levels in the other tomato mutants, notabilis ( not ), and sitiens ( sit ) under drought stress showed that, isoleucine (Ile), valine (Val), leucine (leu), alanine (Ala) and proline (Pro) significantly reduced, while arginine (Arg) and lysine (Lys) were increased in the leaves. Histidine (His), serine (Ser) and glycine (Gly) didn’t change significantly.
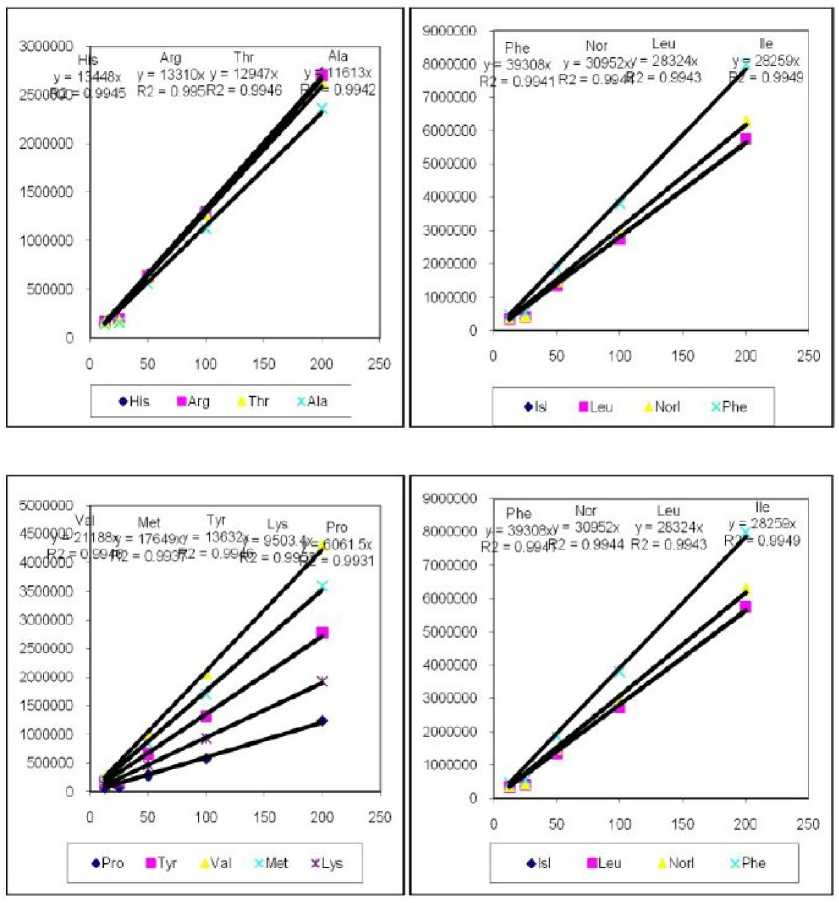
Figure 1. Free amino acids standard curves fractions identified by HPLC analysis
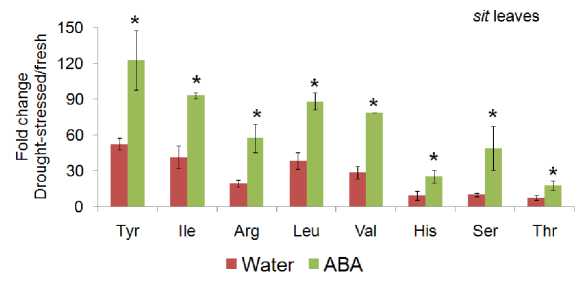
Figure 2 : Amino acid contents in drought stressed tomato mutant ‘sitiens’ (sit) rescued by exogenous ABA feeding in comparison with water fed control. Significant differences were observed in amino acids content in ABA fed leaf mutants. Mean ± SD of n = 3 to 4. *, P<0.05, relative to wild-type plant tissue, Student’s t test.
