Anti-tumor, anti-edematous and analgesic effects of transcranial magnetic therapy in complex treatment of brain tumors of a high degree of malignancy
Автор: Popov Ivan A., Shikhlyarova Alla I., Rostorguev Eduard E., Frantsiyants Elena M., Rozenko Lyudmila Y., Iozefi Dmitry Y., Gusareva Marina A., Zhukova Galina V., Kit Oleg I., Engibaryan Marina A.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 17, 2020 года.
Бесплатный доступ
The results obtained in this study have demonstrated a possibility to increase the efficacy of the complex treatment of patients with brain tumors of a high degree of malignancy with the use of factors of the magnetic-wave nature, including low-intensity PMF and ELF MF, which can be integrated into the adjuvant therapy scope.
Glioblastomas, electromagnetic fields, apoptosis, neurogenic pain, anti-edematous effect, correction of phsychosomatic and vascular disorders
Короткий адрес: https://sciup.org/148311472
IDR: 148311472 | DOI: 10.12710/cardiometry.2020.17.2229
Текст научной статьи Anti-tumor, anti-edematous and analgesic effects of transcranial magnetic therapy in complex treatment of brain tumors of a high degree of malignancy
Ivan А. Popov, Alla I. Shikhlyarova, Eduard Е. Rostorguev, Elena М. Frantsiyants, Lyudmila Y. Rozenko, Dmitry Y. Iozefi, Marina А. Gusareva, Galina V. Zhukova, Oleg I. Kit, Marina A. Engibaryan. Anti-tumor, anti-edematous and analgesic effects of transcranial magnetic therapy in complex treatment of brain tumors of a high degree of malignancy. Cardiometry; Issue 17; November 2020; p.22-29; DOI: 10.12710/cardiometry.2020.17.2229; Available from:
At present, observed is a higher rate of detecting malignant brain tumors (MBT) [1,2,3,4]. Among the primary brain tumors, the glial tumors are found in 35,5% of the cases, and glioblastomas account for 80% of all high-grade gliomas (HGG); they both show the 22 | Cardiometry | Issue 17. November 2020
most severe malignance degree – Grade III-IV [5] and are at the same time the most prevalent type of the adult brain tumors [6, 7].
Despite the fact that MBT have a small share in the total structure of malignant tumors with accounting for 2%, this sort of the cancer tumors is associated with a high lethality rate, when a survival median time may reach a few months only [8,9]. A pronounced high lethality is recorded in individuals aged under 30 (10,44%), which reaches 20% referred to tumors of all localizations, respectively, and which is ranked in the second place according to this indicator [8]. In general, in accordance with the data supplied by the Association of Russian Oncologists (2014), the 50% survival for patients with malignant gliomas does not exceed 1 year [10]. In this case, we should mention that quality of life of these patients in their post-surgery period is considerably deteriorated due to the starting surgery and drops dramatically after chemoradiotherapy.
An aggressive progression of malignant gliomas in the brain and their resistance to treatment are due to uncontrollable proliferation of tumor cells, disorders in the regulation of apoptosis and intense processes of angiogenesis [11,12,13]. As a consequence of the intensive proliferation, an abnormal increase in the number of malignant cells and in the tumor sizes takes place; appearing hypoxia is accelerated; the endothelium is damaged; thrombosis develops [14]. In the MBT pathogenesis, edema of the far perifocal area of the tumor bed plays a large part that causes compression of the involved brain structures at the stage of radiotherapy. As a result from later radiation damages, neurocognitive disorders, dementias, and finally post-radiation necroses develop. Considering the above, it should be stated that prevention of the clinical symptoms and especially retardation and avoidance of further growth of the tumor within the far perifocal area of the tumor bed, mitigation of developing post-radiation edema syndrome, pain relief and improvement of quality of life in this sort of cancer patients are challenging issues in treatment of the brain malignant gliomas. Searches for new ways of the accompanying therapy in neuro-oncology show new candidates for adjuvant treatment methods to be developed like neutron therapy [18,19], radio-sensibilisation of the tumor [20,21,22], brain tumor brachytherapy [23,24], and the TTF therapy involving the use of electrical fields. However it is not always the case with the avoidance of adverse local and systemic disorders in CNS that results in a decline in the resistance, shortening of survival time and deterioration of the life quality in the patients. Fresh opportunities are opened up by a noninvasive technology of transcra-nial magnetotherapy (TMT) as an additional method in treatment in the rehabilitation of patients with intramedullary tumors in the early post-surgery period [25]. Besides, in order to increase the efficacy of magnetotherapy with extremely low frequency magnetic field (ELF MF), designed and developed has been a scientific basis for the activation therapy, the principles of which have been generalized and translated into oncological clinical practice [26,27,28,29]. Therefore it seems reasonable to design a new technology of the accompanying activation therapy which represents a combination of advanced techniques of the transcranial local (referred to the tumor bed) pulsed and the central (projected on the hypothalamus) ELF MF influence at the stages of the complex therapy including radiation therapy of malignant glial tumors.
