Acute myocardial infarction following COVID-19 vaccination: a cause or a coincidence?
Автор: Elheet A.A., Farrag M.H., Elkeliei M.M., Alabdali A.M.
Журнал: Cardiometry @cardiometry
Рубрика: Case report
Статья в выпуске: 22, 2022 года.
Бесплатный доступ
Background. Acute Myocardial Infarction (AMI) is a fatal condition with a subsequent variety of complications. Recently, COVID-19 vaccine has become an essential precaution to avoid infection. However, it is uncommon for AMI to manifest as a result of the COVID-19 vaccination. Methods. Thirty-two years old man, previously healthy, come to the emergency department with a four-hour history of chest discomfort after Covishield (AstraZeneca) vaccination. He was neither hypertensive, diabetic, or smoker and lacked any other typical risk factors for cardiovascular disease. Results. This case describes an adverse response to the COVID-19 vaccination that has the potential to be life-threatening. He had received his first dose of the Covishield vaccination five days prior. Extensive anterior STEMI was diagnosed post-cardiac arrest. He had primary percutaneous coronary intervention (PCI) to recanalize an occluded proximal LAD. Conclusions. Additional screenings for young individuals should be considered before administering the COVID-19 (AstraZeneca) vaccine as a preventive step if reports of significant adverse events in older adults continue.
Covid-19, acute myocardial infarction, stemi, astrazeneca, vaccination
Короткий адрес: https://sciup.org/148324414
IDR: 148324414 | DOI: 10.18137/cardiometry.2022.22.143146
Текст научной статьи Acute myocardial infarction following COVID-19 vaccination: a cause or a coincidence?
Ahmed A. Elheet, Mohamad H. Farrag, Mohamed M. Elke-liei, Ali M. Alabdali. Acute myocardial infarction following COVID-19 vaccination: a cause or a coincidence?. Cardiome-try; Issue 22; May 2022; p. 143-146; DOI: 10.18137/cardiome- try.2022.22.143146; Available from: issues/no22-may-2022/acute-myocardial-infarction
On December 30, 2020, emergency authorization of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (ChAdOx1 nCov-19, AstraZeneca) was granted, based on strong phase three data demonstrating a 76.0% efficacy rate in preventing symptomatic COVID-19 starting 22 days after the first dose, in healthy volunteers. In extremely rare situations (about 1 in 100,000 persons), the vaccination may cause blood clots when combined with low platelet counts. As of April 14, 2021, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) reported an overall incidence of thromboembolic events of 7.9 per million doses (less than 1 in 100,000 people) with concurrent low platelets 1.
Method: Since it was a case report so case presentation is as follows:
Case Presentation
Thirty-two years old man, previously healthy, presented to the emergency department with a four-hour history of retrosternal typical chest discomfort, profuse sweating, and vomiting. He was neither hypertensive, diabetic, or smoker and lacked any other usual risk factors for cardiovascular disease. The patient was admitted with ventricular fibrillation (VF). Cardiopulmonary resuscitation by a bystander was initiated, and he got a 200 joules direct current shock from an automated electrical defibrillator for VF and restored spontaneous circulation. Due to the patient’s failure to achieve complete consciousness during ROSC, he was intubated and mechanically ventilated. ST-segment elevation in anterior leads was reported after Electrocardiogram (ECG), (Figure 1A).
After ST-elevation ACS was diagnosed, the Cardiac Cath Lab was immediately activated for primary PCI. Initial blood tests revealed a normal complete blood count, coagulation, and renal function. Initially, troponin was 100 ng/mL and increased to > 50.000 ng/ mL six hours later. Transthoracic echocardiography was performed prior to the procedure (Figure 1B) and revealed a 25% reduction in left ventricular ejection fraction, 120 ml ESV, and 160 ml EDV, with significant hypokinesis of the apex, anterior, and lateral segments.