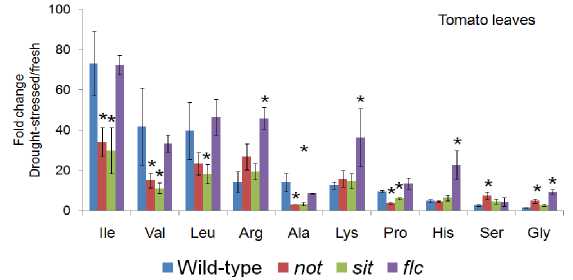
Figure 3: Illustration of branched chain amino acids of drought stressed leafs of tomato mutants defective in ABA biosynthesis are significantly reduced compared to wild-type. Foldchanges in amino acids were calculated by comparing amino acids from fresh tissues to dehydrated tissues. Mean ± SD of n = 3 to 4. Abbreviation (*): P<0.05, relative to wildtype plant tissue, Student’s t test. Concentrations were measure as pmol/mg.
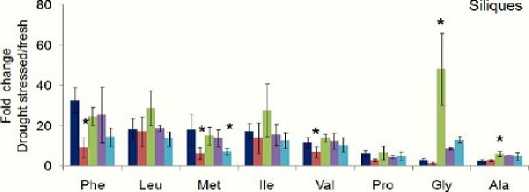
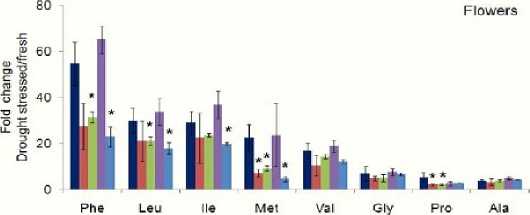
Phe Leu Met lie Vai Pro Gly Ala
■ Col-O "aba2 2 "aba3-1 *aao3 1 "nced3-4
Figure 4: Amino acid contents in dehydrated flowers and cliques were significantly reduced in ABA-biosynthetic mutant ( aba2-2 , aba3-1 , aao3-1 and nced3-4 ) plants comparing with wild type Col-0 plants. Fold-changes in amino acids were calculated by comparing amino acids from fresh tissues to dehydrated tissues.Mean ± SD of n = 3 to 4. * P<0.05, relative to wild-type plant tissue, Student’s t test. Concentrations were measure as pmol/mg.
Within tomato mutants, the most abundant amino acids were isoleucine (Ile), leucine (leu), arginine (Arg) and Val which are also the greatest amino acid shown fluctuations in concentration level in response to leaves water potential in each mutants. Fluctuation of other amino acids within and among mutants can be noticed for valine (Val), arginine (Arg) and leucine (leu). However, here was a constant low level of Proline (Pro) amino acid occurs at water stress.
Amino acids fold-change in drought stressed Arabidopsis leaves as well as reproductive tissues of ABA-defective mutants ( aba2-2 , aba3-1 , aao3-1 and nced3-4 ) were significantly reduced when compared to Arabidopsis wild-type (Col-O). There is no different in the type of these changed amino acids upon stress water treatment, although fold changes are different among the different plant tissues. Unlike leaves, amino acid contents in dehydrated flowers and siliques were significantly reduced in mutants defective in ABA biosynthesis. Reproductive tissues are more sensitive to drought stress than leaf tissues. Level of amino acids changes or fluctuations in amino acids concentration in response to water stresses is high in flower tissues rather than siliques. In addition, this fluctuation appeared clearly within methionine (Met) and glycine (Gly) in within the four mutants as shown in (fig. 4). Aba3-1 mutant is less affected by ABA defection in siliques tissues. On the other hand, the most abundant amino acids are phenylalanine (Phe), leucine (leu), and isoleucine (Ile) in both flowers and sliques tissues. However, the level of these amino acids are higher in flowers than siliques and leave tissues.
Arabidopsis and tomato mutants defective in ABA biosynthesis are expected to be lesser drought tolerant, as it is shown in figure 2 in which branched chain amino acids of drought stressed leaves of tomato mutants defective in ABA biosynthesis are significantly reduced compared to wild-type.
