Alarmones as bacterial persistence factor
Автор: Sidorov R.Yu., Tkachenko A.G.
Журнал: Вестник Пермского университета. Серия: Биология @vestnik-psu-bio
Рубрика: Микробиология
Статья в выпуске: 4, 2023 года.
Бесплатный доступ
The treatment of bacterial infections with antibiotics is significantly complicated because of the adaptive mechanisms employed by bacteria. Chronic and recurrent infections are often linked to bacterial persistence, biofilm formation, and antibiotic tolerance. These processes result in reduced metabolic activity, rendering bacteria insensitive to conventional antibiotics that primarily target actively growing cells. The stringent response, regulated by (p)ppGpp alarmone molecules, serves as a stress adaptation mechanism. It is conserved across numerous bacterial species and plays an important role in long-term survival under nutrient-depleted conditions. (p)ppGpp alarmones also play a significant role in bacterial persistence and the formation of biofilms. The pursuit of novel antibacterial agents that specifically target (p)ppGpp synthesis, thereby inhibiting the stringent response, presents a promising strategy in the battle against bacterial infections. In this context, alarmone synthetase inhibitors emerge as promising candidates for clinical application, as they have demonstrated their effectiveness in suppressing bacterial survival mechanisms, inhibiting biofilm formation, and reducing antibiotic tolerance and bacterial persistence.
Bacterial persistence, antimicrobial resistance, alarmones, (p)ppgpp, dormancy, rsh, alarmone synthetase inhibitors
Короткий адрес: https://sciup.org/147242769
IDR: 147242769 | УДК: 579.61 | DOI: 10.17072/1994-9952-2023-4-356-366
Текст обзорной статьи Alarmones as bacterial persistence factor
Bacterial persistence
Bacterial persistence is a phenomenon characterized by bacterial cells displaying reduced sensitivity to antibiotics forming a distinct subpopulation known as "persisters" within a bacterial population. Despite being genetically identical to the regular cells, persisters possess the ability to endure antibiotic exposure by entering a dormant state. Subsequently, they can reactivate their growth when conditions become favorable [Brauner et al., 2016].
Even when environmental conditions are optimal for bacterial growth, a presence of slow-growing and nondividing cells within the bacterial population is observed [Grimbergen et al., 2015]. This adaptation can be interpreted as a bet-hedging strategy. The majority of the population directs its efforts towards maximizing resource utilization and rapid expansion, while a smaller fraction of persister cells acts as a form of insurance, preparing for potential future challenges or stressors [Kaldalu et al., 2016].
The efficacy of conventional antibiotics is contingent not only upon their direct interaction with cellular targets but also on the ensuing cascade of metabolic disturbances [Stokes et al., 2019]. For instance, antibiotics such as ampicillin, kanamycin, and norfloxacin, despite targeting different bacterial cell components, trigger a common phenomenon—the generation of hydroxyl radicals. These radicals can inflict damage upon macromolecules and, consequently, lead to bacterial cell death, a phenomenon not observed with bacteriostatic antibiotics
[Kohanski et al., 2007]. Therefore, mitigating the activity of an antibiotic target in persister cells may prove crucial in preventing the accumulation of toxic metabolites.
The minimum inhibitory concentration (MIC) test typically utilized to determine antimicrobial resistance is not suitable for assessing persistence, because persister cells do not proliferate in the presence of the antibiotic but are able to survive in a dormant state [Brauner et al., 2018]. To analyze persistence, as well as tolerance, killing curves (time-kill curves) in the presence of an antibiotic are used (Fig. 1). An antibiotic is added to the bacterial culture in a concentration exceeding the MIC, and the number of surviving cells in the culture is measured depending on the time of incubation with the antibiotic. In the case of non-resistant cells, a two-phase curve is observed. The first phase reflects the intensive killing of the general population of actively growing cells, and the second phase is characterized by the slow killing of a small fraction of persisters [Lewis, 2010].
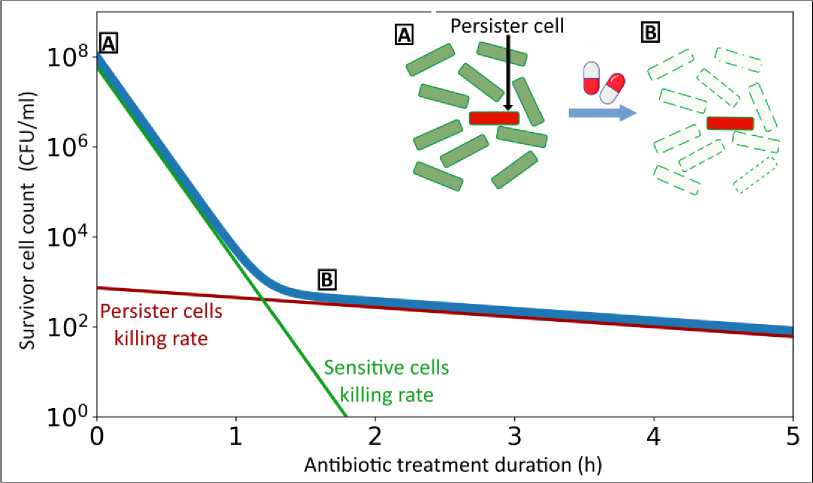
Fig. 1. The typical killing curve of a non-resistant strain culture exposed to a bactericidal antibiotic:
f ( t )= io 8 * e- 10 t ) +io 3 * e- °-5 tt (1)
In some bacterial species, the eradication of persister cells within a population can be achieved by repeated culture reinoculation during the early exponential phase, thereby preventing the culture from transitioning into the stationary or lag phase. This phenomenon has been demonstrated in Escherichia coli , Pseudomonas aeruginosa , and Staphylococcus aureus [Keren et al., 2004]. However, it is only partially characteristic of other bacterial species, such as Mycolicibacterium smegmatis [Bhaskar et al., 2018]. This observation underscores the important role of stationary or lag phase stresses in the development of a persister metabolic state. Numerous studies have demonstrated the inducing influence of various stressors on the emergence of persister cells. This effect is noticeable in response to oxidative stress [Wu et al., 2012], acid stress [Hong et al., 2012], or change of carbon source [Amato et al., 2013; Amato & Brynildsen, 2015; Mok et al., 2015].
