Adaptive reactions as a guideline in evaluation of metabolite therapy
Автор: Maevsky E.I., Grishina E.V., Zakarov A.M., Vasilyeva A.A., Bogdanova L.A., Simonov A.B., Homutov A.A.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 26, 2023 года.
Бесплатный доступ
Unilateral oophorectomy (UOE) of female rats caused severe stress with an increase in the duration of the non-reproductive period (NRP) - the metestrus and diestrus phases, and a shortening of the reproductive period (RP) - the proestrus and estrus phases in the estrus cycle (a symptom of hormonal deficiency). 5 weeks after UOE, the signs of stress persisted: a decrease in the ratio of lymphocytes to the neutrophilic leukocytes in the peripheral blood and the weight ratio of thymus to the adrenal glands, as well as a decrease in the activity of succinate dehydrogenase, which is determined cytobiochemically in peripheral blood cells. A two-week administration of a succinate-containing composition with B vitamins (SCCV) to control and UOE rats increased the ratio of lymphocytes to neutrophils and weight ratio of the thymus to adrenal glands above the intact level - a sign of the “activation” state according to Garkavi and Kvakina and restored the ratio of NRP/RP. At the same time, a significant increase in SDH activity was found in animal peripheral blood lymphocytes. The study demonstrates the possibility to reverse severe stress state to the “activation” state with the help of metabolic therapy containing the combination of SCC and B-Vit.
Unilateral oophorectomy, succinate-containing composition, b vitamins, estrous cycle, stress, cytobiochemical analysis
Короткий адрес: https://sciup.org/148326339
IDR: 148326339 | DOI: 10.18137/cardiometry.2023.26.4654
Текст научной статьи Adaptive reactions as a guideline in evaluation of metabolite therapy
Imprint
ometry.2023.26.4654; Available from: http://www.cardiometry. net/issues/no26-february-202/adaptive-reactions-guideline
Applying medicine, physiotherapeutic effects and biologically active supplements (BAS) in a number of cases demand to obtain objective operational information, i. e. to have feedback from a specific subject. The variability of the functional state inherent in biological objects, and especially in humans, requires the use of dynamic assessment of the body’s response. The adequacy and effectiveness of preventive and therapeutic measures largely depend on this. Especially difficult are the assessments of small, relatively weak – mild means of metabolite therapy, formed on the basis of natural substrates, metabolic products and vitamins.
Direct interaction with the staff of the Rostov-on-Don Research Institute of Oncology (RRIO) in 19701980 made it possible to get acquainted and use the criteria and approaches developed by Garkavi L. H., Kvakina E. B. and Ukolova M. A. in the process of discovery and study of activation therapy. These approaches turned out to be quite simple and informative. As early as the beginning of 1976, L. H. Garka-vi and E. B. Kvakina [1] made a lecture at the school of modeling physiological processes, organized by A. M. Molchanov. The lecture presented the main criteria for assessing the development of response nonspecific reactions of the whole organism to the effects of various nature. According to the developed concept, the body’s responses were classified as a cyclic series of states; “training” – “activation” – “acute and chronic stress”- “areactivity zone” = “unresponsiveness”. Schematically this cycle is shown in Fig. 1.
The “training” state is characterized by an almost calm picture of white blood. All indicators remain within the physiological norm: segmented leukocytes in the range of 55-65%, lymphocytes are within the lower normal zone (less than 27%). The increase in the number of monocytes reflects the approach to the transition to the next state associated with an increase in the intensity of exposure.
In the “activation” state, two zones are distinguished: calm activation (CA) and increased activation (IA). In CA, the total number of leukocytes can reach 9000, eosinophils – 2-7%, segmented leukocytes 47-65%, lymphocytes 27-33%. In IA, the total number of leukocytes can be in the same range, eo-
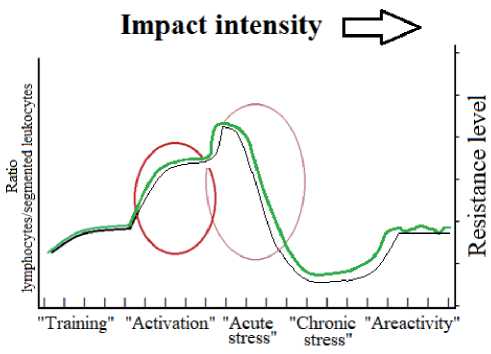
Fig. 1 Stages of the response reactions of the whole organism to the action of an increasing stimulus. Green line – ratio of lymphocytes to segmented leukocytes, black line – resistance level, oval areas – “activation” and “stress”.
sinophils – at the level of 0.5-2%, segmented leukocytes – less than 47%, lymphocytes – from 33 to 45%, monocytes – from 2 to 8%. Further intensification of the impact on the object or increase of the “irritant” dose leads to “stress” state.