DISCUSSION
It is widely known that, drought stress triggers a wide range of plant drought-resistance-related genes regulating the synthesis of osmotically active compounds such as the phytohormone, abscisic acid ( Ramachandra-Reddy et al, 2004) and specific proteins/enzymes known for their roles in stress toleration through stomatal closure, ROS-scavenging, membrane protection (Chaves et al, 2004), detoxification and alleviation of cell damage during dehydration (Bartels and Sunkar, 2005) and relevant amino acids (Vinocur and Altman, 2005). In the current study, the increased contents of total amino acids detected in ABA rescued tomato species exposed to drought were previously reported by (Zhang and Kirkham, 1996; Yadav et al, 1999). The observed data are also in agreement with the previous studies which showed a marked increase in the amount of free amino acids due to water stress (Schubert et al., 1995) such as aspartate, alanine and glutamate in stressed leaves of wheat (Barnett and Naylor, 1966; Routley, 1966), proline (Stewart, 1972 and 1977; Singh et al., 1973; Karamanos et al. 1983; Zagdanska, 1984 and Li et al, 1997), glutamate (A), isoleucine (B), methionine (C), valine (D) and proline (E) in leaves of the cotton (Navaro-Izzo, 1990), methionine (Yang and Adams, 1980), aspartate, glutamate, proline, alanine and valine (Zagdanska, 1984), Glu, Pro, Arg, Asn and Orn has in ponderosa pine (Vance and Zaerr, 1990). Arginine (Vance and Zaerr, 1990).
Exogenous application ABA rescues reduced amino acid phenotypes of “sit” mutant but not that of “not’ and ‘flc’ so that, Tyr, Ile, Arg, Leu, Val, His, Ser and Thr are categorized as ABA-dependent amino acids. A general consensus resulting from using abscisic acid mutants in “sit” tomato mutant subjected to drought is that expression of various free amino acids is mediated by ABA-dependent and
ABA-independent pathways to regulate the
(AMINO ACIDS 9) (AA-ABA 1) (Bray, 2002) in which ABA that plays an important role in physiological regulation of ABA-responsive genes (Finkelstein et al., 2002). Therefore, we can come to conclude that ABA is directly involved in either up or down regulation of certain amino acids involved in response to environmental drought stress. Moreover, amino acids that are not affected by ABA during drought tolerance in both plants seems to be regulated through ABA independent pathway. Previously mentioned physiological significance of amino acids accumulation the presence of water deficit plants were attributed to increase in osmotic potential (Navari-Izzo et al, 1990), synthesis of specific drought-resistance enzymes rich in Gly, Pro and other hydrophilic amino acids, adjustment of N metabolism (Stewart and Larher, 1980; Vance and Zaerr, 1990), a long-distance transport and storage form of nitrogen in plant trees such as Arg and Asp (Vance and Zaerr, 1990), maintaining energy fluxes of the chloroplast such as aspartate, glutamate, proline, alanine and valine (Zagdanska, 1984), protection of several enzymes against heat denaturation and other destructive effects of drought stress such as proline and valine (Aspinall and Paleg et al, 1981; Irigoyen et al., 1992 ; Raggi, 1994), osmotic adjustment of the cell such as proline (Brhada et al., 1997) and involvement in metabolic synthesis of different precursors during water stress in plant such as (2-oxobutyrate and pyruvate, by isoleucine and valine (Val) respectively (Bryan, 1980) and precursors or activators of phytohormones and growth substances such as, ethylene synthesis from methionine (Yang and Adams, 1980). Similarly, amino Acids are. In addition, free amino acids were reported to act as osmolytes in abiotic stress response of many plants such as, leucine and
187 valine that are coordinately regulated are induced many-fold during osmotic stress and their accumulation has biological significance under abiotic stress (Joshi et al. 2010).
