Activities of antioxidant enzymes in mesophyll and bundle sheath cell chloroplasts of maize plants (Zea mays L.) exposed to salt stress
Автор: Aliyeva Nahida
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Биологические науки
Статья в выпуске: 11 т.6, 2020 года.
Бесплатный доступ
Antioxidant defense systems have been studied in the mesophyll (MC) and bundle sheath cell (BSC) chloroplasts of maize ( Zea mays L.) leaves cultivated in an artificial climate chamber under various concentrations (0%, 1%, 2%, 3%) of NaCl. The amounts of some of the main products of lipid peroxidation malondialdehyde (MDA) and reactive oxygen species hydrogen peroxide (H2O2) as well as activities of superoxide dismutase (SOD) and ascorbate peroxidase (APO) were determined in MC and BSC chloroplasts. BSC chloroplasts were found to be more tolerant to salt stress compared with MC chloroplasts. The MDA amount increased in both chloroplasts. H2O2 was found to be localized mainly in MC chloroplasts at various NaCl concentrations. The SOD and APO activities increased in both chloroplasts of the plants exposed to salt stress.
Maize, mesophyll chloroplasts, bundle sheath chloroplasts, malondialdehyde, hydrogen peroxide, superoxide dismutase, ascorbate peroxidase
Короткий адрес: https://sciup.org/14117703
IDR: 14117703 | УДК: 577.1 | DOI: 10.33619/2414-2948/60/05
Текст научной статьи Activities of antioxidant enzymes in mesophyll and bundle sheath cell chloroplasts of maize plants (Zea mays L.) exposed to salt stress
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 577.1
Salt stress is one of the ecological hazards sharply limiting plant growth and productivity. High salinity impedes plant growth and development mostly due to osmotic stress and toxicity [1]. In arid and semi-arid regions, plants are more exposed to salt stress because of the high degree of evaporation leading to water loss. The cessation of growth of many plants under salt stress is closely related to the decrease in photosynthesis [2]. High salinity decreases the photosynthetic activity of plants and due to the chlorophyll degradation, chloroplast substrate is damaged leading to the destruction of membranes and enzymatic proteins [3]. Moreover, secondary stresses, such as oxidative stress, are frequently accompanied by osmotic stress and ion toxicity that are harmful to plant cells due to the accumulation of reactive oxygen species (ROS). ROS can significantly damage membrane lipids, proteins, nucleic acids, and photosynthetic pigments. Therefore, antioxidant and photosynthetic abilities of plants are very important for the normal growth and development under salt stress [4].
Plants possess enzymatic antioxidant mechanisms having the ability to confront the negative effects of salt stress. Enzymatic antioxidants include superoxide dismutase (SOD) and ascorbate peroxidase (APO) [5]. Usually, the inconsistency between the formation of radical species and cell antioxidant defense systems results in the emergence of oxidative stress [6].
Superoxide dismutase (SOD) converts O 2- anion into less reactive species O 2 and H 2 O 2. Based on metal cofactors located at the enzyme active site SODs are classified into 3 members: Fe-SOD, Mn-SOD, and Cu/ZnSOD [7]. As a result of the SOD enzymatic reactions, H 2 O 2 and O 2 are formed [8]. To avoid the harmful effect of H 2 O 2 , it should be removed from the cell at once. Because H 2 O 2 forms the highly reactive OH radical in the presence of certain metal ions and chelates. The regulation of superoxide dismutases plays a vital role in combating oxidative stress caused by biotic and abiotic factors, and the ability of plants to survive under environmental stress [9].
Ascorbate peroxidase (APO) plays an important role in the antioxidant system of plants by breaking down H 2 O 2 . The APO family consists of at least five different isoforms in the soluble form, including stromal, cytosolic, and apoplastic enzymes that bind to the thylakoid and microsomal membranes [5].