“Acute stress” (AS) according to H. Selye [2] is characterized by leukocytosis, aneosinophilia, lymphopenia and neutrophilia. Approximate quantitative parameters of AS for a human: the number of leukocytes – more than 9000, eosinophils – 0, segmented leukocytes – more than 65%, lymphocytes – at the level of 20% and below.
In “chronic stress”, the number of leukocytes and eosinophils can be low, normal or elevated, the number of stab leukocytes is usually higher than normal, segmented leukocytes – more than 65%, lymphocytes – less than 20%. The authors [1] note that the combination of eosinophilia with lymphopenia is an unfavorable sign indicating depletion of the glucocorticoid function of the adrenal glands.
The ratio between the number of lymphocytes and segmented neutrophils (LF/SN) is considered as the “main indicator”. This ratio has the highest rate in the zone of increased activation and then in descending order: calm activation, training, and stress. Gradual changes in the recurring triads of adaptive responses: training, activation and stress are “probably associated with the existence of several (more than a dozen) response ranges in the conditions of a whole organism. ”
It was found that between these triads of adaptive reactions (between the stress of the previous range and the training of the next one) there is a kind of “un- responsiveness”. Experience has shown that in order to get out of the zone of “unresponsiveness”, it is necessary either to significantly reduce or significantly increase the intensity of exposure. To move from an existing reaction to the next one, let’s say from “training” to “calm activation”, it is necessary to increase the subsequent dose by about 1.5 times.
These states could be consistently repeated with an increase in the intensity of a wide variety of influences at various levels. If we keep in mind the pharmacological effects, then from homeopathic to high allopathic and even toxic doses of drugs. It turned out a kind of helix from a set of stereotypical cyclic states. Experimental studies that were carried out by U. Kaminsky, E. Kosenko and myself in the laboratory of bioenergy under the guidance of Professor M. N. Kondrashova at the Institute of Biological Physics of the USSR Academy of Sciences, together with the staff of the RRIO L. Barsukova, A. Shikhlyarova, gave reason to believe that the development of these stereotyped reactions at different levels – “helix levels”, as the intensity of the influencing factors increases, requires more and more powerful mobilization of energy, plastic and immune resources of the body.
In these studies, the development of activation reactions with all stereotypical manifestations and even a significant increase in thymus mass and the ratio of the normalized weight characteristics of the thymus to the adrenal glands in animals of the late reproductive period in response to microinfluences was most striking. One of these influences was the subcutaneous injection (administration) of an adrenaline solution at a dose of 0.1-1.0 μg (the dose is 3-4 orders of magnitude less than the therapeutic one). After 2-3 weeks, the weight of the thymus in elderly male Wistar rats increased 4-5 times compared to that in animals of the control group, which were injected with the same volume of saline. The apparent simplicity of the analysis required manual counting of blood cells on a smear. Moreover, rats differ from humans and other mammals by a pronounced predominance of the number of lymphoid cells over the number of segmented leukocytes.
In our study, an attempt was made to move away from the notions of physiological adaptive reactions and their alternation, since, on the one hand, it was required to study the consequences of severe operational stress, rather than physiological. On the other hand, the question arose whether the above criteria could
Issue 26. February 2023 | Cardiometry | 47
be acceptable and show something in evaluating the effectiveness of mild metabolic therapy in severe operational stress. However, the experience of metabolic therapy has shown that its implementation should not so much be based on dose criteria, but on the modification of the composition – a set of components of metabolic therapy. The present experimental study was directed to address these questions.
Thus, the purpose of this study was to study the possibility of using the concept of adaptive activation reactions to assess the effectiveness of a variation of metabolic therapy in severely stressed animals.
But before describing the results of the announced experimental study, we should dwell on that part of the history of metabolite therapy that arose and developed for more than half a century thanks to M. N. Kondrashova and her scientific school as a part of physiological mitochondriology. In a simplified and concise form, coupled with the data of modern studies, the development of succinate-dependent metabolic therapy can be described as follows.
-
1 .Originally M. N. Kondrashova found that among the intermediates of the tricarboxylic acid cycle (the Krebs cycle, localized in mitochondria, that are the energy stations of cells in human and animal tissues), the most efficient in terms of energy power is the oxidation of succinic acid (concentrations of approximately 10-3 M succinate).