Both resistance and persistence represent distinct strategies employed by bacteria to counteract antibiotics and are not concurrently active within the same bacterial strain. Nevertheless, over the course of the species' evolution, these strategies can complement each other [Vogwill et al., 2016]. Persister cells undergo evolutionary pressure from antibiotics but manage to survive their effects. As a result, with each new generation, random mutations accumulate in these surviving cells, some of which may confer resistance to the antibiotic. Persistence facilitates the natural selection of such mutations under the direct influence of environmental factors, such as antibiotics, occasionally resulting in the conversion of persistence into resistance [Sebastian et al., 2017]. Therefore, bacteria that are frequently exposed to antibiotics and manage to survive through persistence are at a higher risk of accumulating mutations that provide antimicrobial resistance [Cohen et al., 2013; Mandal et al., 2019].
Persister formation factors
The emergence of persister cells is closely linked to the growth phase of a batch culture. The largest proportion of persister cells forms during the stationary phase, attributable to slower growth rates [Brauner et al., 2016; Salcedo-Sora & Kell, 2020]. Furthermore, persister cells are generated during the lag phase, when growth halts as the bacteria adapt to environmental conditions [Brauner et al., 2016]. Additionally, they are formed at a lower frequency during the exponential phase, partly due to genetic noise and population heterogeneity [Zhang, 2014;
Identifying the genes responsible for the development of persistence is a challenging endeavor that demands a non-trivial approach. A method that has been successful in pinpointing genes linked to fundamental bacterial functions, such as flagella formation, chemotaxis, virulence, which involves screening libraries of single knockouts, has proven unyielding in discovering a single-knockout mutant that fails to generate persister cells [Lewis, 2010]. Nonetheless, in strains with knockouts of certain global transcriptional regulators that influence the expression of numerous genes, a tenfold reduction in the persistence frequency has been observed. These findings point to the cumulative nature of persister cell formation. It appears that persisters are generated through the concerted action of multiple independent and parallel mechanisms, aligning with their adaptive characteristics. An important implication of the polygenic basis for persister cell formation is the impossibility of entirely suppressing it with a single pharmacological compound [Lewis, 2010].
Dormancy
Bacterial dormancy denotes a state in which bacterial cells cease metabolic activity and reproduction for an extended period while remaining viable [Wood et al., 2013]. Dormancy can be regarded as a more pronounced form of growth cessation [Brauner et al., 2016]. Different levels of dormancy depth are discerned based on the duration of the lag phase required for growth resumption [Pu et al., 2019]. The utmost level of dormancy depth is evident in viable but nonculturable bacterial cells (VBNC) [Pu et al., 2019].
Combining fluorescence microscopy and microfluidics techniques, researchers found that cells of a hyperper-sistent hipA7 mutant strain that survived ampicillin action showed no growth before the exposure to the antibiotic [Balaban et al., 2004]. Another study showed that pretreatment with the transcription inhibitor rifampicin or the translation inhibitor tetracycline resulted in increased survival when exposed to the ampicillin and ciprofloxacin antibiotics, linking reduced protein synthesis to persistence [Kwan et al., 2013]. The important role of ATP synthesis as a marker of persistence was demonstrated in a study where cells were exposed to arsenate, which disrupts glycolysis and thus reduces ATP production. Cells with low levels of ATP, characteristic of the stationary phase, had a level of persistence to ciprofloxacin and ampicillin to the same extent as stationary-phase cells [Shan et al., 2017]. In addition, a decrease in intracellular ATP concentration serves as a regulator of the formation of insoluble protein aggregates called aggresomes, which contribute to the formation of dormancy and ultimately persistence [Pu et al., 2019]. The mentioned experimental data clearly indicate the dormant nature of the persistence phenomenon.
Activation of the marRAB operon results in the development of antibiotic insensitivity without impeding growth. Moreover, heightened expression of efflux pumps can decrease the antibiotic burden within a cell through active expulsion. These mechanisms can be instigated by genetic alterations, constituting a manifestation of resistance. Nevertheless, the overexpression of these genes can also stem from random fluctuations or stress, a characteristic of persistence. To delineate these occurrences, the concept of heteroresistance is employed, where only a fraction of the population demonstrates resistant traits [Brauner et al., 2016].
Drawing from the preceding discussion, it becomes evident that dormancy is indeed a distinctive feature of persister cells. However, it's important to note that while dormancy is associated with persistence, the two notions are not entirely synonymous. If a cell is incapable of emerging from a state of profound dormancy, it would not be considered part of the persister population that withstands the antibiotic's effects [Pu et al., 2019].
(p)ppGpp alarmones
A study examining the hipA7 allele, which is associated with a higher persister frequency, found that when the relA and spoT genes are deleted, the hip mutant of E. coli no longer exhibits an increased production of persister cells [Korch et al., 2003]. In E. coli , the relA and spoT genes encode (p)ppGpp synthetase proteins responsible for converting guanosine nucleotides into nucleotide messengers known as (p)ppGpp or alarmones [Bel-jantseva et al., 2017]. The term "alarmones" is derived from a fusion of the words "alarm" and "hormone," reflecting the fact that these regulatory molecules are synthesized within bacterial cells in response to unfavorable environmental conditions, effectively functioning as molecular alarm signals.