The maize plant ( Zea mays L.) is the third most important cereal after rice and wheat and grows in a wide range of soils and climates. It is a C4 plant of the Poaceae family and is moderately sensitive to salt stress [10]. The salt level over 0.25 M damages the maize plant, impedes its growth leading to weakened development [11]. The carbon assimilation process in maize ( Zea mays L.) leaves consists of two cycles. These cycles are carried out through two photosynthetic cells separated and specialized inside the leaf: bundle sheath cells (BSC) compactly located around conductive vessels and mesophyll cells (MC) surrounding them. BSC and MC perform different metabolic functions in maize plants: PEP-carboxylase and C4-carbon fixation pathway operate in MC and RBP-carboxylase and the Calvin cycle operate in BSC [12]. Electron microscopy studies revealed differences in the structure of mesophyll and bundle sheath cells. Thus, mesophyll cells are characterized by a granular structure, stromal thylakoids, and a small number of starch grains, while bundle sheath cells are characterized by an agranal structure and more starch grains. Sodium is a major toxic ion that affects potassium uptake and therefore disrupts the stroma fluctuations that cause water loss and necrosis in maize [13]. Besides, salt stress causes oxidative damage to plant cells by excessive production of active oxygen species [14].
The present study investigated the cellular localization H 2 O 2 , in MC and BSC in maize plants exposed to salinity.
Materials and methods
Plant material and growth conditions: Seedlings of the maize plant ( Zea mays L.), which is a cereal crop, were cultivated in soil under controlled phytotron conditions (photoperiod — 14 h/10 h, t — 26 °C, light intensity — 600 µmol m-2 s-1 and relative humidity of about 70%) based on the method presented by Hasan et al. [15]. Salinity treatment was started after the second leaf blades of the plants were fully developed by supplying 50 ml of 0 (control), 1%, 2% and, 3% NaCl stock solutions every day. Distilled water served as control. The soil was kept moist by the addition of respective stock salt solutions at regular intervals. Each treatment was replicated five times in which a pot was considered as one replicate. After salt treatment for 5 days, the plant samples were taken.
Chloroplast and thylakoid isolation: The mesophyll (M) and bundle sheath (BS) chloroplasts were isolated mechanically according to Edwards and colleagues [16]. The differential centrifugation method was used to isolate subcellular fractions (chloroplasts, etc.) from mesophyll and bundle sheath cells of leaf samples. To obtain assimilating tissues, leaves were detached from stems, washed with distilled water, and cut into small segments with a width of 2–3 mm. These segments were homogenized in 25 mМ HEPES buffer (pH 7.8) containing 0.3 M sucrose, 1 mM EDTA, 15–20 mM 2-mercaptoethanol (buffer A) using MPW-302 (Poland) mechanical disintegrator for 4 sec at 7,000 rev/min.
Hydrogen peroxide (H 2 O 2 ) content: The amount of hydrogen peroxide was determined spectrophotometrically using the Bellinkampi method [17]. Optical density was determined at 560 nm in Thermo Scientific Evolution 350 UV-Vis Spectrophotometer. Standards were prepared using 30% H 2 O 2 .
Determination of the malondialdehyde (MDA) content . The main product of the lipid peroxidation in plant tissues-MDA was determined based on the reaction with thiobarbituric acid (TBT) [18]. 50 µl of the plant material was homogenized after adding 650 µl of TBA reagent. Then supernatant and the obtained mixture were kept in the water bath for 30 min, at 95 °С and cooled using ice. The optical density of the supernatant was determined by the spectrophotometer (λ=532 and 600 nm) after the repeat centrifugation for 10 min, at 15,000g. The MDA content was calculated using an extinction coefficient of 155 (nmol/g-1 fresh weight) with the subtraction of non-specific absorption at 600 nm.
Superoxide dismutase (SOD, EC 1.15.1.1) assay: The enzyme activity was determined at 450 nm using SOD Assay Kit (Sigma, Aldrich).