On the other hand, plant’s response to multiple stresses is more complicated as it cannot be inferred from the response to individual stress (Mittler, 2006). In addition, there is limited information available on this correlation, since there were high number of transcriptional factors regulating drought stress-responsive genes in higher plants (Signal). However, the noticed variation patterns of free amino acid synthesis in the identified mutants comparing to wild types evident for that, ABA transcription factors seems to be co-expressed with genes involved in amino acid metabolism. It was previously reported that accumulate high amounts of ABA induce expression of many transcription factors cascades that lead to expression of many drought stressed induced functional proteins and many branched chain amino acids used by many plants for enhancement of drought toleration (Umezawa et al, 2006). The previous explanations can show the presence of complex interactions mechanism between ABA-induced signaling and drought-stress signaling of related amino acid. In the current study, we have found that amino acids have two different types of responses underlying drought tolerance in plant. The first group seems to be ABA independent in which elevation of these amino acids is probably accompanied by the activation of genes involved in the perception of drought stress and in the transmission of the stress signal. On the other hand, reduced amino acids are categorized within the another group compatible metabolites such as the phytohormone abscisic acid (ABA), sugars, organic acids, amino acids or osmotically active compounds and ions expressed in response to drought stress (Bartels and Sunkar, 2005). Similar results were obtained by (Hanson and Hitz, 1982) on sorghum.
Therefore, it is not surprising to have differences in the amino acid pattern for stress conditions in these
ABA mutants where, signal transduction specific steps are impaired in ABA (AA-ABA 2).
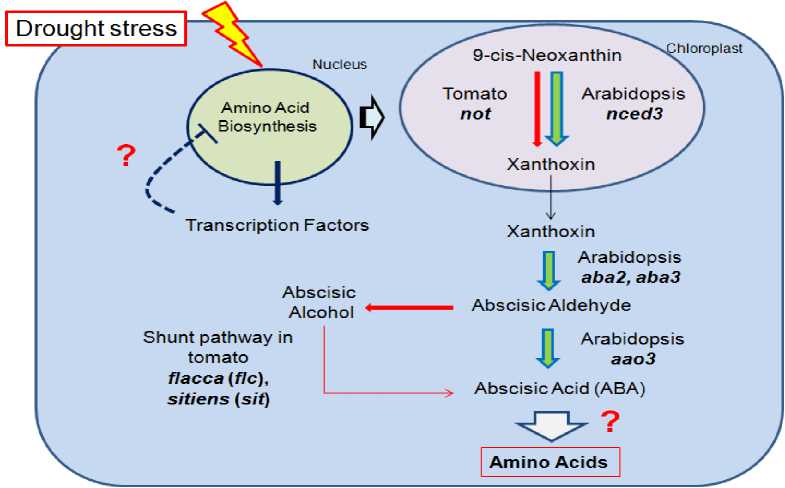
Figure 5: Schematic representation of biosynthetic pathways of abscisic acid in tomato and Arabidopsis. The hormone is synthesized through the shunt pathway via abscisic alcohol precursor in tomato. Alternatively, it can be from the carotenoids precursor,Neoxanthin in Arabidopsis. Mutants of Arabidopsis and tomato defective in ABA biosynthesis are lesser droughts tolerant. Plants, in response to stress, accumulate high amounts of ABA and induce expression of many transcription factors. However, no information is yet known about how ABA or transcription factors are associated with drought stressed induced accumulation of branched chain amino acids.
Consequently, it seems that I have two kinds of transcriptional elements according to amino acids characters of expression under drought stress. Based on literature survey and publicly available database of model Arabidopsis, a hypothetical schematic reprehensive of ABA biosynthetic pathways in tomato and Arabidopsis is illustrated in (fig. 5) that clarify our proposed sight of view regarding complex regulatory mechanisms involved in drought stress toleration regulated by internal signals mediated by abscisic acid (ABA) that involves its control, production, degradation, signal perception, and transduction.