Stress factors can disrupt the optimal rate of the translation elongation process. To address this challenge, cells activate a mechanism known as the stringent response. The classic stringent response in bacteria is triggered when there is a shortage of amino acids. Protein synthesis necessitates a supply of all 20 amino acids. Insufficient levels of even one amino acid within the cell can result in the production of incomplete and nonfunctional proteins. In such instances, the cell initiates the stringent response, which, in the E. coli , is orchestrated by the enzyme RelA, capable of synthesizing (p)ppGpp [Starosta et al., 2014]. When any amino acid becomes scarce, the corresponding uncharged tRNA starts to accumulate within the cell. RelA forms a complex by initially binding to the uncharged tRNA and subsequently to the ribosome. This formation of the RelA-tRNA-ribosome complex activates the alarmone synthetase RelA, ultimately leading to an accumulation of (p)ppGpp within the E. coli cell [Winther et al., 2018]. Alarmones are able to bind RNA polymerase, modulating its selectivity for gene promoters [Mechold et al., 2013]. They suppress the ability of RNA polymerase to interact with GC-rich discriminatory regions of promoters, characteristic of ribosomal RNA and protein genes, which reduces their transcription level, and therefore the rate of protein synthesis [Wagner, 2002; Burgos et al., 2017].
Guanosine nucleotides, namely GTP, GDP, and GMP (guanosine triphosphate, guanosine diphosphate, and guanosine monophosphate), serve crucial roles in intracellular energy and information processes. GTP is utilized by RNA polymerase for RNA strand synthesis. In RNA, the linkage between adjacent nucleotides forms between the 5'-phosphate and the 3'-OH group [Murakami, 2015]. However, as part of the stringent response, guanosine nucleotides are transformed into regulatory molecules known as alarmones. This conversion is catalyzed by (p)ppGpp synthetases, capable of transferring pyrophosphate from ATP to the 3'-OH group of GTP, GDP, or GMP, resulting in the synthesis of guanosine pentaphosphate (pppGpp), guanosine tetraphosphate (ppGpp), and guanosine 5'-mono-3'-diphosphate (pGpp) (Fig. 2) [Syal et al., 2021]. The alarmones pppGpp and ppGpp are typically collectively referred to as (p)ppGpp. However, if pGpp is included, all three alarmones are denoted as (pp)pGpp. Alarmones represent just one instance of regulatory molecules generated from available metabolites within the organism [Irving et al., 2021].
Since the (p)ppGpp molecule was first discovered in 1969 through autoradiography by Cashel and Gallant [Cashel & Gallant, 1969], the comprehension of its cellular functions has significantly broadened beyond the stringent response. In E. coli, the accumulation of (p)ppGpp has far-reaching effects, influencing the expression of approximately 500 genes by stabilizing RpoS, a sigma factor for stationary phase genes [Merrikh et al., 2009]. Moreover, in E. coli cells, (p)ppGpp directly inhibits DNA primase [Maciag et al., 2010; Giramma et al., 2021], thereby restricting DNA replication, suppresses rRNA synthesis, and modulates the transcription of the ribosome modulation factor Rmf, affecting translation [Izutsu et al., 2001]. Collectively, these changes result in a slowdown of cell growth [Pacios et al., 2020]. The set of identified targets affected by (p)ppGpp continues to expand [Kushwaha et al., 2020]. For instance, the interaction of (p)ppGpp with the translation initiation factor IF2 halts protein synthesis [Diez et al., 2020], the suppression of GTPase activity by (p)ppGpp leads to a reduction in the number of mature ribosomes [Corrigan et al., 2016], and (p)ppGpp interaction with HPRT inhibits purine metabolism [Anderson et al., 2019].
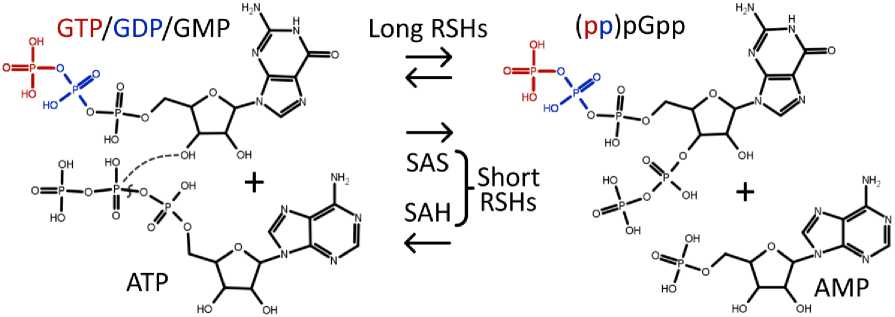
Fig. 2. Alarmone synthesis and hydrolysis:
In the (pp)pGpp synthesis process, pyrophosphate is transferred from ATP to the 3'-OH group of GTP/GDP/GMP, leading to the production of AMP. During hydrolysis, the 3'-pyrophosphate in (pp)pGpp is cleaved, resulting in the restoration of GTP/GDP/GMP. These reactions are catalyzed by enzymes belonging to the RelA/SpoT homolog (RSH) superfamily. Long RSHs are generally capable of both (p)ppGpp synthesis and hydrolysis, while short RSHs are specialized for either synthesis (small alarmone synthetases, or SAS) or hydrolysis (small alarmone hydrolases, or SAH).
