Anatase-silica additive for self-cleaning materials based on cement
Автор: Chernykh T.N., Kiiko P.I., Kriushin M.V.
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Construction materials science
Статья в выпуске: 1 Vol.17, 2025 года.
Бесплатный доступ
Introduction. The cost of anatase additives to concrete can be lowered by synthesizing it and applying it to a mineral substrate that is active against cement. In particular, the use of anatase in mineral powders such as silica shows promise. This paper describes the synthesis of anatase-silica photocatalysts with varying anatase to silica ratios and evaluates their performance in cement-based materials. Materials and methods. The photocatalytic additive was obtained by TiO2 deposition on the microsilica substrate using sol-gel technology. The physicochemical studies included X-ray phase analysis and electron scanning microscopy. To determine the self-cleaning, we used the rhodamine test. Results and discussion. An increase in the TiO2/SiO2 ratio increases the area covered with anatase and the height of the main anatase reflections in the X-ray phase analysis. But the increase in the anatase reflection intensity is not proportional to the change in the TiO2/SiO2 ratio with the same size of anatase crystallites. With an increase in TiO2 relative to SiO2, the self-cleaning efficiency increases and reaches the required level when the ratio of oxides in the additive is slightly less than 1:1. It means that 1 g of TiO2 is enough per specific surface area of the substrate 22 m2 to create an effective photocatalytic additive. Conclusion. The optimal TiO2/SiO2 ratio of 1:1 was revealed by varying the amount of anatase relative to the substrate in the range of TiO2/SiO2 ratios from 1:2 to 1:0.5. The results can be used in the production of technically and economically effective photocatalytic additives for self-cleaning materials based on cement.
Self-cleaning, anatase additives, anatase-silica photocatalysts
Короткий адрес: https://sciup.org/142243352
IDR: 142243352 | DOI: 10.15828/2075-8545-2025-17-1-5-13
Текст научной статьи Anatase-silica additive for self-cleaning materials based on cement
Original article
Self-cleaning building materials are innovative solutions that can themselves of dirt or bacteria without need for human intervention. These materials are particularly effective in facade finishing within the construction industry, especially for buildings and structures located near industrial facilities and highways. The use of selfcleaning materials in facades can significantly extend their service life compared to those using conventional materials.
Many different surface self-cleaning strategies have been developed over the past few decades [1]. The most promising strategy for building materials based on mineral binders is the use of photocatalytically active additives, which decompose organic matter and impart superhydrophilic properties to the surface [2]. Water droplets, forming a thin film on superhydrophilic surfaces, wedge between the base and the pollutants washing them off [3]. The most widespread photocatalyst additives are nano- and micro-sized titanium dioxide (TiO2) in the anatase modification. The anatase form of TiO2 makes additives photocatalytically active at a sufficiently high band gap of 3.2 eV, which corresponds to near ultraviolet light with a wavelength of 390 nm [4–6]. Such radiation is part of the solar spectrum, but the efficiency of the photocatalyst under the influence of visible radiation is less than 10 % [7, 8]. Anatase photocatalyst additives are also very expensive, which impedes their widespread use in construction.
The cost of anatase additives can be reduced by their synthesis through the deposition on a mineral substrate, which can be active with respect to the binder [9–13] or have its own photocatalytic activity to expand the radiation spectrum triggering photocatalysis [14–18].
CONSTRUCTION MATERIALS SCIENCE
The deposition of anatase on mineral powders conventionally used for the production of cement materials as active mineral additives with a highly developed surface and stability up to 550 °C is promising, since the additive is heated to this temperature during synthesis.
An effective and widespread pozzolanic additive in concrete is microsilica. It is a waste product, which determines its low cost. Microsilica has a highly developed surface and ability to bind portlandite, which determines its efficiency and popularity [19–21].
Therefore, obtaining a complex additive from anatase deposited on microsilica for facade cement materials is expedient and economically sound. This work describes the synthesis of anatase-silica photocatalysts with different anatase and silica ratios and assesses their efficiency for cement-based materials.