-
2 .Further there was presented the cycle of development of rest-activity-recovery processes at the mitochondrial level: during the transition from rest to activity up to hypoxic load, when succinate dehydrogenase (SDH) is activated, the enzyme system responsible for the oxidation of succinate. An increase in the
-
3 .An indicator of SDH activity under conditions of a whole organism with a fairly acceptable approximation for practice can be the cytobiochemical determination of SDH activity in lymphocyte mitochondria on a peripheral blood smear [3].
-
4 .Experimental and clinical practice has shown that extremely small (milligram) doses of exogenous succinate have therapeutic efficacy. Therefore, the effects of exogenous (delivered as preparations or dietary supplements) succinate in the conditions of the whole organism cannot be due to its “fuel” role as a substrate for energy metabolism. Because of that special studies were conducted that revealed the signal “information” role of the succinate molecule. Moreover, the studies carried out jointly with V. M. Dilman [4] showed that one of the most important objects that perceive the succinate signal is the hypothalamus, the center of neurohumoral regulation.
-
5 .Finally, in 2004 He et al. [5] discovered that succinate is a ligand for the exorphan cell receptor QRP91. Succinate affinity for the receptor is at the level of 104-10-5 M. As a result of ligand-receptor interaction, the level of intracellular calcium ions, Ca2+, increases, which contributes to the mobilization of many intracellular processes (Fig. 2). This receptor is now called SUCNR1 and is considered by many researchers to be the stress receptor. SUCNR1 was found not only in cardiomyocytes, cells of the receptor zones of the juxtaglomerular apparatus of the nephron and carotid
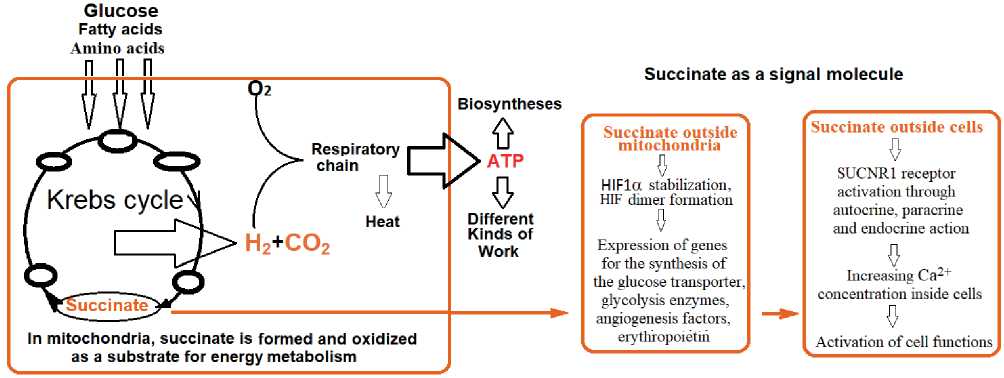
Fig. 2.Scheme of Succinate functions as an energetic substrate in mitochondria (Mt) and signal molecule outside Mt and in extracellular space. Ovals in the Krebs cycle are dehydrogenases.
-
6 .An important factor was the discovery of the role of extramitochondrial succinate (at a concentration of about 10-4-10-5M) as a stabilizer of the cytosolic hypoxia-induced factor HIF1a. The HIF dimer initiates the expression of genes responsible for the de novo synthesis of enzymatic and signaling adaptation systems in oxygen deficiency. The discovery of HIF and its functional role was awarded the 2019 Nobel Prize [6].
intensity of the load up to deenergization is accompanied by the inhibition of SDH and the accumulation of endogenous succinate. Oxidation of accumulate succinate plays a key role in recovery during the rest period after exercise.
artery, but also in many other cells, including hepatocytes, dendritic and macrophage cells of the immune system, and even in cells of brown fat and retina.
However, as soon as the signaling action of extra-mitochondrial and extracellular succinate was discovered, a wave of works began showing the harm of increasing the concentration of succinate (there are several hundred such works, see NCBI, PubMed). Indeed, in a number of pathologies such as diabetes mellitus (especially with severe hyperglycemia), obesity, irritable bowel syndrome, hypertension, various manifestations of the metabolic syndrome, a decrease in SDH activity and accumulation of endogenous succinate were often found. Moreover, reperfusion tissue damage after prolonged ischemia has also been associated with the oxidation of excess endogenous succinate. And in such types of neoplasms as pheochromocytoma, the triad of paraganglioma-pheochromocytoma-interstitial gastric cancer or interstitial kidney cancer, severe genetic defects in the SDH β-subunit were found. In addition, it became clear that for some types of neoplasms, the anoxic accumulation of endogenous succinate is a kind of adaptive strategy that allows, through the stabilization of HIF, to increase blood flow, substrate delivery and metastasis.