Ascorbate peroxidase (APX, EC 1.11.1.11) assay: The activity of the enzyme was determined spectrophotometrically based on the decomposition of H 2 O 2 by the ascorbate peroxidase enzyme for 1 min, at 290 nm [19]. The reaction medium consisted of 0.1 mM EDTA (pH 8.0), 0.05 mM ascorbic acid, 0.1 mM H 2 O 2 , 50 mM Na-Phosphate (pH 7.6) buffer and 100 µl of the enzymatic extract. The APO activity was estimated based on the decline in the optic density during the first 30 sec of the reaction and was expressed in mmol ascorbate/(mg protein min) at the extinction coefficient (ɛ) of 2.8 mM-1cm-1
Statistical analysis: The paper presents data of three experiments carried out in three replicates. Calculations, graphs, and their descriptions were performed using the applications Microsoft Office Word 7 and Excel 7 for Windows XP.
Results and discussion
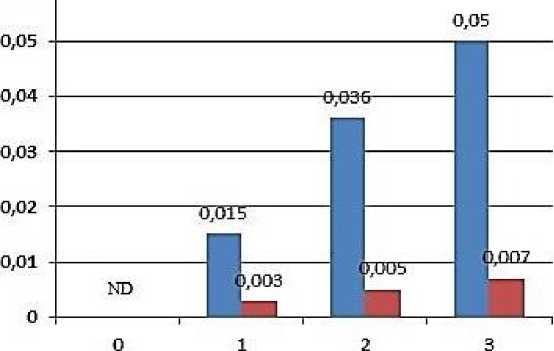
H2O2 plays a dual role in plants. At low concentrations, it acts as a secondary signal molecule that participates in the stimulus signal and raises the level of tolerance to various stresses. On the other hand, H2O2 accumulation causes membrane lipid peroxidation when it reaches a high concentration [5]. Hydrogen peroxide a form of reactive oxygen species, was not found in the MC and BSC chloroplasts of the control plant in maize leaves during our experiments. H2O2 was found in MC chloroplasts under the influence of salt stress. Whereas it was not found in the chloroplasts of BSC (Figure 1).
о
О о р ад
Е
■ м
■ BS
NaCI (%)
Figure 1. Changes in the H2O2 content in mesophyll and bundle sheath chloroplasts of the maize plants exposed to salt stress. ND, not detectable.
As seen, H 2 O 2 is formed in the mesophyll and bundle sheath chloroplasts exposed to salt stress. In chloroplasts, H 2 O 2 is assumed to be formed around photosystem II (FS II), which is located mainly in the granular structure [20]. In NADP-ME-type C4 plants, the granular structure of BSC chloroplasts is generally less common and therefore, FS II activity is less or absent in BSC chloroplasts [21]. Thus, H 2 O 2 is less formed in BSC and these cells are more tolerant to salt stress than MC. Less H 2 O 2 amounts in BSC compared with MC were observed also in the maize plants exposed to low temperatures [22]. These results can be attributed to both structural and physiological differences between MC and BSC chloroplasts.
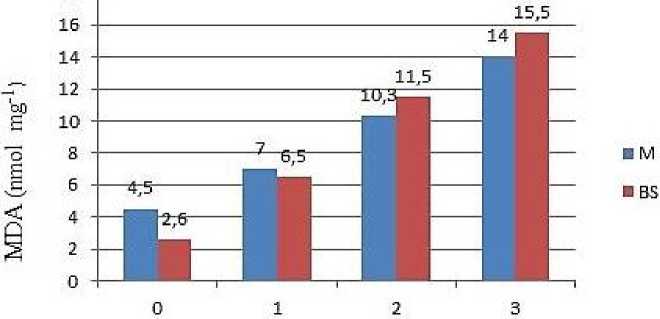
Malondialdehyde is distributed differently in the chloroplasts of mesophyll and bundle sheath cells of maize leaves. Thus, its amount was greater in MC chloroplasts compared with BSC chloroplasts in control plants. Under salt stress, the MDA amount increased in both of BSC and MC chloroplasts (Figure 2).
NaCI (%)
Figure 2. Changes in the MDA content in mesophyll (M) and bundle sheath (BS) chloroplasts of the maize plants exposed to salt stress.