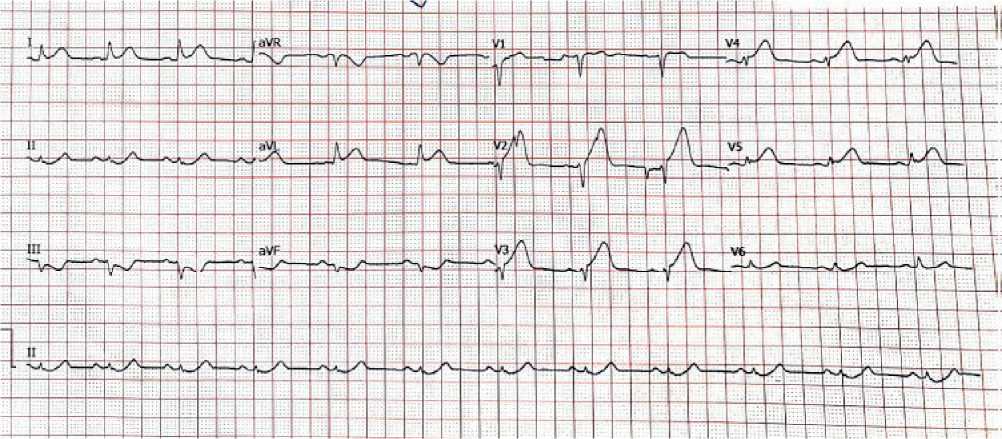
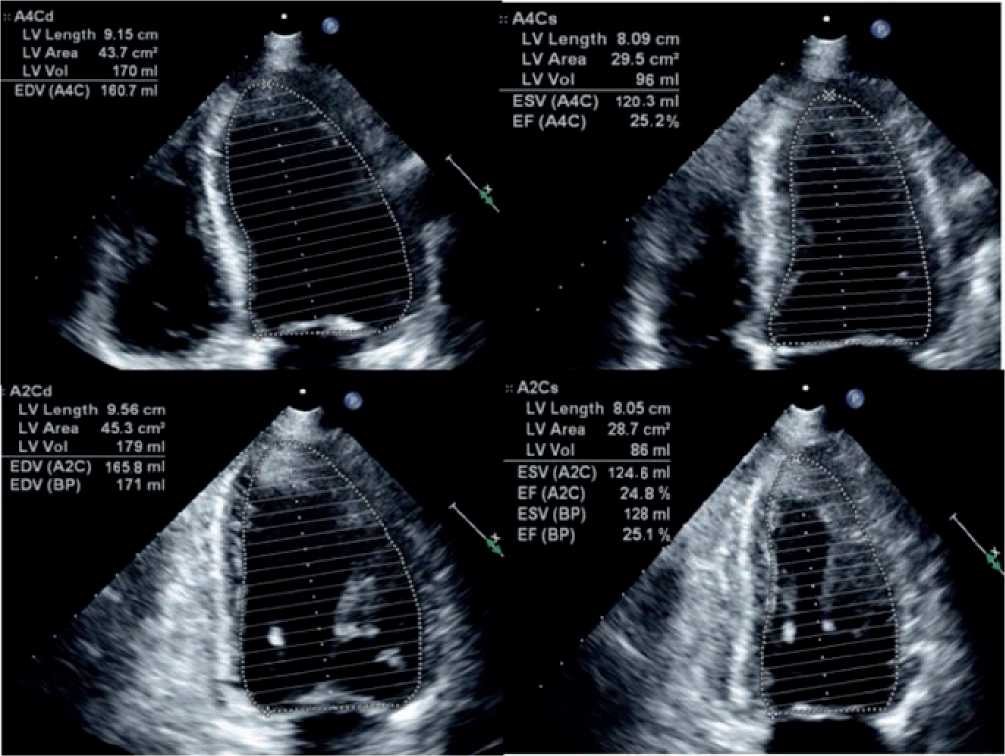
Figure 1B: TTE by Simpson’s method in apical 4 chamber and apical 2 chamber.
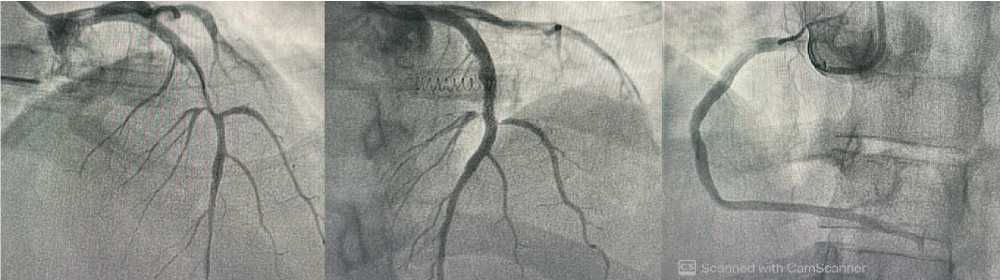
The trans-radial coronary angiography revealed a completely blocked proximal LAD artery with normal branches. Following LAD wiring and mild predilatation with a low-pressure balloon (SeQuent, 2.5×15mm), a proximal complete LAD lesion developed, and one DES (Coroflex ISAR NEO, 3×32mm)
144 | Cardiometry | Issue 22. May 2022
was placed with final TIMI 3 flow and no distal embolization (Figure 2A-C). Due to the anticipated significant risk of systemic cardioembolic events, thrombus aspiration was not done. The patient recovered full consciousness two hours later and was successfully extubated. The patient was hospitalized for 48 hours
A
B
C
Figure 2. left coronary angiogram after wiring of total LAD showed proximal to mid segment subtotal stenosis (A), Post successful PCI to LAD (B), normal RCA(C).
in the critical care unit (ICU). No further adverse events occurred, and after three days, the patient was discharged. On discharge, the patient was prescribed a one-year course of dual antiplatelet treatment (DAPT) consisting of ticagrelor (90 mg) twice daily and aspirin (100 mg) daily.