Upon water stress, higher plant’s response, Arabidopsis and tomato, trigger ABA synthesis from a carotenoids compounds, 9-cis-epoxycarotenoids (2,3+ Amino acids 7) through contribution of zeaXanthin epoxidase, 9-cis-epoxycarotenoid dioxygenase (NCED: Thompson et al., 2000) and abscisic aldehyde oxidase under drought-stressed conditions (Mahajan and Tuteja, 2005; Chaves et al, 2004 + Amino acids 7). (AMINO ACIDS 9).). ABA then play major role in up-regulation of drought stress-responsive genes expression as well as other osmoprotective compounds involved in an ABA-dependent pathway such as amino acids involved in stress toleration (Shinozaki and Yamaguchi-Shinozaki, 2000; Mahajan and Tuteja, 2005; Zhu et al., 2002 + AA-ABA3). On the other hand, transcription factors such as DREB family (Stockinger et al., 1997; Kasuga et al., 1999)) that include four main kinds: APETALA2/EREBP, bZIP, WRKY, and MYB [70-79 + SIGNAL]. They induce the expression of stress tolerance genes and hence activated the expression of stress tolerance genes involved in an ABA-independent pathway, leading to improvement of tolerance to drought stress (Stockinger et al., 1997; Kasuga et al., 1999). In Arabidopsis thaliana, it was we reported that a R2R3-type MYB transcription factor, MYB96, regulates drought stress response by integrating ABA signaling (Drought resistance 4).
The protein of most different genes coding drought-resistance is rich in Gly, Pro and other hydrophilic amino acids, but the content of Trp and Cys is lower than others (Li et al, 1997+ Signal). Drought responsive genes like RD (response to dehydration), COR (cold responsive), LEA (late embryogenes. abundant)/dehydrin-like, and aquaporin genes (Seki et al., 2001).
CONCLUSION
-
1- The increase in free proline may have contributed to the osmotic adjustment.
-
2- Isoleucine and valine present the same response profile to water stress, with progressive increases in concentration with lower water potentials.
-
3- The increase in methionine levels may also be an indicator of water stress in plant tissues.
ACKNOWLEDGEMENTS
We gratefully acknowledge funding from Boyce Thompson Institute at Cornell University and for providing the T-DNA insertion mutants and other lab facilities.
Список литературы ABA biosynthesis defective mutants reduce some free amino acids accumulation under drought stress in tomato leaves in comparison with arabidopsis plants tissues
- Aspinall D and Paleg LG.1981.Proline accumulation: Physiological aspects. In: The Physiology and Biochemistry of Drought Resistance in Plants, Academic Press, Paleg 220 Mohamed et al. LG, Aspinall D (Eds.), Sydney, pp. 205-241.
- Barnet, N.M and Naylor, A.W, 1966. Amino acid and protein metabolism in Bermuda grass during water stress. Plant Physiology, 41,1222.
- Bartels D, Sunkar R. (2005). Drought and salt tolerance in plants. Crit Rev Plant Sci, 24:23-58.
- Bray EA. (2002). Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ 25:153-161.
- Brhada, F., Poggi, M.-C., Le Rudulier, D., (1997). Choline and glycine betain uptake in various strains of Rhizobia isolated from nodules of Vicia faba var. Major and Cicer arietinum L. Modulation by salt, chloline and glycine betaine. Curr. Microbiol. 34, 167-172.
- Bruce BW, Gregory OE, Barker TC. (2002). Molecular and physiological approaches to maize improvement for drought tolerance. J. Exp. Bot., 53: 13-25.
- Bryan JK. (1980). Synthesis of the aspartate family and branchedchain amino acids. In EE Conn, PK Stumpf, eds, The Biochemistry of Plants, Vol 5. Academic Press, New York, pp 403-452.
- Chaves MM, Oliveira MM. (2004). Mechanisms underlying plant resistance to water deficits: prospects for water-saving agriculture. J Exp Bot, 55: 2365-2384.
- Colmer, T.D, T.J. Flowers and R. Munns. (2006). Use of wild relatives to improve salt tole.rance in wheat. J. Exp. Bot., 57: 1059-1078.
- De Smet I, Vassileva V, De Rybel B, Levesque MP, Grunewald W, Van Damme D, Van Noorden G, Naudts M, Van Isterdael G, De Clercq R, et al., (2008). Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594-597.