Furthermore, research has demonstrated an association between elevated levels of (p)ppGpp and the development of tolerant and persistent cells [Rodionov & Ishiguro, 1995; Hobbs & Boraston, 2019]. P. aeruginosa mutants deficient in the spoT and dksA genes, which exhibit heightened (p)ppGpp levels, display the suppression of negative DNA supercoiling. This suppression restricts DNA replication and transcription, ultimately conferring tolerance to ofloxacin and ciprofloxacin [Viducic et al., 2006]. Alarmones enhance antibiotic tolerance in Vibrio cholerae by diminishing the production of reactive oxygen species in response to antibiotic exposure [Kim et al., 2018]. Furthermore, the role of (p)ppGpp in the emergence of resistance to penicillin and vancomycin antibiotics has been elucidated [Wu et al., 2010].
Bacteria residing in the deeper layers of biofilms confront restricted nutrient availability, prompting the activation of the stringent response. It has been established that the persistence of P. aeruginosa and E. coli cultivated within biofilms is contingent on (p)ppGpp. In the Δ relA Δ spoT mutant of P. aeruginosa , which exhibits impaired (p)ppGpp production, the protective capacity of biofilms against antibacterial agents diminishes [Nguyen et al., 2011]. The influence of (p)ppGpp on biofilm formation has also been documented in Pseudomonas putida [Liu et al., 2017], Helicobacter pylori [Zhao et al., 2021], M. smegmatis [Gupta et al., 2015], and Mycobacterium tuberculosis [Gupta et al., 2021].
The alarmone (p)ppGpp plays a crucial role in growth rate control in E. coli [Potrykus et al., 2011]. Growth rate control ensures that the ratios of total RNA to DNA and total RNA to protein within a cell increase as the number of cell divisions per hour rises. The (p)ppGpp-deficient strain lacks the ability to regulate growth rate entering the stationary phase without achieving metabolic equilibrium [Potrykus et al., 2011; Fernández-Coll et al., 2020]. Given that a decreased level of (p)ppGpp hampers cellular adaptation to the stationary phase, blocking the stringent response systems can prevent the development of the persister cell phenotype, which is characteristic of this growth phase.
Nonetheless, even in the (p)ppGpp-deficient background, bacterial cells can still generate persisters, albeit in reduced quantities. Studies have revealed that augmented production of toxins such as MqsR, MazF, GhoT, and YafQ in E. coli Δ relA Δ spoT cells still results in increased persistence. Therefore, (p)ppGpp is not an absolute prerequisite for the formation of persisters. However, when these toxins are expressed in the presence of (p)ppGpp, a statistically significant increase in the number of persisters is observed [Chowdhury et al., 2016].
The stringent response is a mechanism of adaptation to stress, conserved among different bacterial species, and is involved in long-term survival during nutrient starvation, biofilm formation, virulence, antibiotic tolerance and persistence in M. tuberculosis [Warner & Mizrahi, 2006;
Alarmone synthetase inhibitors
The development of novel drugs plays an important role in the battle against tuberculosis. Over the last decade, novel anti-tuberculosis drugs, such as bedaquiline and delamanid, have been introduced. These drugs feature novel mechanisms of action aimed at addressing multidrug resistance [Li et al., 2019]. While novel mechanisms can address resistance, they represent a temporary solution unless measures are taken to shorten treatment duration and control latent tuberculosis in its advanced stages. Antibacterial agents targeting persistent and tolerant cells, along with biofilms, hold promise as potential solutions to these challenges. The stringent response inhibition emerges as a promising strategy for the treatment of tuberculosis infection [Danchik et al., 2021].
A novel class of antibacterial compounds that inhibit the (p)ppGpp synthesis in bacteria holds the potential to address late phases of infection and tackle the issue of persistence [Kushwaha et al., 2019]. Compounds that inhibit (p)ppGpp synthesis demonstrate limited activity against actively growing bacterial cells but can efficiently target late bacterial cultures, where the proportion of slowly growing and dormant cells increases [Dutta et al., 2019]. This feature presents a potential remedy for the issue posed by conventional antibiotics, which, despite their effectiveness against actively growing cells, have limited influence on non-growing cells.
The class of alarmone synthetase inhibitors comprises structurally diverse compounds, including relacin and its analogs, vitamin C, GSK-X9, and DMNP [Sinha et al., 2023]. Relacin effectively inhibited the sporulation of Bacillus anthracis , the causative agent of anthrax, and impeded biofilm formation in Bacillus subtilis [Wex-selblatt et al., 2012]. Relacin analogs AC and AB disrupted long-term cell survival in M. smegmatis cultures under nutrient-starved conditions. These compounds suppressed biofilm formation in both M. smegmatis and M. tuberculosis and also disrupted pre-existing biofilms [Syal et al., 2017]. The X9 inhibitor effectively mitigated tolerance to isoniazid induced by nutrient starvation [Dutta et al., 2019]. Exposure to X9 replicated the survival deficiencies observed in the M. tuberculosis strain with the rel Mtb gene deletion. Furthermore, DMNP demonstrated activity against M. smegmatis stationary phase cells and possessed the capability to interfere with biofilm formation in this bacterium [Tkachenko et al., 2021]. The ability of these compounds to suppress biofilm formation, impair long-term survival in nutrient-starved conditions, and reduce tolerance and persistence indicates the clinical potential of alarmone synthetase inhibitors for the treatment of bacterial infections.