With such circumstances many works are devoted to finding new pathological properties of the increased succinate level. Not many researchers have the courage, sober approach to the problem, the desire and knowledge to figure out how and under what conditions succinate acts as a dangerous foe or friend that has a therapeutic effect. Although even a superficial and, moreover, a thorough analysis clarified the situation as a whole. Since we are talking about a signal regulatory effector, then it should be evaluated as signals in the regulation cascades are evaluated. In the body, receptor signals of any nature (metabolic, hormonal, mediator, ionic or electro-chemical) have primarily an informational function, intermittent, short and even impulsive. If the signal turns from intermittent into a long-term effect, then it loses the nature of an infor- mational regulator, regardless of what the effector is: catecholamines, acetylcholine, glutamate, or a change in the electrochemical gradient. Such an impact, except for harm and damage to the functional-structural organization of the receiving object of the recipient, does not cause anything else. Absolutely the same situation develops with endogenous succinate. For example, during short-term ischemia, low-intensity short stress, acute ischemia/hypoxia or hyperglycemia, the appearance of succinate outside mitochondria and outside cells is an information signal for mobilizing oxygen delivery and activation of cell and tissue functions.
But, if genetic or hormonal, metabolic, microbiome and chronic ischemic circumstances cause a significant and long-term (in some cases for life) increase in the concentration of endogenous succinate, then this only leads to the progression of the pathology. The means of combating this trouble must necessarily include finding ways to inhibit the extra formation of endogenous succinate and activate the oxidation of endogenous succinate in order to prevent a long-term increase in its concentration.
The succinate situation when a dose of external – exogenous succinate enters the body, basically, cannot reproduce the described pathological circumstances, even when administered in courses. For example, succinate entering orally or directly into the bloodstream as part of dietary supplements or medicine is able to cause an extremely short-term (no more than 10 minutes) and very small excess of the level of extracellular and extramitochondrial succinate within the physiological norm. This is a fact well established back in 1958 [7]. It has been fully confirmed by a subsequent set of pharmacokinetic studies, the latest of which is dated 2021 [8]. Therefore, exogenous succinate can only have a regulatory informational effect, but not a pathological impact. Obviously, this is the main difference between the action of exogenous succinate and the key point in answering the question: “is succinate a foe or a friend? “ [9]. The information component, which is not drowned in a pathological excess of succinate, obviously has a positive effect, in contrast to succinate overflow.
In connection with the important role of SDH activity in the oxidation of succinate and maintaining its normal level, we recall a number of works performed in the scientific school of M. N. Kondrashova, and in particular, directly by us. It was shown that a short
Issue 26. February 2023 | Cardiometry | 49
“physiological” level of immobilization stress or acute hypoxia contribute to the activation of SDH, as well as the “activation” reactions caused by ultra-low doses of adrenaline or exogenous succinate [10].
In the experiment, we took female rats for two reasons. Firstly, even in the late reproductive and post-reproductive period, they retain the cyclical changes in the cells of the vaginal epithelium, reflecting the residual ovulatory activity, that is, the functioning of the axis hypothalamus – pituitary gland – ovaries. Secondly, even H. Selye drew attention to the fact that the ovulatory cycle is drastically disturbed and interrupted during severe stress of any genesis. Therefore, according to the ratio of the phases of the residual ovulatory cycle, monitored by the cytology of the vaginal smear, it is possible to objectively assess the real situation in dynamics after unilateral ovariectomy and the possibility of correcting this stressful state with the help of a succinate-containing composition (SCC). A substrate composition was taken as the SCC, recreating the previously developed dietary supplement “Enerlit-Klima” (Russia) and AMBEREN (USA).
For an objective assessment of stress and the course of SCC at the end point of the study, the above-described characteristics of the triad according to Garka-vi and Kvakina [1] were used according to the main indicator, the ratio of LF/SN and according to such an indicator of stress as the weight ratio of the thymus and adrenal glands, introduced by H. Selye [2].
As is known, stress activation of the regulatory cascade of the hypothalamus-pituitary-adrenal glands is accompanied by excessive formation of ACTH and, accordingly, hyperproduction of glucocorticosteroids. These changes are easily registered by hypertrophy of the adrenal glands and a decrease in the weight of the thymus.
Intravital assessment of SDH activity, as already mentioned, with the cytobiochemical (CB) method, which enables us to evaluate the redox transformations triggered by exogenous substrates in immobilized peripheral blood cells after special processing of blood smears. This indicator, being integrative, reflects to some extent shifts in the hormonal, energy and immunological status of the whole organism [3].