- Deak, K.I. and Malamy, J.E. (2005). Osmotic regulation of root system architecture. Plant J. 43, 17-28.
- Finkelstein RR, Gampala SS, Rock CD. (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14 (suppl.): S15-S45.
- Flowers TJ. (2004). Improving crop salt tolerance. J Exp Bot 55: 307-319.
- Gubler, F., Millar, A.A., and Jacobsen, J.V. (2005). Dormancy release, ABA and pre-harvest sprouting. Curr. Opin. Plant Biol. 8: 183-187.
- Hanson AD, Hitz WD. (1982). Metabolic responses of mesophytes to plant water deficits. Annu. Rev. Plant Physiol. 33: 163-203.
- Irigoyen JJ, Emerich DW and Sanchez-Diaz M. (1992). Alfalfa leaf senescence induced by drought stress: photosynthesis, hydrogen peroxide metabolism, lipid peroxidation and ethylene evolution. Physiol Plant 84: 67-72.
- Jander G, Norris S. R, Joshi V, Fraga M, Rugg A, Yu S, Li L, Last R. L, (2004). Application of a high-throughput HPLC-MS/MS assay to Arabidopsis mutant screening; evidence that threonine aldolase plays a role in seed nutritional quality, Plant Journal. 39 (3): 465-475.
- Joshi J, Joung JG, Fei Z, and Jander G. (2010). Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids, 39(4): 933-947
- Joshi V and Jander G. (2009). Arabidopsis methionine gamma-lyase is regulated according to isoleucine biosynthesis needs but plays a subordinate role to threonine deaminase. Plant Physiol, 151: 367-378.
- Joshi, M.V., Bruzzone L. and Chaudhuri, S. (2006). A model-based approach to multiresolution fusion in remotely sensed images. IEEE Trans. Geosci. Remote Sens., 44: 2549-2562.
- Karamanos, A. J., Drossopoulos, J. B., Niavis, C. A. (1983). Free proline accumulation during development of two wheat cultivars with water stress. J. Agric. Sci., Camb. 100, 429-439.
- Kasuga, M., Liu, Q., Miura, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999). Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 17, 287?291.
- Landry LG, Chapple CCS, Last RL. (1995). Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol 109: 1159-1166.
- Li NY, Gao JF. (1997). Effect of Osmotic Stress on Isoelectric Point and Subun it Composition of Proteins in Shoots of Wheat. Acta Univ. Agric. Boreali-occidentalis.; 25: 6-11.
- Mahajan S, Tuteja N. (2005). Cold, salinity and drought stresses: an overview. Archives of Biochemistry and Biophysics 444, 139-158.
- Mittler R. (2006). Abiotic stress, the field environment and stress combination, Trends Plant Sci. 11: 15-19.
- Munns, R. and Sharp, R.E. (1993). Involvement of abscisic acid in controlling plant growth in soils of low water potential. Aust. J.Plant Physiol. 20, 425-437.
- Nambara E, Kawaide H, Kamiya Y, Naito S. (1998). Characterization of an Arabidopsis thaliana mutant that has a defect in ABA accumulation: ABA dependent and ABA-independent accumulation of free amino acids during dehydration. Plant Cell Physiol 39: 853-858.
- Navari-Izzo F, Quartacci MF, Izzo R. (1990). Water-stress induced changes in protein and free amino acids in field grown maize and sunflower. Plant Physiol Biochem 28: 531-537.
- Raggi, V., (1994). Changes in free amino acids and osmotic adjustment in leaves of water stressed bean. Physiol. Plant 91, 427-434.
- Ramachandra-Reddy A, Chaitanya KV, Vivekanandan M. (2004). Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol, 161: 1189-1202.
- Ribaut J-M. and Poland D. Ribaut J-M, Ba?nziger M, Setter T, Edmeades G, Hoisington D. (2004). Genetic dissection of drought tolerance in maize: a case study. In: Nguyen H, Blum A, eds. Physiology and biotechnology integration for plant breeding. New York: Marcel Dekker Inc., 571-611.