METHODS AND MATERIALS
To synthesize the photocatalyst, tetrabutoxytitanium (PromKhimPerm LLC, TU 6-09-2738-09) was used as a source of TiO2 and MK-85 microsilica (ChEMK JSC, GOST R 58894-2020) with a mass fraction of SiO2 of 85 % and a specific surface area of 12.5 m2/g was used as a substrate for TiO2 deposition. OP-10, a non-ionic surfactant (Soyuzkhimprom CJSC), was used for the uniform distribution of TiO2 over the substrate surface.
The photocatalytic additive was obtained by TiO2 deposition on the substrate using sol-gel technology [9]. The synthesis included the following four stages: the dissolution of the surface-active additive (OP-10) in ethyl alcohol in a magnetic stirrer for 5 minutes; the sequential introduction of microsilica powder and tetrabutoxytitanium into the mixture in the magnetic stirrer for 30 minutes; drying (gel aging) for 24 hours in a laboratory chamber dryer at 115 ºC; and roasting in a laboratory chamber furnace for two hours at 550 ºC.
White Portland cement PCB 1-500D0 GOST 965-89 (Cemiks LLC) was used as a binder.
The physicochemical studies included X-ray phase analysis using an Ultima IV X-ray diffractometer and electron scanning microscopy using a Jeol JSM-7001F scanning electron microscopy complex, EDS Oxford INCA X-max 80, WDS Oxford INCA WAVE, EBSD, and HKL. While preparing for the analysis, additive samples were not crushed to preserve their structure, which allows the indirect assessment of the coating of microsilica particles with anatase.
To determine the self-cleaning of hardened cement paste, we used the rhodamine test described in the Italian standard UNI11259-2016 [22], which quantifies the self-cleaning efficiency and assesses whether the material is self-cleaning. We used an irradiation lamp with a wavelength of 390nm and an organic dye – rhodamine B. During the tests, some of the samples were dyed with a solution of rhodamine B at a concentration of 4·10-4mol/l in an amount of 3.84 ml/cm2. The samples were photographed and placed under a UV lamp. After 4 and 26 hours, the samples were photographed to record the color change. Photographing parameters included complete darkness and a flash as a light source. Camera settings included the focal distance of f/1.8, ISO400 light sensitivity, white balance 4000, and a shutter speed of 1/250 sec. The surface color (dye degradation efficiency) was measured using ImageJ software according to RGB coordinates. They are converted into the CIELAB system using L* a* b* color coordinates, where L is luminosity, a and b are colorimetric coordinates representing the color tone measurement in a two-dimensional plane. The color change between the source color of the material and the color with the applied dye is calculated by:
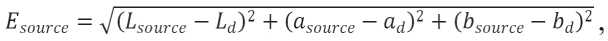
where Lsouce, asource, bsource are the CIELAB coordinates of the source color of the material in the photograph; and Ld, ad, bd are the CIELAB coordinates of the dyed surface color in the photograph immediately after applying the dye.
The color change between the source color of the material and the color with the applied dye after irradiation for t hours is calculated by:
Ef = J (-^source — ^t)2 + (-^source — &> + (bsource — Ъ^ , (2)
where L*souce, a* source , b*source are the CIELAB coordinates of the source color of the material in the photograph after t hours of irradiation; and Lt, at, bt are the CIELAB coordinates of the dyed surface color in the photograph after t hours of irradiation.
The calculation of the color degradation efficiency (%) is:
盛=
|Et — ^source
^source
If the indicator reaches 20% after 4 hours and 50% after 26 hours, the material is considered self-cleaning.
RESULTS AND DISCUSSION
The ratio of components used to synthesize the anatase-silica additive was selected based on the TiO2 and SiO2 ratio upon completion of synthesis. Table 1 presents the number of components per laboratory synthesis.
The surface coating of microsilica with anatase was assessed using electron microscopy (Fig. 1).