Discussion
This case examines an unpleasant MI that occurred following the administration of an AstraZeneca vaccination. According to the Centers for Disease Control (CDC), over 800,000 individuals suffer from MIs each year 2, with 33% or more occurring in individuals 75 years or older 3. Due to the fact that older persons receive priority for the vaccination, it is possible that older adults will continue to present to the emergency room with MIs following their COVID immunization. On the other hand, our case is unique in being young and free of risk factors. This fact does not indicate that the COVID-19 vaccination is directly responsible for MIs, but it may be a contributor by increasing pressure on the heart. AstraZeneca phase III study in the US revealed statistically significant vaccination effectiveness of 79% and 100% in avoiding symptomatic COVID-19 infection and preventing severe illness and hospitalization, respectively. Recently, there have been rumors, stray media stories, and preprints of post-vaccination vascular thromboembolic catastrophes, particularly with the AstraZeneca vaccine. 4,5,6. With the aid of an independent neurologist, the independent data safety monitoring board (DSMB) performed a thorough examination of thrombotic events and cerebral venous sinus thrombosis (CVST). The DSMB found no evidence of an elevated thrombosis or thrombotic events risk among the 21,583 individ- uals who received at least one vaccination dose. CVST-specific incidents were not found in this study 1. While no trial has been conducted to explicitly examine MI prevalence among COVID-19 vaccinated individuals, several assumptions could be advanced. To begin, it may exacerbate underlying atheromatous plague by increasing thrombotic state post-vaccine. Greinacher et al. hypothesized that vaccine-induced prothrombotic immune thrombocytopenia, a condition comparable to heparin-induced thrombocytopenia, was responsible for the post-vaccination thrombotic phenomena 6. Secondly, Boivin et al. noted that there is a demand-supply mismatch in the frail heart following immunization. Thirdly, platelet transfection with mRNA or a viral vector-based vaccination is a remote potential 8. Fourthly, it might be a case of vasospastic allergic myocardial infarction in reaction to the vaccination, termed Kounis syndrome 8,9. In our situation, we hypothesize that post-vaccination thrombotic phenomena occurred on preexisting CAD. However, at this point, it would be flawed to establish a causative link between the COVID-19 vaccination and MI. Given that vaccine is being provided in large numbers and MI is a very common emergency, this might be a coincidental or even idiosyncratic reaction. Thus, each situation should be carefully evaluated prior to discussing with the patient’s attendant or the general public.
Conclusion
This study reports a myocardial infarction case following AstraZeneca COVID-19 vaccination first dose. Until the vaccine manufacturers provide further data or many case reports or case series documenting more severe side effects are available, it is doubtful to conclude a major danger presented to young adults getting the vaccination. Meanwhile, healthcare practitioners should be mindful of the false or exaggerated danger claims associated with the COVID-19 vaccination, which might cause the general public to rush to the judiciary. Any related research should be done with caution, not to exaggerate the findings. Moreover, additional screenings for young individuals should be considered before administering the COVID-19 vaccine as a preventive step if reports of significant adverse events in older adults continue.
Acknowledgments
The authors are thankful to the Department of Cardiology, Alhada and Taif Armed Forces Hospitals and Mahala cardiac center, specialized medical councils, Ministry of Health and Population, Egypt for the continuous support towards this research study.
Ethical Consent
Patient included in the study provided informed consent.
Funding
No funding was used to conduct current study.
Conflict of interest
The authors declare no conflict of interest.
Список литературы Acute myocardial infarction following COVID-19 vaccination: a cause or a coincidence?
- Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARSCoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet. 2021; 9;397(10269):99-111.
- DOI: 10.1016/S0140-6736(20)32661-1
- Fryar CD, Chen TC, Li X. Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999-2010. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2012.
- Yazdanyar, A., & Newman, A. B. (2009). The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clinics in geriatric medicine, 25(4), 563.
- DOI: 10.1016/j.cger.2009.07.007
- Wise, Jacqui. "Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots". (2021).
- DOI: 10.1136/bmj.n699
- Chatterjee S, Ojha UK, Vardhan B, Tiwari A. Myocardial infarction after COVID-19 vaccination-casual or causal?. Diabetes & metabolic syndrome. 2021;15(3):1055.
- DOI: 10.1016/j.dsx.2021.04.006
- Greinacher, Andreas, Thomas Thiele, Theodore E. Warkentin, Karin Weisser, Paul Kyrle, and Sabine Eichinger. "A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination". (2021).
- DOI: 10.21203/rs.3.rs-362354/v2
- Boivin, Zachary, and Jennifer Martin. "Untimely myocardial infarction or COVID-19 vaccine side effect". Cureus 13, no. 3 (2021).
- DOI: 10.7759/cureus.13651
- Kounis, Nicholas G., Andreas Mazarakis, Grigorios Tsigkas, Sotiris Giannopoulos, and John Goudevenos. "Kounis syndrome: a new twist on an old disease". Future Cardiology 7, no. 6 (2011): 805-824.
- DOI: 10.2217/fca.11.63 EDN: PMYUXZ
- Kounis, Nicholas G., et al. "Allergic reactions to current available COVID-19 vaccinations: pathophysiology, causality, and therapeutic considerations". Vaccines 9, no. 3 (2021): 221.
- DOI: 10.3390/vaccines9030221 EDN: QZTRKY