Conclusion
Bacterial persistence serves as an important bacterial strategy to reduce susceptibility to antibiotics. In contrast to antimicrobial resistance, persister cells cannot actively grow in the presence of antibiotics; instead, they transition into a slowly growing or completely dormant state. This shift can be advantageous because conventional antibiotics primarily target metabolic processes in actively growing cells, leaving persisters capable of surviving antibiotic exposure. Research has unveiled a link between heightened (p)ppGpp levels and the emergence of tolerant and persistent bacterial cells. The stringent response, a stress adaptation mechanism, which is conserved in many bacterial species, is instrumental in long-term survival under nutrient-depleted conditions and contributes to processes such as biofilm formation, virulence, antibiotic tolerance, and persistence. The pursuit of novel antibacterial agents that specifically target (p)ppGpp synthesis, thus inhibiting the stringent response, represents a promising approach to combat bacterial infections. Alarmone synthetase inhibitors show great potential for clinical application in this context, as they have demonstrated their effectiveness in suppressing bacterial survival mechanisms, inhibiting biofilm formation, and reducing antibiotic tolerance and bacterial persistence.
Список литературы Alarmones as bacterial persistence factor
- Amato S.M., Orman M.A., Brynildsen M.P. Metabolic control of persister formation in Escherichia coli. Mol Cell. V. 50, No. 4 (2013): pp. 475-87. DOI: 10.1016/j.molcel.2013.04.002.
- Amato S.M., Brynildsen M.P. Persister heterogeneity arising from a single metabolic stress. Curr Biol. V. 25, No. 16 (2015): pp. 2090-8. DOI: 10.1016/j.cub.2015.06.034.
- Anderson B.W., Liu K., Wolak C., Dubiel K., She F. et al. Evolution of (p)ppGpp-HPRT regulation through diversification of an allosteric oligomeric interaction. Elife. 8:e47534 (2019). DOI: 10.7554/eLife.47534.
- Balaban N.Q., Merrin J., Chait R., Kowalik L., Leibler S. Bacterial persistence as a phenotypic switch. Sci-ence. V. 305, No. 5690 (2004): pp. 1622-5. DOI: 10.1126/science.1099390.
- Beljantseva J., Kudrin P., Jimmy S., Ehn M., Pohl R. et al. Molecular mutagenesis of ppGpp: turning a RelA activator into an inhibitor. Nature Scientific Reports. V. 7, 41839 (2017). DOI: 10.1038/srep41839.
- Bhaskar A., De Piano C., Gelman E., McKinney J.D., Dhar N. Elucidating the role of (p)ppGpp in myco-bacterial persistence against antibiotics. IUBMB Life. V. 70, No. 9 (2018): pp. 836-844. DOI: 10.1002/iub.1888.
- Brauner A., Fridman O., Gefen O., Balaban N.Q. Distinguishing between resistance, tolerance and persis-tence to antibiotic treatment. Nature Reviews Microbiology. V. 14 (2016): pp. 320–330. DOI: 10.1038/nrmicro.2016.34.
- Burgos H.L., O'Connor K., Sanchez-Vazquez P., Gourse R.L. Roles of transcriptional and translational control mechanisms in regulation of ribosomal protein synthesis in Escherichia coli. J Bacteriol. V. 199, No. 21 (2017): e00407-17. DOI: 10.1128/JB.00407-17.
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Na-ture. V. 221, No. 5183 (1969): pp. 838-41. DOI: 10.1038/221838a0.
- Chowdhury N., Kwan B.W., Wood T.K. Persistence increases in the absence of the alarmone guanosine tetraphosphate by reducing cell growth. Scientific Reports. V. 6 (2016): 20519. DOI: 10.1038/srep20519.
- Cohen N.R., Lobritz M.A., Collins J.J. Microbial persistence and the road to drug resistance. Cell Host Microbe. V. 13, No. 6 (2013): pp. 632-42. DOI: 10.1016/j.chom.2013.05.009.
- Corrigan R.M., Bellows L.E., Wood A., Gründling A. ppGpp negatively impacts ribosome assembly af-fecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc Natl Acad Sci USA. V. 113, No. 12 (2016): E1710-9. DOI: 10.1073/pnas.1522179113.
- Dahl J., Kraus C.N., Boshoff H.I., Doan B., Foley K., Avarbock D. et al. The role of RelMtb-mediated ad-aptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA. V. 100 (2003): pp. 10026–10031. DOI: 10.1073/pnas.1631248100a.
- Danchik C., Wang S., Karakousis P.C. Targeting the Mycobacterium tuberculosis stringent response as a strategy for shortening tuberculosis treatment. Front Microbiol. V. 12 (2021): 744167. DOI: 10.3389/fmicb.2021.744167.
- Davis K.M., Isberg R.R. Defining heterogeneity within bacterial populations via single cell approaches. Bioessays. V. 38, No. 8 (2016): pp. 782-90. DOI: 10.1002/bies.201500121.
- Diez S., Ryu J., Caban K., Gonzalez R.L. Jr., Dworkin J. The alarmones (p)ppGpp directly regulate trans-lation initiation during entry into quiescence. Proc Natl Acad Sci USA. V. 117, No. 27 (2020): pp. 15565-15572. DOI: 10.1073/pnas.1920013117.
- Dutta N.K., Klinkenberg L.G., Vazquez M.-J. et al. Inhibiting the stringent response blocks Mycobacte-rium tuberculosis entry into quiescence and reduces persistence. Sci Adv. V. 5, No. 3 (2019): eaav2104. DOI: 10.1126/sciadv.aav2104.
- Ehrt S., Schnappinger D., Rhee K.Y. Metabolic principles of persistence and pathogenicity in Mycobacte-rium tuberculosis. Nat Rev Microbiol. V. 16, No. 8 (2018): pp. 496–507. DOI: 10.1038/s41579-018-0013-4.