MDA is an effective indicator of cell oxidative damage [23]. NaCl causes an increase in the MDA amount in MC and BSC chloroplasts of the maize plant [24]. Among organelles, chloroplasts of mesophyll cell are considered the most sensitive to salt stress because they are the strongest source of ROS production. Thylakoid membranes contain high amounts of unsaturated fatty acids, and membrane damage is partially associated with a decrease in unsaturated lipids, which leads to a decrease in the stability of membrane proteins [25]. Malondialdehyde is a product of peroxidation of unsaturated fatty acids with phospholipids, and lipid peroxidation levels have been used as an indicator of damage caused by free radicals to cell membranes under stress. The malonaldehyde content was also expected to be low in BSC chloroplasts under salt stress, as ROS was significantly lower. Thus, the disruption of thylakoid membranes observed in MC chloroplasts of salt-exposed plants may be attributed to lipid peroxidation (Figure 1). Despite the similar lipid oxidation levels in both tissue types of stressed plants, the BSC chloroplast structure was better preserved. The results show that lipid peroxidation does not necessarily lead to structural damage. Halliwell [26] also suggested that lipid peroxidation would not be the primary mechanism of tissue damage under oxidative stress.
The activities of superoxide dismutase and ascorbate peroxidase increased in both mesophyll and bundle sheath cells of maize plants exposed to 1%, 2%, 3% NaCl (after the emergence of the 2th leaf) compared with the control plants. The activity of SOD and APO reaches a maximum in plants exposed to 2% NaCl, and a significant decrease was observed at 3% NaCl. Spectrophotometric analysis of the activity of antioxidant enzymes in mesophyll and bundle sheath cells showed higher activity of enzymes in bundle sheath cells than in mesophyll cells (Figure 3).
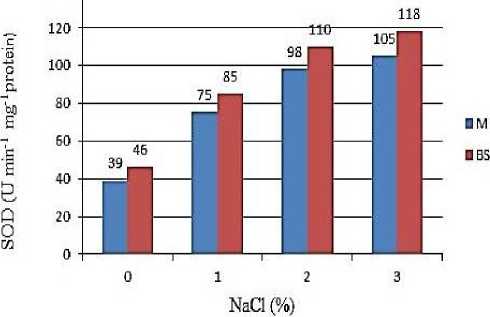
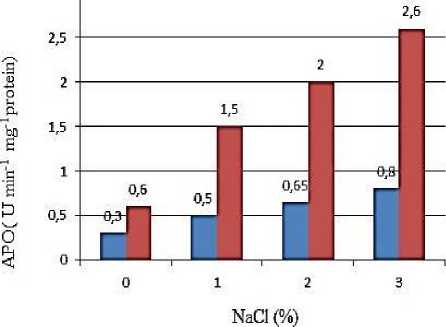
Figure 3. Activities of SOD and APO in mesophilic (M) and bundle sheath (BS) chloroplasts of maize leaves under various NaCl concentrations.
As can be seen from the Figure 3, the activity of SOD is equally increased in the mesophyll and bundle sheath chloroplasts of the leaves of the maize plant exposed to salt stress. APO activity was more common in bundle sheath chloroplasts than in mesophyll. Similar results were obtained by Omoto et al. [24]. According to the authors, in maize seedlings watered with 3% NaCl for 5 days, the activity of superoxide dismutase (after emerging of the 4th leaf) increased compared with the control in both mesophyll and bundle sheath cells. The ascorbate peroxidase activity was higher in BSC exposed to salt stress. Studies have shown that the structure of mesophyll cells in NADP-type (NADP-ME) C4 plants is more sensitive to salt stress than that of BSC [15].
The activities of the SOD and APO enzymes, the main components of antioxidant defense systems in MC and BSC, isolated from maize leaves, increase compared with the control at low salt concentrations and were partially inhibited at high salt concentrations. Based on the results of the experiments, the SOD and APO enzymes localized in BSC play a major role in salt tolerance of the maize plant [27]. The same results were obtained in the experiments performed with chloroplasts of the maize leaves exposed to short-term water stress. The increased SOD and APO activities under the influence of light, causing oxidative stress, were also observed [28]. Thus, according to the results of the study, the SOD and APO enzymes localized in BSC play a key role in the adaptation of the maize plant to stress.