The aim of this study is to increase the efficacy of the complex treatment of patients with the malignant brain gliomas by employing a combination of the pulsed and extremely low frequency transcranial magnetotherapy in the early post-surgery period at the stage of the radiotherapy, based on some experimental developments of the modes of the applied factors and some studies on certain mechanisms of their actions and effects.
Materials and methods
-
1. Fundamental research work. Our experiments have been conducted with the use of most advanced high-tech equipment designed for molecular genetics studies and with applications of actions produced by a pulsed magnetic field with different intensities, based on an original approach to development of certain frequency algorithms close to the endogenous rhythms in the human brain.
-
2. Gamma therapy has been provided with Ther-atronEquinox produced by BestTheratronics, one of the Cobalt-60 based external beam systems. The radiation treatment area measuring 12х18 cm has been matched the well plate dimensions, and the gamma radiation dose for each well has reached 10 Gy.
-
3. A pulsed electromagnetic exposure has been produced with therapy extended-type device Neu-ro-MS/D manufactured by Neurosoft using the frequency mode as follows: F= 0,3 Hz (5min.) -3,0 Hz (1min.) - 9,0 Hz (1min.), t total= 7 min with different induction parameters B= 300 mT and 15mT.
-
4. Clinical research. The basis of this research work is data on 50 patients with malignant glial supratentorial tumors of the brain, which have received treatment in the Neuro-oncology Department in FSHI at the Oncology Research Center, the Ministry of Healthcare of the Russian Federation, within the period between 2018 and 2020 inclusive. All research reports and records have been prepared in accordance with the ethical standards of The Declaration of Helsinki (1964, Revision 2013) and approved by the Ethics Committee at the Rostov Research Institute of Oncology of the Ministry of Healthcare of Russia (Research Ethics Compliance Findings Record No.19 dd.06.10.2017). All patients involved therein have given their written
-
5. Scheme of complex treatment. At stage one, surgery has been performed that has been supported by advanced high-tech approaches such as craniotomy including a reconstructive cranioplasty, cytoreductive removal of the tumor limited by the visible area of the healthy tissues. 2 weeks after surgery, a post-operative contrast-enhanced MRI scan has been produced, and the required clinical laboratory tests have been completed. In case of repeat admissions, the patients were subjected to the conform radiotherapy (with an energy of 6 MEV) with Linear Accelerator Varian Novalis for treating deep-seated tumors, according to the classical dose fractionation with delivery of a single focused dose of 2 Gy till reaching a focused dose total of 60 Gy. The radiotherapy sessions have been conducted one time a day to cover 5 consecutive days a week, with the treatment course duration of 6 weeks.
-
6. Design of original technology of applying tran-scranial magnetotherapy Course 1 of the original TMT has involved 10 sessions, starting from after-sur-
24 | Cardiometry | Issue 17. November 2020
In our experimental trials we have used human glioblastoma cell culture T98G. The T98G cell culture has been maintained in the 12-well-plate (JetBioFil, China) at a temperature of 37 Сº under controlled atmosphere conditions with 5% CO2 and 95% humidity in humidified cell culture incubator CB 150 (Binder, Germany); for this purpose, used has been condi- tioned medium RPMI-1640 (Biolot, Russia) containing 10% of the fetal calf serum (Biolot, Russia) and 50 µg/ml Gentamicin (Biolot, Russia).
Upon reaching 75-80% of confluence, the conditioned medium has been removed and replaced with the fresh medium in all wells.