- Fernández-Coll L., Maciag-Dorszynska M., Tailor K., Vadia S., Levin P.A. et al. The absence of (p)ppGpp renders initiation of Escherichia coli chromosomal DNA synthesis independent of growth rates. mBio. V. 11, No. 2 (2020): e03223-19. DOI: 10.1128/mBio.03223-19.
- Giramma C.N., DeFoer M.B., Wang J.D. The alarmone (p)ppGpp regulates primer extension by bacterial primase. J Mol Biol. V. 433, No. 19 (2021): 167189. DOI: 10.1016/j.jmb.2021.167189.
- Gong W., Wu X. Differential diagnosis of latent tuberculosis infection and active tuberculosis: a key to a successful tuberculosis control strategy. Front Microbiol. V. 12 (2021): 745592. DOI: 10.3389/fmicb.2021.745592.
- Grimbergen A.J., Siebring J., Solopova A., Kuipers O.P. Microbial bet-hedging: the power of being differ-ent. Curr Opin Microbiol. V. 25 (2015): pp. 67-72. DOI: 10.1016/j.mib.2015.04.008.
- Gupta K.R., Kasetty S., Chatterji D. Novel functions of (p)ppGpp and cyclic di-GMP in mycobacterial physiology revealed by phenotype microarray analysis of wild-type and isogenic strains of Mycobacterium smegmatis. Appl Environ Microbiol. V. 81, No. 7 (2015): pp. 2571–8. DOI: 10.1128/AEM.03999-14.
- Gupta K.R., Arora G., Mattoo A., Sajid A. Stringent response in mycobacteria: from biology to therapeu-tic potential. Pathogens. V. 10, No. 11 (2021): 1417. DOI: 10.3390/pathogens10111417.
- Harms A., Maisonneuve E., Gerdes K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science. V. 354, No. 6318 (2016): aaf4268. DOI: 10.1126/science.aaf4268.
- Henry T.C., Brynildsen M.P. Development of Persister-FACSeq: a method to massively parallelize quan-tification of persister physiology and its heterogeneity. Sci Rep. V. 6 (2016): 25100. DOI: 10.1038/srep25100.
- Hobbs J.K., Boraston, A.B. (p)ppGpp and the stringent response: an emerging threat to antibiotic thera-py. ACS Infect Dis. V. 5, No. 9 (2019): pp.1505-1517. DOI: 10.1021/acsinfecdis.9b00204.
- Hong S.H., Wang X., O'Connor H.F., Benedik M.J., Wood T.K. Bacterial persistence increases as envi-ronmental fitness decreases. Microb Biotechnol. V. 5, No. 4 (2012): pp. 509-522. DOI: 10.1111/j.1751-7915.2011.00327.x.
- Huaman M.A., Sterling T.R. Treatment of Latent Tuberculosis Infection-An Update. Clin Chest Med. V. 40, No. 4 (2019): pp. 839-848. DOI: 10.1016/j.ccm.2019.07.008.
- Irving S.E., Choudhury N.R., Corrigan R.M. The stringent response and physiological roles of (pp)pGpp in bacteria. Nat Rev Microbiol. V. 19, No. 4 (2021): pp. 256-271. DOI: 10.1038/s41579-020-00470-y.
- Izutsu K., Wada A., Wada C. Expression of ribosome modulation factor (RMF) in Escherichia coli re-quires ppGpp. Genes Cells. V. 6, No. 8 (2001): pp. 665-76. DOI: 10.1046/j.1365-2443.2001.00457.x.
- Kaldalu N., Hauryliuk V., Tenson T. Persisters – as elusive as ever. Appl Microbiol Biotechnol. V. 100, No. 15 (2016): pp. 6545-6553. DOI: 10.1007/s00253-016-7648-8.
- Keren I., Kaldalu N., Spoering A., Wang Y., Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiology Letters. V. 230 (2004): pp. 13-18.
- Kester J.C., Fortune S.M. Persisters and beyond: mechanisms of phenotypic drug resistance and drug tol-erance in bacteria. Crit Rev Biochem Mol Biol. V. 49, No. 2 (2014): pp. 91-101. DOI: 10.3109/10409238.2013.869543.
- Kim H.Y., Go J., Lee K.M., Oh Y.T., Yoon S.S. Guanosine tetra- and pentaphosphate increase antibiotic tolerance by reducing reactive oxygen species production in Vibrio cholerae. J Biol Chem. V. 293, No. 15 (2018): pp. 5679-5694. DOI: 10.1074/jbc.RA117.000383.
- Klapper I., Dockery J. Mathematical Description of Microbial Biofilms. SIAM Review. V. 52, No. 2 (2010): pp. 221-265. DOI: 10.1137/080739720.
- Klinkenberg L.G., Lee J., Bishai W.R., Karakousis P.C. The stringent response is required for full virulence of Mycobacterium tuberculosis in Guinea pigs. J. Infect. Dis. V. 202 (2010): pp. 1397-1404. DOI: 10.1086/656524.
- Kohanski M.A., Dwyer D.J., Hayete B., Lawrence C.A., Collins J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. V. 130, No. 5 (2007): pp. 797-810. DOI: 10.1016/j.cell.2007.06.049.
- Korch S.B., Henderson T.A., Hill T.M. Characterization of the hipA7 allele of Escherichia coli and evi-dence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol. V. 50, No. 4 (2003): pp. 1199-213. DOI: 10.1046/j.1365-2958.2003.03779.x.
- Kushwaha G.S., Oyeyemi B.F., Bhavesh N.S. Stringent response protein as a potential target to intervene persistent bacterial infection. Biochimie. V. 165 (2019): pp. 67-75. DOI: 10.1016/j.biochi.2019.07.006.