The microphotograph of the additive with the minimum TiO2 content (Fig. 1a, composition #1 as per Table 1) shows TiO2 on the surface of the microsilica
CONSTRUCTION MATERIALS SCIENCE particles in separate areas covering no more than 10 % of the surface. The elemental analysis also shows a relatively small amount of titanium relative to silica, which confirms that TiO2 is insufficient to cover the surface of the microsilica particles. An increase in the TiO2/SiO2 ratio increases the area covered with anatase (Fig. 1b, 1c, and 1d). The elemental analysis spectra also show that the amount of TiO2 relative to SiO2 increases, which confirms an increase in the area of anatase distribution over the surface of the microsilica particles. Notably, even if the amount of anatase relative to microsilica is at the maximum of the range, silica reflections do not disappear completely, i.e. the particles are not completely covered with anatase.
X-ray phase analysis confirms the formation of TiO2 in the required anatase modification, while with an increase
Table 1. The ratio of initial components to synthesize the additives
|
TiO2/SiO2 ratio in the additive |
Ethyl alcohol, g |
Surfactant OP-10, g |
Tetrabutoxytitanium, g |
Microsilica, g |
|
1:2 |
36.0 |
4.0 |
7.17 |
4.0 |
|
1:1 |
72.0 |
4.0 |
14.33 |
4.0 |
|
1:0.67 |
108.0 |
4.0 |
21.50 |
4.0 |
|
1:0.5 |
144.0 |
4.0 |
28.67 |
4.0 |
|
1:0 |
36.0 |
4.0 |
7.17 |
0.0 |
b) TiO2/SiO2 1:1
а) TiO2/SiO2 1:2
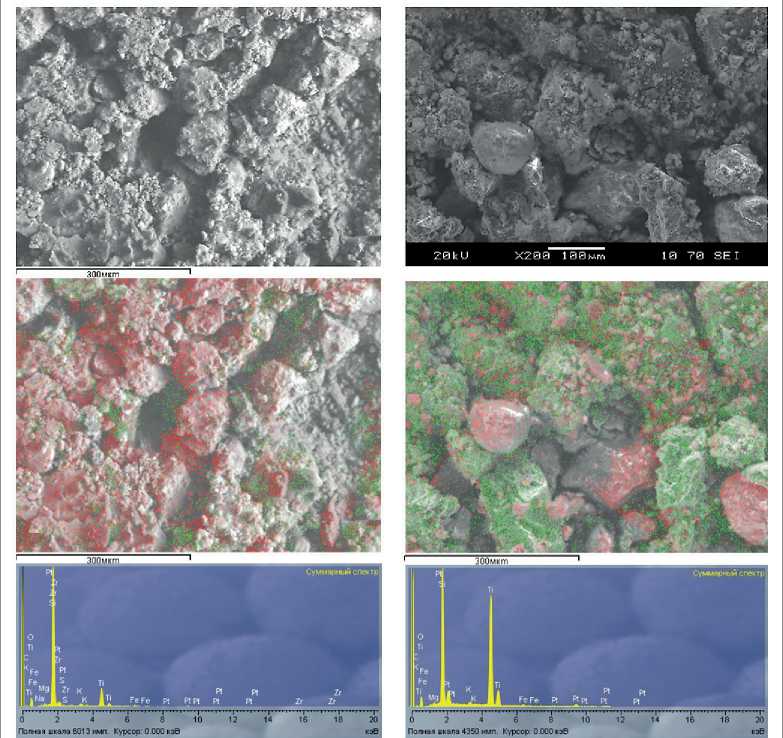
Fig. 1. Microphotographs of additives with different TiO2/SiO2 ratios
CONSTRUCTION MATERIALS SCIENCE
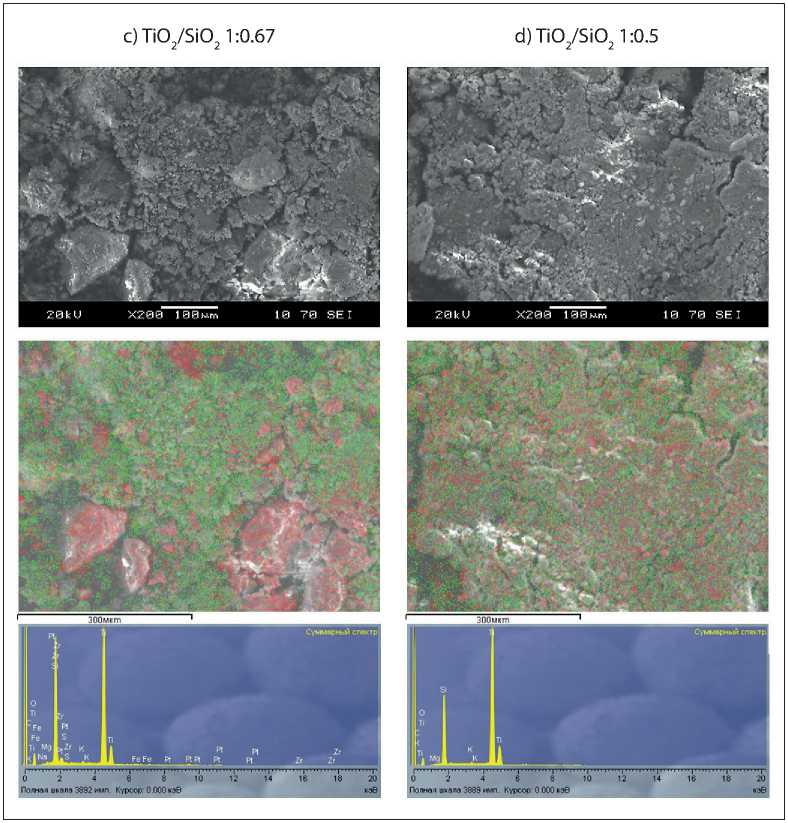
-
Fig. 1. The End
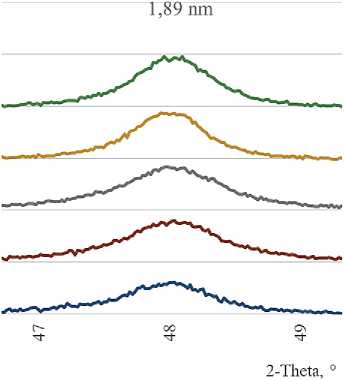
in the TiO2/SiO2 ratio in the diffraction patterns, the height of the main anatase reflections naturally increases (Fig. 2).
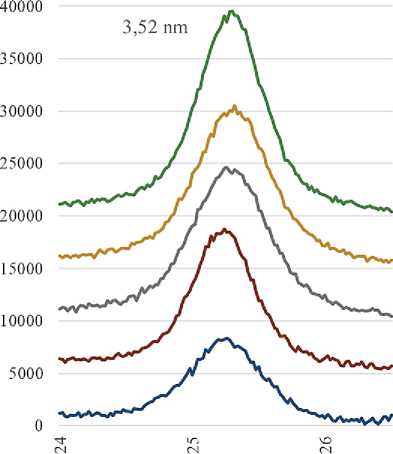
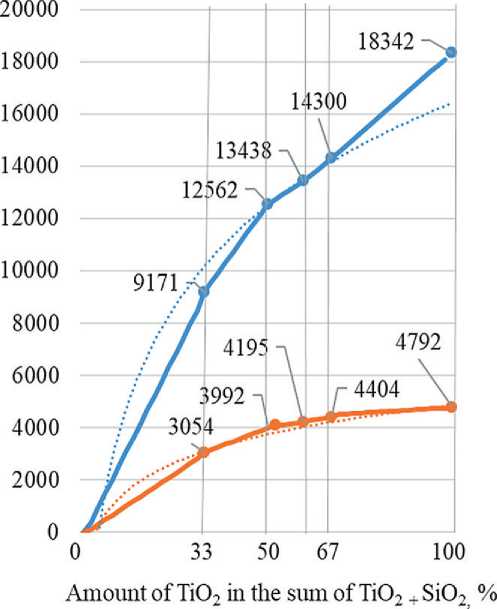
We also found that the increase in the anatase reflection intensity in the X-ray phase analysis of the additives with an undisturbed deposited layer is not proportional to the change in the TiO2/SiO2 ratio. The resulting dependence has a form close to logarithmic (Fig. 3).