In this case, an important point is that the basis for this original innovative development, representing a technology of delivering exposure of pulsed magnetic field (IMF) and extremely low frequency magnetic field (ELF MF) is the activation therapy designed to induce the desired response patterns built upon the principles of interactions of living systems with factors of the wave nature. First and foremost we have taken into account a high sensitivity of biological systems to low-intensity actions and exposures, focusing on an approximation to synchronization of the oscillatory activity of the factors of the endogenous and exogenous nature, a construction of an algorithm of frequencies with adhering to the principle of multiplicity and an application of the mathematical exponential function law, when varying the dose (intensity) of the exposure.
informed consent to be involved in research with processing their personal data.
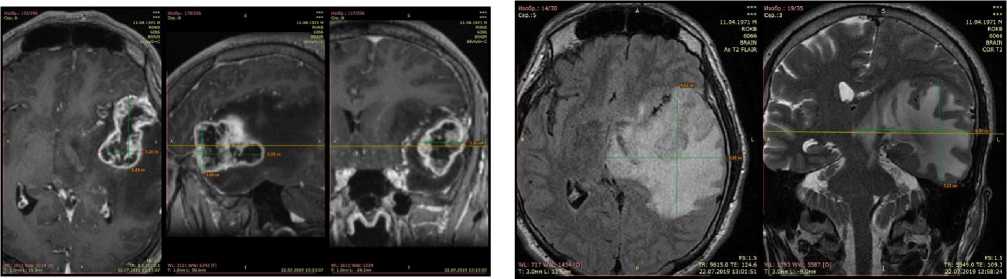
According to the randomization requirement, the patients have been divided into 2 groups: the main test group covering 25 patients suffering from the malignant brain gliomas in functionally critical regions (the region of central gyri, temporal lobe of the dominant hemisphere), which have received the standard surgery treatment supplemented with 2 TMT courses: one course at the early post-surgery stage and another at the stage of the radiotherapy (RT). The reference group has included 25 patients with malignant glial supratentorial tumors of the brain, who have received the standard complex therapy that involves surgery and radiation therapy, but without the TMT supplement. Before surgery, the patients have undergone the following examinations: neurological examinations, the brain MRI with the BRAVO sequence with the intravenous injection of a contrast agent, complete blood count (CBC) and biochemical blood test, ECG supported by therapeutist’s consultation, examination of external respiration function and electroencephalogram imaging. The initial levels of the neurological deficit have been assessed with the use of the Barthel scores, the Karnofsky Performance Status Scale, the Montreal Cognitive Assessment (MoCA), the NIH Stroke Scale/Score (NIHSS); we have identified disorders of cognitive functions, quality of life and functional independence in the said cohorts of the patients.
gery day 2 till day 6, to be performed every day (5 sessions) and subsequently at a day interval till the patient’s hospital discharge. Course 2 of the accompanying TMT has consisted of 15 sessions, which have been conducted at a day interval throughout the entire period of radiotherapy. Each TMT session has incorporated two actions: the first action has been produced in the morning with magnetotherapy equipment Gradient-4M, which has induced ELF MF applied to the projection of the hypothalamus in an algorithm of frequencies as follows: 0,3 Hz (t=5 min.) – 3 Hz (t=1 min.) – 9 Hz (t=1 min) with an induction (B) from 3 to 1mT (in the mode according to the exponential law). The second therapy exposure has been delivered 2,5-3 hours later with the use of PMF device Neuro-MSD (manufactured by Neurosoft, Ivanovo, Russia) for treating the perifocal area of the far tumor bed using the frequency mode as follows: 0,3 Hz (t=5 min.) – 3 Hz (t=1 min.) -9 Hz (t=1 min.) with B=15mT. In the RT course, the first therapy action has been provided in the morning followed by the RT conform session conducted 1-1,5 hour later with a single fractioned dose of 2 Gy; subsequently, upon expiration of 1-1,5 hours, the second exposure to PMF has been delivered.
Results of research
We have conducted 3 sets of experiments with human glioblastoma cell culture T98G delivered by the Russian National Cell Culture Collection at the Research Institute of Cytology of the Russian Academy of Sciences.
The selection of the required parameters of the pulsed magnetic field combined with radiotherapy has demonstrated the advantage of the PMP with an induction of 15 mT over 300 mT recorded 24 hours after the exposure. It has manifested itself in a rise of lethality of cell culture T98G practically by 3 times as against the reference (see Table 1 herein). While the contribution of the radiotherapy to the suppression of the tumor growth has been expected, the independent effect produced by PMF could not be anticipated, since it has exceeded the reference level of lethality by 2,3 – 2,8 times and reduced the values of the mythot-ic index by 3,7 times that is evidence of the access of this action to the intimate mechanisms of the tumor growth.