- Kushwaha G.S., Patra A., Bhavesh N.S. Structural analysis of (p)ppGpp reveals its versatile binding pat-tern for diverse types of target proteins. Front Microbiol. V. 11 (2020): 575041. DOI: 10.3389/fmicb.2020.575041.
- Kwan B.W., Valenta J.A., Benedik M.J., Wood T.K. Arrested protein synthesis increases persister-like cell formation. Antimicrob Agents Chemother. V. 57, No. 3 (2013): pp. 1468-1473. DOI: 10.1128/AAC.02135-12.
- Lewis K. Persister cells. The Annual Review of Microbiology. V. 64 (2010): pp. 357-72. DOI: 10.1146/annurev.micro.112408.134306.
- Li Y., Sun F., Zhang W. Bedaquiline and delamanid in the treatment of multidrug-resistant tuberculosis: Promising but challenging. Drug Dev Res. V. 80, No. 1 (2019): pp. 98-105. DOI: 10.1002/ddr.21498.
- Liu H., Xiao Y., Nie H., Huang Q., Chen W. Influence of (p)ppGpp on biofilm regulation in Pseudomonas putida KT2440. Microbiol Res. V. 204 (2017): pp. 1-8. DOI: 10.1016/j.micres.2017.07.003.
- Maisonneuve E., Gerdes K. Molecular mechanisms underlying bacterial persisters. Cell. 157, No. 3 (2014): pp. 539-48. DOI: 10.1016/j.cell.2014.02.050.
- Mandal S., Njikan S., Kumar A., Early J.V., Parish T. The relevance of persisters in tuberculosis drug dis-covery. Microbiology. V. 165, No. 5 (2019): pp. 492-499. DOI: 10.1099/mic.0.000760.
- Maciag M., Kochanowska M., Lyzeń R., Wegrzyn G., Szalewska-Pałasz A. ppGpp inhibits the activity of Escherichia coli DnaG primase. Plasmid. V. 63, No. 1 (2019): pp. 61-7. DOI: 10.1016/j.plasmid.2009.11.002.
- Mechold U., Potrykus K., Murphy H., Murakami K.S., Cashel M. Differential regulation by ppGpp versus pppGpp in Escherichia coli. Nucleic Acids Res. V. 41, No. 12 (2013): pp. 6175-89. DOI: 10.1093/nar/gkt302.
- Merrikh H., Ferrazzoli A.E., Lovett S.T. Growth phase and (p)ppGpp control of IraD, a regulator of RpoS stability, in Escherichia coli. J Bacteriol. V. 191, No. 24 (2009): pp. 7436-46. DOI: 10.1128/JB.00412-09.
- Mok W.W., Orman M.A., Brynildsen M.P. Impacts of global transcriptional regulators on persister me-tabolism. Antimicrob Agents Chemother. V. 59, No. 5 (2015): pp. 2713-9. DOI: 10.1128/AAC.04908-14.
- Murakami K.S. Structural biology of bacterial RNA polymerase. Biomolecules. V. 5, No. 2 (2015): pp. 848-64. DOI: 10.3390/biom5020848.
- Nguyen D. et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. V. 334 (2011): pp. 982-986. DOI: 10.1126/science.1211037.
- Orman M.A., Brynildsen M.P. Dormancy is not necessary or sufficient for bacterial persistence. Antimi-crob Agents Chemother. V. 57, No. 7 (2013): pp. 3230-3239. DOI: 10.1128/AAC.00243-13.
- Pacios, O., Blasco, L., Bleriot, I. et al. (p)ppGpp and its role in bacterial persistence: new challenges. Anti-microb Agents Chemother. V. 64, No. 10 (2020): e01283-20. DOI: 10.1128/AAC.01283-20.
- Potrykus K., Murphy H., Philippe N., Cashel M. ppGpp is the major source of growth rate control in E. coli. Environmental Microbiology. V. 13, No. 3 (2011): pp. 563-575. DOI: 10.1111/j.1462-2920.2010.02357.x.
- Prax M., Bertram R. Metabolic aspects of bacterial persisters. Front Cell Infect Microbiol. V. 4 (2014): 148. DOI: 10.3389/fcimb.2014.00148.
- Primm T.P., Andersen S.J., Mizrahi V., Avarbock D., Rubin H., Barry C.E. The stringent response of My-cobacterium tuberculosis is required for long-term survival. J Bacteriol. V. 182, No. 17 (2000): pp. 4889-4898. DOI: 10.1128/jb.182.17.4889-4898.2000.
- Pu Y., Li Y., Jin X., Tian T., Qi M., et al. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Molecular Cell. V. 73 (2019): pp. 143-156. DOI: 10.1016/j.molcel.2018.10.022.
- Rodionov D.G., Ishiguro E.E. Direct correlation between overproduction of guanosine 3',5'-bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli. J Bacteriol. V. 177, No. 15 (1995): pp. 4224-9. DOI: 10.1128/jb.177.15.4224-4229.1995.
- Salcedo-Sora J. E., Kell D.B. A quantitative survey of bacterial persistence in the presence of antibiotics: towards antipersister antimicrobial discovery. Antibiotics. V. 9, No. 8 (2020): 508. DOI: 10.3390/antibiotics9080508.
- Sebastian J. et al. De novo emergence of genetically resistant mutants of Mycobacterium tuberculosis from the persistence phase cells formed against antituberculosis drugs in vitro. Antimicrob Agents Chemother. V. 61, No. 2 (2017): e01343-16. DOI: 10.1128/AAC.01343-16.
- Shan Y., Gandt A.B., Lewis K. et al. ATP-dependent persister formation in Escherichia coli. mBio. V. 8, No. 1 (2017): e02267-16. DOI: 10.1128/mBio.02267-16.