The analysis of the width of the diffraction anatase reflections at their half-height shows that the variations in the peak width are insignificant, which indicates that the size of the anatase crystallites is close and reaches 125–145 nm for hkl 101 and 118–155 nm for hkl 200. Taking into account the specific surface area of microsilica, anatase particles of this size cannot cover the microsilica surface completely. However, for creating a photocatalytic additive, the uniform distribution of anatase over the surface in no more than one layer is more important than covering the substrate completely to ensure the maximum interaction area with a minimum amount of TiO2.
To identify the optimal TiO2/SiO2 ratio in the additive for self-cleaning, we carried out a rhodamine test on tablet samples of hardened cement paste (made of normal-thickness paste) with additives in an amount of 1%. Photographs of the samples before and after 26-hour irradiation are shown in Figure 4; the results of image processing are shown in Table 2 and in Figure 5.
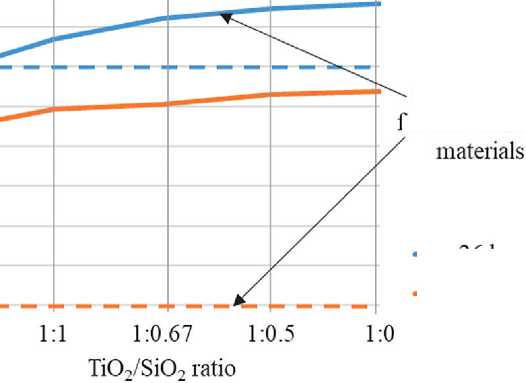
According to the dependence, with an increase in TiO2 in relation to SiO2 in the composition of the samples, the self-cleaning efficiency increases and reaches the required level at the oxide ratio in the additive of slightly less than 1:1, which corresponds to 1 g of TiO2 consumption per specific surface area of microsilica of about 22 m2. A higher distribution of the photocatalytic additive over the surface of the microsilica substrate increases the efficiency of the additive but also increases the cost.
CONSTRUCTION MATERIALS SCIENCE
—1:2 —1:1 —1:0.67 —1:0.5 —1:0
uQS/du.a^ISUOUI
-
Fig. 2. Main diffraction anatase reflections in diffraction patterns
TiO2/SiO2 ratio
1:2 1:1 1:0.5
1:0
о^и^д^^ISSWI
^^_3.52 ши
^^^^ 1.89 іші
Logaritlimic (3.52 шіі) Logai'ithmic (1.89 urn)
Fig. 3. The dependence of the peak height on the TiO2/SiO2 ratio in the initial mixture for the synthesis
CONSTRUCTION MATERIALS SCIENCE
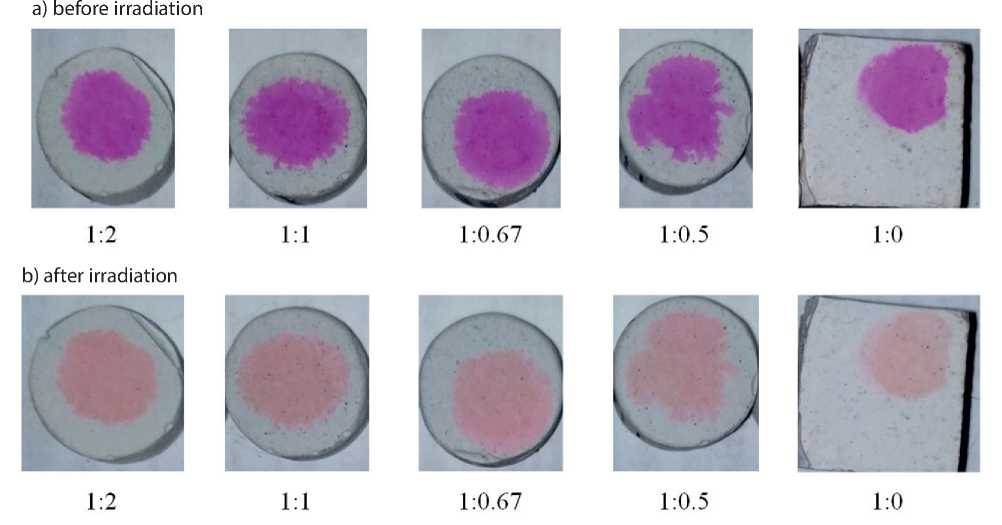
Fig. 4. Photographs of cement samples before and after 26-hour irradiation
Table 2. Color degradation on the cement sample surface
:2
required level for self-cleaning
^—26 hours
^—4 hours
Fig. 5. The dependence of color degradation on the cement sample surface on the TiO2/SiO2 ratio
CONSTRUCTION MATERIALS SCIENCE
CONCLUSION
We synthesized a series of anatase-silica additives by depositing anatase on the surface of microsilica using sol-gel technology, varying the amount of anatase relative to the substrate in the range of TiO2/SiO2 ratios from 1:2 to 1:0.5. Electron microscopy showed that anatase is distributed over the surface of the microsilica in a uniform layer and the surface coverage area increases with an increase in the amount of microsilica. In the range of oxide ratios, microsilica particles are not completely covered with anatase, but over most of the surface. This is confirmed by a nonlinear increase in anatase reflections