- Routley R. 1966. "On a Significance Theory." Australasian Journal of Philosophy 44: 172-209.
- Sahi C., Singh A., Blumwald E., Grover A. 2006. Beyond osmolytes and transporters: novel plant salt-stress tolerance-related genes from transcriptional profiling data. Physiologia Plantarum 127: 1-9.
- Schubert A, Restagno M, Novello V, Peterlunger E. (1995). Effects of shoot orientation on growth, net photosynthesis, and hydraulic conductivity of Vitis vinifera L. cv. Cortese. American Journal of Enology and Viticulture 46, 324-328.
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K. (2001). Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. The Plant Cell 13, 61-72.
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M. (2003). Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in Plant Biology 6, 410-417.
- Shinozaki, K. and Yamaguchi-Shinozaki, K. (2000). Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 3, 217?223.
- Shinozaki,K., Yamaguchi-Shinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. J. Exp Bot. 58, 221-227.
- Singh, T. N., Aspinall, D., Paleg, L. G. Boggess, S. F. (1973). Stress metabolism. II. Changes in proline concentration in excised plant tissues. Aust. J. Biol. Sci., 26, 57-63.
- Stewart CR. (1972). Proline content and metabolism during rehydration of wilted excised leaves in the dark. Plant Physiol. 50: 679-681.
- Stewart CR, Boggess SF, Aspinall D, Paleg G. (1977). Inhibition of proline oxidation by water stress. Plant Physiol. 59: 930-932.
- Stewart GR, Larher F. (1980). Accumulation of amino acids and related compounds in relation to environmental stress. In "The Biochemistry of Plants" (BJ Miflin ed), Vol. 5, Academic Press, New York, pp. 609-635.
- Stockinger EJ, Gilmour SJ, Thomashow MF. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA, 94: 1035-1040.
- Thompson, A.J., Jackson, A.C., Parker, R.A., Morpeth, D.R., Burbidge, A., and Taylor, I.B. (2000). Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cisepoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol. Biol. 42, 833-845.
- Umezawa, T., Fujita, M., Fujita, Y.,Yamaguchi-Shinozaki, K. and Shinozaki, K. (2006). Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future Curr. Opin.Biotech. 17, 113-122.
- Vance N. C. and Zaerr J. B. (1990). Analysis by HPLC of free amino acids extracted from needles of drought-stressed and shaded Pinusponderosa seedlings. Physiol. Plant. 79: 23-30.
- Verslues PE, Zhu JK. (2005). Before and beyond ABA: upstream sensing and internal signals that determine ABA accumulation and response under abiotic stress. Biochem Soc Trans. 33: 375-379.
- Vinocur B, Altman A. (2005). Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol, 16: 123-132.
- Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C, Uchacz T, Sarvas C, Wan J, Dennis DT, McCourt P, Huang Y (2005). Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J., 43: 413-424.
- Yadav, V.K., Gupta, V., Nyflam, Y., (1999). Hormonal regulation of nitrate in gram (Cicer arietinum) genotypes under drought. Indian J. Agric. Sci. 69, 592-595.
- Yang, S.F. and Adams D.O. (1980). Biosynthesis of ethylene, p. 163-175. In: P.K. Stumpf and E.E. Conn (eds.). The Biochemistry of plants. vol. 4. Academic Press, New York.
- Zagdanska, B. (1984). Influence of water stress upon photosynthetic carbon metabolism in wheat (Triticum aestivum) cultivar Kalibri. J. Plant Physiol., 116, 153-160.
- Zhang F, Kirkham MB. (1996). Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytologist 132, 361-373.
- Zhang J. Z., Creelman R. A., and Zhu J-K. (2004). From Laboratory to Field. Using Information from Arabidopsis to Engineer Salt, Cold, and Drought Tolerance in Crops. Plant Physiol. 135: 615-621.
- Zhu, J.K., (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol., 53: 247-273.