Materials and Methods
Experiments were conducted twice on 68 fertile female rats (18-19 months old), Sprague Dawley line, weighing 350-410 g. The animals were divided into
50 | Cardiometry | Issue 26. February 2023
eight groups: 1) intact control (INT), n=9; 2) control after a course of SCC (SCC) n=9; 3) control after a course of B-Vit (B-Vit) n=6; 4) control, after a course of SCC with B vitamins (SCCV), n=9; 5) female rats with unilateral oophorectomy (UOE), n=9; 6) female rats that received a 2-week course of SCC 3 weeks after UOE (UOE+SCC), n=10; 7) female rats that received a 2-week course of B vitamins 3 weeks after UOE (UOE+B-Vit) n=6; 8) female rats that received a B vitamin-enriched SCC course after UOE (UOE+SC-CV), n=10.
UOE was performed by Bairamov A. A. (M. D., professor at The Almazov Medical Center, St. Petersburg) according to the generally accepted methodology. Three weeks after UOE, the SCC was administered in dose of 34.4 mg per kg of animal body mass, perorally, every 24 hours for 2 weeks. We administered such doses, because the dose for rats has to be 6 times higher than the dose for humans as per the guidelines for testing new pharmacological compounds. The succinate-based composition contained 50% ammonium succinate with the rest of the ingredients present in equal amounts: calcium disuccinate, magnesium disuccinate, sodium glutamate, and glycine. B vitamins composition contained vitamins B1, B2 and B6 (BAS called Smart B) in equal amounts and was administered perorally. The B vitamins composition was 1, 5 times higher than the prophylactic daily vitamin dose for a human, in combination with the SCC.
Estrous cycle (EC) phases - proestrus, estrus , metestrus, and diestrus – were determined daily during the entire experiment and were based on the cytology of the vaginal smears, collected at 9: 00 am. Fractions of round nucleated epithelial cells, irregularly shaped non-nucleated cornified cells, and leukocytes were calculated in the total cell pool.
Changes in the animals’ functional state were evaluated based in the integral parameter: ratio of lymphocytes to segmented nuclei neutrophilic leukocytes and weight ratio of thymus to adrenal glands (according to L. Garkavi and E. Kvakina [1] and Hans Selye [2]). Thymus and adrenal glands were taken from decapitated animals and weighed to 0.1 mg. An increase of the value of these ratios reflects the increase in thymus mass, and thus points to the development of “activation” [1]. A decrease of the ratios usually depends on the degradation of thymus and hypertrophy of the adrenal cortex and points to the development of stress [1, 2].
For the cytobiochemical control of Succinate dehydrogenase (SDH) activity [3] blood smears were prepared using a special device in order to get replicable monolayer samples. The smears were dried and then fixed for exactly 30 sec in 60% acetone (in 10mM HEPES, pH 5.2 aqueous solution), then rinsed and dried. Fixed smears were incubated at 37°C for 1 hour in the media that contained 125 mM KCl, 10 mM НEPES (рН 7.2), and 1.22 mM NBT under oxidation of added potassium succinate. Potassium malonate was added to the incubation media in order to inhibit succinate dehydrogenase (SDH). A probe without substrates served as the control; it allowed evaluation of the endogenous substrates (ES) contribution. After incubation with NBT, the smears were rinsed and a nuclear stain was added (0.5% neutral red) for 8 min. Specific formazan color during NBT reaction was determined with a video camera on the Leica DM1000 light microscope connected to the computer. Intensity of formazan staining, normalized to the background levels of unstained cells, was expressed in arbitrary units (AU) calculated using BioImages program. 30 stained cells were evaluated in each smear. The following probes were used: ES – oxidation of exogenous substrates, MAL – 5mM potassium malonate, SUC – 5mM potassium succinate.
Statistical analysis was applied to the collected data, significance calculated using Excel and Orig-inPro. Experimental studies were carried out in accordance with the rules for keeping and caring for animals and the regulations of the order of the Ministry of Health of Russia No. 708n dated August 23, 2010 “On approval of the rules of laboratory practice in the Russian Federation”, ethical principles established by the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes (adopted in Strasbourg on 03/18/1986 and confirmed in Strasbourg on 06/15/2006). The design of the experiment was approved by the ethics committee of the Institute for Theoretical and Experimental Biophysics of the Russian Academy of Sciences in accordance with “Guide for the Care and Use of Laboratory Animals, 8th edition” National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Washington (DC): Nat. Acad. Press (US); 2011.