in diffraction patterns with an increase in the TiO2/SiO2 ratio and by calculating the surface coverage area taking into account the 118–155 nm size of anatase crystallites, if we assume that anatase crystallites are distributed over the surface in a single layer.
With an increase in TiO2 relative to SiO2, the selfcleaning efficiency increases and reaches the required level when the ratio of oxides in the additive is slightly less than 1:1, which means that 1 g of TiO2 is enough per specific surface area of the substrate of about 22 m2 to create an effective photocatalytic additive. The results can be used in the production of technically and economically effective photocatalytic additives for self-cleaning cements.
Список литературы Anatase-silica additive for self-cleaning materials based on cement
- Ragesh P., Anand Ganesh V., Nair S. V., Nair A.S. A review on “self-cleaning and multifunctional materials.” Journal of Materials Chemistry A. 2014; 2(36): 14773-14797. https://doi.org/10.1039/c4ta02542c
- Topçu I.B., Akkan E., Uygunoğlu T., Çalişkan K. Self-Cleaning Concretes: An Overview. Journal of Cement Based Composites. 2020; 1(2): 6-12. https://doi.org/10.36937/cebacom.2020.002.002
- Neves J.C., Mohallem N.D.S., Viana M.M. Self-cleaning materials: Concepts, properties and applications. Revista Virtual de Quimica. 2021; 13(2). https://doi.org/10.21577/1984-6835.20210003
- Castro-Hoyos A.M., Rojas Manzano M.A., Maury-Ramírez A. Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials. Coatings. 2022; 12(7): 1-21. https://doi.org/10.3390/coatings12070968
- Hamidi F., Aslani F. TiO2-based photocatalytic cementitious composites: Materials, properties, influential parameters, and assessment techniques. Nanomaterials. 2019; 9(10): 1-33. https://doi.org/10.3390/nano9101444
- Padmanabhan N.T., John H. Titanium dioxide based self-cleaning smart surfaces: A short review. Journal of Environmental Chemical Engineering. 2020; 8(5): 1-22. https://doi.org/10.1016/j.jece.2020.104211
- Li X., Simon U., Bekheet M.F., Gurlo A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies. 2022; 15(15): 1-52.
- Wang Z., Liu Y., Huang B., Dai Y., Lou Z., Wang G., Zhang X., Qin X. Progress on extending the light absorption spectra of photocatalysts. Physical Chemistry Chemical Physics. 2014; 16(7): 2758-2774. https://doi.org/10.1039/c3cp53817f
- Strokova, V. V, Gubareva, E.N., Baskakov, P.S., Ogurtsova, Y.N., Antonenko, M.V, Abzalilova, A.V. Fotokataliticheskaya aktivnost` kompozicionnogo materiala, poluchennogo metodom zol`-gel` osazhdeniya tio2 na kremnezemny`j nositel`[Photocatalytic activity of composite material obtained by the method of sol-gel deposition of tio 2 on silica support] Vestnik texnologicheskogo universiteta. 2020; 23(10): 5–9 (In Russ.)