Conclusion
Our results showed that the content of H 2 O 2 in MC was higher than in BSC. The differential response of MC and BSC chloroplasts to salt stress in maize may be related to the amount of hydrogen peroxide formed in these cells. M cells are more sensitive to salt stress, and hydrogen peroxide is mainly accumulated in these cells leading to oxidative damage. More tolerance of BSC chloroplasts to salt stress compared with MС chloroplasts is not attributed to the amount of salt accumulated in these cells.
Acknowledgement: This work was supported by the Science Development Foundation under the President of the Republic of Azerbaijan — Grant №: 1-2016-1 (26)-71/02/3.
Список литературы Activities of antioxidant enzymes in mesophyll and bundle sheath cell chloroplasts of maize plants (Zea mays L.) exposed to salt stress
- Zhu J. K. Regulation of ion homeostasis under salt stress // Current opinion in plant biology. 2003. V. 6. №5. P. 441-445. DOI: 10.1016/S1369-5266(03)00085-2
- Diao M., Ma L., Wang J., Cui J., Fu A., Liu H. Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system // Journal of plant growth regulation. 2014. V. 33. №3. P. 671-682. DOI: 10.1007/s00344-014-9416-2
- Shu S., Yuan L. Y., Guo S. R., Sun J., Yuan Y. H. Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress // Plant Physiology and Biochemistry. 2013. V. 63. P. 209-216. DOI: 10.1016/j.plaphy.2012.11.028
- Munns R., Tester M. Mechanisms of salinity tolerance // Annu. Rev. Plant Biol. 2008. V. 59. P. 651-681. DOI: 10.1146/annurev.arplant.59.032607.092911
- Noctor G., Foyer C. H. Ascorbate and glutathione: keeping active oxygen under control // Annual review of plant biology. 1998. V. 49. №1. P. 249-279. DOI: 10.1146/annurev.arplant.49.1.249
- Jiang C., Zu C., Lu D., Zheng Q., Shen J., Wang H., Li D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress // Scientific reports. 2017. V. 7. P. 42039.
- DOI: 10.1038/srep42039
- Mittler R. Oxidative stress, antioxidants and stress tolerance // Trends in plant science. 2002. V. 7. №9. P. 405-410.
- DOI: 10.1016/S1360-1385(02)02312-9
- Luis A., Corpas F. J., López-Huertas E., Palma J. M. Plant superoxide dismutases: function under abiotic stress conditions // Antioxidants and antioxidant enzymes in higher plants. Cham.: Springer, 2018. P. 1-26.
- DOI: 10.1007/978-3-319-75088-0_1
- Gill S. S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants // Plant physiology and biochemistry. 2010. V. 48. №12. P. 909-930.
- DOI: 10.1016/j.plaphy.2010.08.016
- Maas E. V., Hoffman G. J., Chaba G. D., Poss J. A., Shannon M. C. Salt sensitivity of corn at various growth stages // Irrigation Science. 1983. V. 4. №1. P. 45-57.
- DOI: 10.1007/BF00285556
- Menezes-Benavente L., Kernodle S. P., Margis-Pinheiro M., Scandalios J. G. Salt-induced antioxidant metabolism defenses in maize (Zea mays L.) seedlings // Redox report. 2004. V. 9. №1. P. 29-36.
- DOI: 10.1179/135100004225003888
- Von Caemmerer, S., & Furbank, R. T. (2003). The C4 pathway: an efficient CO2 pump. Photosynthesis Research, 77(2-3), 191. :1025830019591
- DOI: 10.1023/A
- Sumer A. Evidence of sodium toxicity for the vegetative growth of maize during the first phase of salt stress // J. App. Bot. 2004. V. 78. P. 135-139.
- De Azevedo Neto A. D., Prisco J. T., Enéas-Filho J., de Abreu C. E. B., Gomes-Filho E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes // Environmental and Experimental Botany. 2006. V. 56. №1. 87-94.