Results and Discussion
Effects of unilateral oophorectomy and succinate-based compositions on the function state of animals
UOE changed estrous cycle structure in female rats. Usually, to quantify changes in the estrous cycle, the changes in the lengths of each of the 4 phases are considered separately. However, in our case, evaluation of the statistical significance of the postoperative changes turned out to be overly complex because of the small samples of control and intact animals; the animals were included in the experiment at the same time, and it was almost impossible to get uniform groups of females with synchronized estrous cycle phases. Conducting the surgery and administering the substrate compositions during the same phase of the cycle in all animals would have significantly lengthened the study, and factors that are difficult to control would be at play – time and age trends, changes in the weather and temperature, food etc. In order to get more homogenous data, which can be statistically analyzed, instead of comparing the length of each estrous cycle phase, we grouped the phases in pairs, separating non-reproductive and reproductive periods (NRP and RP), and used the ratio of the NRP to RP length as a quantitative parameter. NRP was made up of total lengths of metestrus and diestrus, RP – proestrus and estrus. The NRP/RP length ratio turned to be a significantly more uniform parameter.
Among the female rats in the 3 groups that have not undergone UOE, the NRP/PR ratios were similar to those in the intact animals, regardless of which substrate composition was administered (left 3 columns in Fig. 3).
UOE increased the NRP/RP ratio as the result of longer NRP and shorter RP. Such a change in the estrous cycle probably reflected insufficient estradiol production by the remaining ovary, although it is difficult to imagine that potential of one ovary in fertile animals is not enough to compensate consequences of UOE (5 weeks after the surgery) via hyperactivity and hypertrophy. Most likely, the cause of insufficient activity of the remaining ovary is due to the disruption of its functional control by the hypothalamus and the pituitary as a consequence of post-operative stress [2]. If this assumption is correct, then the succinate-based substrate composition can be viewed as a potential
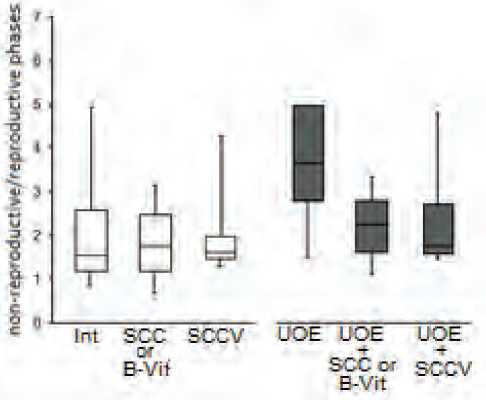
Fig. 3.Changes in the ratios of the non-reproductive period length (sum of metestrus and diestrus phases) to the reproductive period length (sum of proestrus and estrous phases) in female rats that have undergone unilateral ovariectomy (UOE) and received a treatment course of succinate-containing composition (SCC) or B vitamins (B-Vit) and mixture of SCC with B vitamins (SCCV). Tukey’s boxplot.
corrector of estrous cycle; this was shown by Dilman et al [4] in the experiments using succinate and glutamate, and later supported by our team in the study evaluating possibility of correcting estrous phase ratios in female rats of post-reproductive age using a SCC [11].
Indeed, administration of the SCC to the rats that have undergone UOE significantly improved the situation: the NRP/RP ratio decreased to the levels observed in the control animals (Fig. 3). Therefore, a course of the SCC stimulated compensatory hyperactivity of the remaining ovary. Noteworthy is the absence of a positive result after administration of the vitamin composition without the SCC (not shown in Fig. 3); the combination of B vitamins and the SCC (SCCV) successfully corrected the NRP/RP ratio (Fig. 3).
Thus, an assumption that unrealized potential of the remaining ovary may be due to the dysfunction in the control system (hypothalamus-pituitary-ovarian axis) that occurs under stress when oscillation rhythm is disrupted and formation and secretion into the blood stream of follicle stimulating and luteinizing hormones are suppressed, can be considered substantiated. According to Selye [2], such disruptions can be consequences of an intense increase in the synthesis of adrenocorticotropic hormone that occurs under stress. This leads to the need to quantitatively evaluate degrees of developing stress caused by UOE and the effects of the substrate compositions on this process.
Additionally in order to detect stress state in alive animals, we used the ratio of lymphocytes to neutrophilic leukocytes in peripheral blood (Fig. 4). It turned out that without oophorectomy, the change in this ratio does not change significantly after courses of SCC, B vitamins (B-Vit), SCCV. Ovariectomy led to a sharp drop in this ratio. The effects of B-Vit and the SCC alone were not statistically significant, while the course of combined exposure to the SCC with B-Vit significantly increased this ratio.