- Drelich J., Marmur A. Physics and applications of superhydrophobic and superhydrophilic surfaces and coatings. Surface Innovations. 2014; 2(4): 1-17. https://doi.org/10.1680/si.13.00017
- Gubareva E.N., Strokova V.V., Ogurtsova Y.N., Baskakov P.S., Singh L.P. Composition and properties of tio2 sol to produce a photocatalytic composite material. Key Engineering Materials. 2020; 854: 45-50. https://doi.org/10.4028/www.scientific.net/KEM.854.45
- Labuzova M.V., Gubareva E.N., Ogurtsova Y.N., Strokova V.V. Ispol`zovanie fotokataliticheskogo kompozicionnogo materiala v cementnoj sisteme [Use of the Photocatalytic Composite Material in the Cement System]. Stroitel’nye Materialy. 2019; 770(5): 16–21. https://doi.org/10.31659/0585-430X-2019-770-5-16-21 (In Russ.)
- Son B.T., Long N.V., Nhat Hang N.T. Fly ash-, foundry sand-, clay-, and pumice-based metal oxide nanocomposites as green photocatalysts. RSC Advances. 2021; 11(49): 30805-30826. https://doi.org/10.1039/d1ra05647f
- Cong Y., Zhang J., Chen F., Anpo M., He D. Preparation, photocatalytic activity, and mechanism of nano-TiO2 Co-doped with nitrogen and iron (III). Journal of Physical Chemistry C. 2007; 111(28): 10618-10623. https://doi.org/10.1021/jp0727493
- Khairy M., Zakaria W. Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egyptian Journal of Petroleum. 2014; 23(4): 1-8. https://doi.org/10.1016/j.ejpe.2014.09.010
- Lezner M., Grabowska E., Zaleska A. Preparation and photocatalytic activity of iron-modified titanium dioxide photocatalyst. Physicochemical Problems of Mineral Processing. 2012; 48(1): 193-200.
- Pal B., Sharon M., Nogami G. Preparation and characterization of TiO2/Fe2O3 binary mixed oxides and its photocatalytic properties. Materials Chemistry and Physics. 1999; 59(3): 254-261. https://doi.org/10.1016/S0254-0584(99)00071-1
- Torres-Luna J.A., Sanabria N.R., Carriazo J.G. Powders of iron(III)-doped titanium dioxide obtained by direct way from a natural ilmenite. Powder Technology. 2016; 302: 254-260. https://doi.org/10.1016/j.powtec.2016.08.056
- Arakelyan G.A., Karapetyan A.K., Badalyan M.M., Ghahramanyan A.A., Makarov E.M. Increasing the Efficiency of Fine-Grained Lightweight Concrete Using Complex Additives. Journal of Architectural and Engineering Research. 2022; 2: 3-8. https://doi.org/10.54338/27382656-2022.2-001
- Karlina A.I., Karlina Y.I., Gladkikh V.A. Analysis of Experience in the Use of Micro- and Nanoadditives from Silicon Production Waste in Concrete Technologies. Minerals. 2023; 13(12): 1-34.
- Žvironaitė J., Pundienė I., Gaidučis S., Kizinievič V. Effect of different pozzolana on hardening process and properties of hydraulic binder based on natural anhydrite. Journal of Civil Engineering and Management. 2012; 18(4): 530-536. https://doi.org/10.3846/13923730.2012.702126
- Amrhein K., Stephan D. Principles and test methods for the determination of the activity of photocatalytic materials and their application to modified building materials. Photochemical and Photobiological Sciences. 2011; 10(3): 338–342. https://doi.org/10.1039/c0pp00155d