- Sinha S.K., Neethu R.S., Devarakonda Y., Rathi A., Regatti P.R. et al. Tale of Twin Bifunctional Second Messenger (p)ppGpp Synthetases and Their Function in Mycobacteria. ACS Omega. V. 8, No. 36 (2023): pp. 32258-32270. DOI: 10.1021/acsomega.3c03557.
- Starosta A.L., Lassak J., Jung K., Wilson D.N. The bacterial translation stress response. FEMS Microbiol Rev. V. 38, No. 6 (2014): pp. 1172-201. DOI: 10.1111/1574-6976.12083.
- Stokes J.M., Lopatkin A.J., Lobritz M.A., Collins J.J. Bacterial metabolism and antibiotic efficacy. Cell Metab. V. 30, No. 2 (2019): pp. 251-259. DOI: 10.1016/j.cmet.2019.06.009.
- Syal K., Flentie K., Bhardwaj N., Maiti K., Jayaraman N. et al. Synthetic (p)ppGpp analogue is an inhibi-tor of stringent response in mycobacteria. Antimicrob Agents Chemother. V. 61, No. 6 (2017): e00443-17. DOI: 10.1128/AAC.00443-17.
- Syal K., Rs N., Reddy M.V.N.J. The extended (p)ppGpp family: new dimensions in stress response. Curr Res Microb Sci. V. 2 (2021): 100052. DOI: 10.1016/j.crmicr.2021.100052.
- Tkachenko A.G., Kashevarova N.M., Sidorov R.Yu., Nesterova L.Yu., Akhova A.V. et al. A synthetic diterpene analogue inhibits mycobacterial persistence and biofilm formation by targeting (p)ppGpp synthetases. Cell Chemical Biology. V. 28, No. 10 (2021): pp. 1420-1432.e9. DOI: 10.1016/j.chembiol.2021.01.018.
- Viducic D., Ono T., Murakami K. et al. Functional analysis of spoT, relA and dksA genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol Immunol. V. 50, No. 4 (2006): pp. 349-57. DOI: 10.1111/j.1348-0421.2006.tb03793.x.
- Vogwill T., Comfort A.C., Furió V., MacLean R.C. Persistence and resistance as complementary bacterial adaptations to antibiotics. J Evol Biol. V. 29, No. 6 (2016): pp. 1223-1233. DOI: 10.1111/jeb.12864.
- Wagner R. Regulation of ribosomal RNA synthesis in E. coli: effects of the global regulator guanosine tetraphosphate (ppGpp). J Mol Microbiol Biotechnol. V. 4, No. 3 (2002): pp. 331-40.
- Warner D.F., Mizrahi V. Tuberculosis chemotherapy: the influence of bacillary stress and damage re-sponse pathways on drug efficacy. Clin Microbiol Rev. V. 19(3) (2006): 558-70. DOI: 10.1128/CMR.00060-05.
- Weiss L.A., Stallings C.L. Essential roles for Mycobacterium tuberculosis Rel beyond the production of (p)ppGpp. J. of Bacteriol. V. 195, No. 24 (2013): pp. 5629-38. DOI: 10.1128/JB.00759-13.
- Wexselblatt E., Oppenheimer-Shaanan Y., Kaspy I. et al. Relacin, a novel antibacterial agent targeting the stringent response. PLoS Pathog. V. 8, No. 9 (2012): e1002925. DOI: 10.1371/journal.ppat.1002925.
- Winther K.S., Roghanian M., Gerdes K. Activation of the stringent response by loading of RelA-tRNA complexes at the ribosomal A-site. Mol Cell. V. 70, No. 1 (2018): pp. 95-105.e4. DOI: 10.1016/j.molcel.2018.02.033.
- Wood T.K., Knabel S.J., Kwan B.W. Bacterial persister cell formation and dormancy. Applied and Envi-ronmental Microbiology. V. 79, No. 23 (2013): 7116-21. DOI: 10.1128/AEM.02636-13.
- Wu J., Long Q., Xie J. (p)ppGpp and drug resistance. J Cell Physiol. 224. No. 2 (2010): pp. 300-4. DOI: 10.1002/jcp.22158.
- Wu Y., Vulić M., Keren I., Lewis K. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother. V. 56, No. 9 (2012): pp. 4922-6. DOI: 10.1128/AAC.00921-12.
- Yan J., Bassler B.L. Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe. V. 26, No. 1 (2019): pp. 15-21. DOI: 10.1016/j.chom.2019.06.002.
- Zhang Y. Persisters, persistent infections and the Yin–Yang model. Emerging Microbes and Infections. V. 3, No. 1 (2014): e3. DOI: 10.1038/emi.2014.3.
- Zhao Y., Cai Y., Chen Z., Li H., et al. SpoT-mediated NapA upregulation promotes oxidative stress-induced Helicobacter pylori biofilm formation and confers multidrug resistance. Antimicrob Agents Chemother. V. 65, No. 5 (2021): e00152-21. DOI: 10.1128/AAC.00152-21.
- Zheng E.J., Stokes J.M., Collins J.J. Eradicating bacterial persisters with combinations of strongly and weakly metabolism-dependent antibiotics. Cell Chem Biol. V. 27, No. 12 (2020): pp. 1544-1552.e3. DOI: 10.1016/j.chembiol.2020.08.015.
- Zamakhaev M.V., Goncharenko A.V., Shumkov M.S. [Toxin-antitoxin systems and bacterial persistence (Review)]. Appl. Biochem. Microbiol. V. 55, No. 6 (2019): pp. 571-581. (In Russ.). DOI: 10.1134/S055510991906014X.