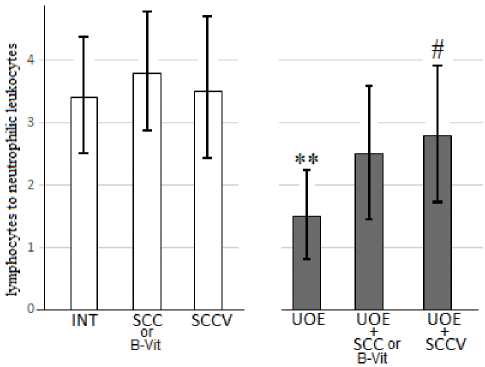
Fig. 4.Effects of the 2-week course of SCC or B vitamins composition and mixture of SCC with B vitamins (SCCV) on the ratio of lymphocytes to neutrophilic leukocytes in peripheral blood. in the control animals (left 3 light charts) and animals that have undergone UOE (right 3 dark charts). Confidence intervals values are shown (SE x tn). ** – difference in comparison with a group of intact rats, p<0.01; # – difference in comparison with UOE group, p<0.05).
Interestingly, the cytobiochemical activity of SDH in peripheral blood cells in animals without ovariectomy significantly increased under the influence of a mixture of SCC and B-Vit (Fig. 5A). After ovariectomy, SDH activity dropped sharply, and the administration of the SCC and B-Vit alone did not increase SDH activity, while the combined use of the SCC and B-Vit contributed to an increase in SDH activity (Fig. 5B).
We calculated the correlation between SDH activity and the ratio of lymphocytes/neutrophils and found that the correlation coefficient for eight groups (intact; control + SCC; control + B-Vit; control + SCCV; UOE; UOE + SCC; UOE + B-Vit; UOE + SCCV) equals 0.7 (p<0.05). Therefore, both indicators: cytobio-chemically determined activity of SDH and the ratio of lymphocytes/neutrophils indicate the anti-stress effectiveness of the combination of SCC and B-Vit, which provided an improvement in the ratio of the duration of non-reproductive phases to reproductive
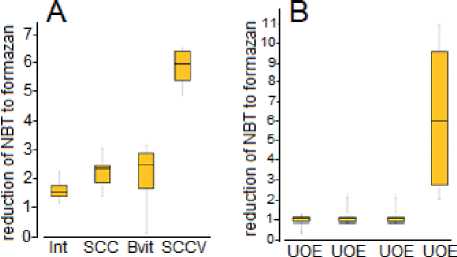
see Bvit SCCV
Fig. 5.Cytobiochemical test: reduction of NBT to formazan by SDH at the Succinate oxidation, 10 mM in the peripheral blood lymphocytes. A – control group of rats; B – group after unilateral ovariectomy (UOE).
ones (Fig. 3) in favor of the reproductive phases. It can be assumed that this is due to both a change in the hypothalamus-pituitary-ovarian axis and in the regulatory axis of the hypothalamus-pituitary-adrenal glands.
After decapitation of the animals we determined the ratio of the normalized weight of the thymus to the weight of the adrenal glands in animals and found some increase in this indicator in intact animals after a separate administration of the SCC or B-Vit, and only their combination increased this thymus/ adrenal gland ratio in control animals (Fig. 6). After UOE, this ratio is sharply reduced and it cannot be increased with the help of BAS, either SCC or B-Vit, but their combination significantly increased the ratio
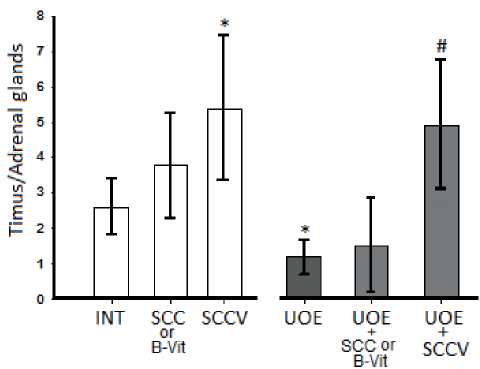
Fig. 6.Effects of the 2-week course of the SCC or B vitamins composition and the mixture of SCC with B vitamins (SCCV) on the Timus/Adrenal glands weight ratio in the control animals (left 3 light charts) and animals that have undergone UOE (right 3 dark charts). Confidence intervals values are shown (SE x tn). Number of animals in each group 9.(* difference in comparison with a group of intact rats, p<0.05; # – difference in comparison with a group of UOE, p<0.05).
of thymus/adrenal glands. The correlation coefficient between the ratio of lymphocytes/neutrophils and the ratio of thymus/adrenals is 0.8 (p<0.05). Most surprisingly, the correlation coefficient between SDH activity and the thymus/adrenal ratio is 1 (p<0.01), which is rarely observed in biological experiments.