- DOI: 10.1016/j.envexpbot.2005.01.008
- Hasan R., Ohnuki Y., Kawasaki M., Taniguchi M., Miyake H. Differential sensitivity of chloroplasts in mesophyll and bundle sheath cells in maize, an NADP-malic enzyme-type C4 plant, to salinity stress // Plant production science. 2005. V. 8. №5. P. 567-577.
- DOI: 10.1626/pps.8.567
- Gardeström P., Edwards G. E. Isolation of mitochondria from leaf tissue of Panicum miliaceum, a NAD-malic enzyme type C4 plant // Plant physiology. 1983. V. 71. №1. P. 24-29.
- DOI: 10.1104/pp.71.1.24
- Bellincampi D., Dipierro N., Salvi G., Cervone F., De Lorenzo G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants // Plant physiology. 2000. V. 122. №4. P. 1379-1386.
- DOI: 10.1104/pp.122.4.1379
- Shaw M., DeLine R., Klein D. V., Ross T. L., Young D. M., Zelesnik G. Abstractions for software architecture and tools to support them // IEEE transactions on software engineering. 1995. V. 21. №4. P. 314-335.
- DOI: 10.1109/32.385970
- Mittal S., Kumari N., Sharma V. Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes // Plant Physiology and Biochemistry. 2012. V. 54. P. 17-26.
- DOI: 10.1016/j.plaphy.2012.02.003
- Yamane K., Rahman M. S., Kawasaki M., Taniguchi M., Miyake H. Pretreatment with antioxidants decreases the effects of salt stress on chloroplast ultrastructure in rice leaf segments (Oryza sativa L.) // Plant production science. 2004. V. 7. №3. P. 292-300.
- DOI: 10.1626/pps.7.292
- Romanowska E., Drożak A., Pokorska B., Shiell B. J., Michalski W. P. Organization and activity of photosystems in the mesophyll and bundle sheath chloroplasts of maize // Journal of plant physiology. 2006. V. 163. №6. P. 607-618.
- DOI: 10.1016/j.jplph.2005.06.007
- Pastori G., Foyer C. H., Mullineaux P. Low temperature-induced changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves // Journal of Experimental Botany. 2000. V. 51. №342. P. 107-113.
- DOI: 10.1093/jexbot/51.342.107
- Jain M., Mathur G., Koul S., Sarin N. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.) // Plant Cell Reports. 2001. V. 20. №5. P. 463-468.
- DOI: 10.1007/s002990100353
- Omoto E., Nagao H., Taniguchi M., Miyake H. Localization of reactive oxygen species and change of antioxidant capacities in mesophyll and bundle sheath chloroplasts of maize under salinity // Physiologia Plantarum. 2013. V. 149. №1. P. 1-12.
- DOI: 10.1111/ppl.12017
- Thomas P. G., Dominy P. J., Vigh L., Mansourian A. R., Quinn P. J., Williams W. P. Increased thermal stability of pigment-protein complexes of pea thylakoids following catalytic hydrogenation of membrane lipids // Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1986. V. 849. №1. P. 131-140.
- DOI: 10.1016/0005-2728(86)90104-0
- Halliwell B. Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts // Chemistry and Physics of lipids. 1987. V. 44. №2-4. P. 327-340.
- DOI: 10.1016/0009-3084(87)90056-9
- Tan M., Lu J., Zhang A., Hu B., Zhu X., Li W. The Distribution and Cooperation of Antioxidant (Iso)enzymes and Antioxidants in Different Subcellular Compartments in Maize Leaves during Water Stress // Journal of Plant Growth Regulation. 2011. V. 30. №3. P. 255-271.
- DOI: 10.1007/s00344-010-9189-1
- Romanowska E., Buczyńska A., Wasilewska W., Krupnik T., Drożak A., Rogowski P.,.. Zienkiewicz M. Differences in photosynthetic responses of NADP-ME type C4 species to high light // Planta. 2017. V. 245. №3. P. 641-657.
- DOI: 10.1007/s00425-016-2632-1