Therefore, based on the four measurements – ratio of non-reproductive and reproductive estrous cycle period lengths, ratio lymphocytes to neutrophils, ratio of thymus and adrenal weights, and the quantitation of NBT reaction that occurs during succinate oxidation – we determined that the most effective way to correct functional state of female rats with and without UOE is to use a relatively short course of succinate-based composition enriched with vitamins (SCCV). The effect of SCCV can be described as an anti-stress effect. Noteworthy is the fact that a course of SCCV provided a complete compensatory activation of the remaining ovary, which was evidenced by a decrease of the non-reproductive to reproductive phases length ratio of the estrous cycle. The discovery that a SCCV course supports activity of the hormonal system was also apparent in the CBC analysis; there was a significant increase in the SDH activity – increase in the formazan color formation that occurs when added succinate is oxidized.
Thus, we found that with the help of metabolic therapy, it is possible to ensure the correction of not only the functional state under physiological conditions, but also to ensure the maintenance of the functional state of animals under conditions of severe postoperative stress. At the same time, judging by all the measured indicators, there is a development of an “activation” reaction against the background of severe stress, that is, in fact, a reverse movement along the scale shown in Fig. 1.
Conclusions
-
1. A two-week course of the succinate-based composition with B-vitamins resulted in a significant increase in the ratio of lymphocytes to neutrophilic leucocytes and the weight ration of the thymus to adrenal gland in female rats; this points to a development of “activation” state – increased resistance to harmful stimuli and stressors.
-
2. Following unilateral ovariectomy, we observed changes in the estrous cycle phases – an increase in the length of the non-reproductive period and shortening of the reproductive period; this points
Issue 26. February 2023 | Cardiometry | 53
-
3. A two-week course of the succinate-based composition with B vitamins administered 3 weeks after unilateral oophorectomy resulted in the development of “activation” state and recovery of the estrous cycle almost to that of the intact animals.
-
4. The cytobiochemical analysis showed an increase of SDH activity, measured in the nitroblue tetrazol reduction, that reflects the development of “activation” state after a course of the succinate-based composition with B vitamins in the control and UOE groups.
-
5. The study demonstrates the possibility to reverse severe stress state to the “activation” state with the help of metabolic therapy containing the combination of SCC and B-Vit.
to hormonal deficiency that typically occurs under strong stress.
Список литературы Adaptive reactions as a guideline in evaluation of metabolite therapy
- Garkavi L. H., Kvakina E. B., Ukolova M. A. Adaptive reactions and resistance of the organism. 3rd edition, revised. Rostov-on-Don: Publish. Rostov university, 1990.223 p.
- Selye H. In Vivo: The Case for Supramolecular Biology. // New York: Liveright, 1967.168p.
- Kondrashova M. N., Zakharchenko M. V., N. V. Khunderyakova, Maevsky E. I. Cytobiochemical method for determining the activity of succinate dehydrogenase, oxidation of endogenous succinic acid, signaling action of micromolar concentrations of succinic acid, its use for quantitative assessment of the level of adrenergic regulation in the body, medium and kit for implementing the method. RF patent No. 2364868.Published: 20.08.2009.
- Dil’man V. M. Anisimov V. N. Kondrashova M. N. // Effect of succinic and glutamic acids on the sensitivity of the hypothalamo-gonadotropic system to inhibitory effect of estrogens in old rats. / Farmakol Toksikol. 1976, vol. 39(5), p. 540-543.[in Russian].
- PMID: 800765
- He W., Miao F. J., Lin D. C., Schwandner R. T., Wang Z., Gao J. et al. Citric acid cycle intermediates as ligands for orphan G-proteincoupled receptors. Nature, 2004, Vol. 429, рр. 188-193.
- The Nobel Prize in Physiology or Medicine 2019: Gregg L. Semenza/ Hypoxia-Inducible Factors in Physiology and Medicine. https: //www. nobelprize. org/prizes/ medicine/2019/semenza/facts.
- Nyhan W. L., Busch H. Metabolic Patterns for Succinate- 2-C4 in Tissues of Tumor-bearing Rats // Cancer Research Vol. 18, November, 1958. p 1203 - 1208.
- Hass D. T., Bisbach C. M., Robbings B. M. et al. Succinate metabolism uncouples retinal pigment epithelium mitochondria / BioRxiv.
- Maevsky E. I., Kondrashova M. N. // The succinate fraction of respiration - the most sensitive characteristic of mitochondria in case of small changes in the physiological state of animals // In book: Mitochondrial processes in the temporal organization of vital activity. Pushchino. 1978, pp. 24-32.
- Maevsky E. I., Peskov A. B., Uchitel M. L., Pogorelov A. G., Saharova N. Y., Vihlyantseva E. F., Bogdanova L. A., Kondrashova M. N. A succinate-based composition reverses menopausal symptoms without sex hormone replacement therapy // Adv. Gerontol. - 2008.Vol. 21 № 2 - 298-305.