Our analysis of the molecular genetics data has shown there is a considerable impact produced by
Table 1. Cell counting, lethality and mythotic index under different experiment conditions upon expiration of twenty four hours from actions/exposures
|
Live cells |
Dead cells |
A total cell count |
Lethality |
Mythotic index |
|
|
Reference |
961666,7± 27556,3 |
58166,66 ± 4342,8 |
1019833,3 ± 30653,5 |
5,7% |
6,6% |
|
10 Gy |
510000,0 ± 35087,2* |
116833,3 ± 8887,2* |
626833,33 ± 42369,9* |
18,6% |
4,4% |
|
15 mT |
975000 ± 40875,8 |
153833,3 ± 8150,3* |
1128833,3 ± 45156,3 |
13,6% |
1,8% |
|
300 mT |
1148000 ± 31763,0 |
156400,0 ± 8920,6* |
1087000,0 ± 95432,1 |
14,4% |
2,8% |
|
10 Gy+15 mT |
760000 ± 47128,7 |
165500,0 ± 6815,6* |
925500,0 ± 46752,4 |
17,9% |
3,5% |
|
10 Gy+300 mT |
813333,33 ± 21464,9 |
143333,3 ± 3873,9* |
956666,7 ± 23988,6 |
15% |
3,1% |
Note: * - statistically significant differences from the reference, р ≤0,05
PMF on the expression of the apoptosis genes. Upon expiration of 24 hours from the exposure to PMF with an induction of 15 mT we have recorded the strongest response for locus P53, which has exceeded the reference by 1,9 times, as well as for loci Bcl2 and Casp3 by 1,8 and 1,4 times, respectively (see Table 2 herein).
The completed analysis of the efficacy of the biotropic parameters of PMF has made it possible to translate this method into clinical practice using the designed program-assisted mode of the double tran-scranial action that involves ELF MF and TMT.
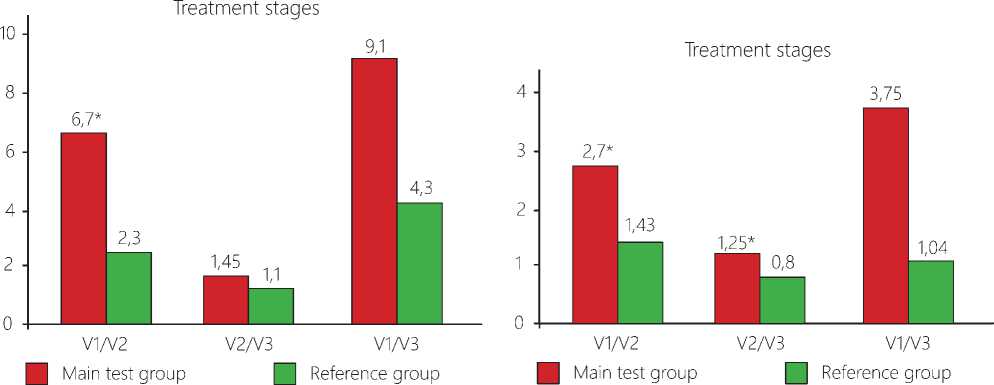
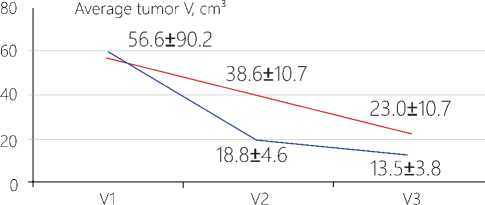
Change in the tumor volume. When assessing the data on the neuro-vizualization and three-dimensional estimation of the tumor volume before surgery (V1), no significant differences between the data in the main test group and those in the reference cohort have been found (р=0,7661). At the TMT stage, in the post-surgery period, we have observed in the patients a decrease in absolute values of the residual tumor volume in the patients of the main test group by 2,0 times as against the reference group. A similar dynamics has been also traced when computing differences between the groups in the V1/V2 ratios (see Figure 1 herein). At the stage of radiotherapy these differences have been anticipated due to a high efficacy of the gamma therapy that has supported the suggestion on indifference of an interaction of the physical factors of the ionizing and non-ionizing nature.
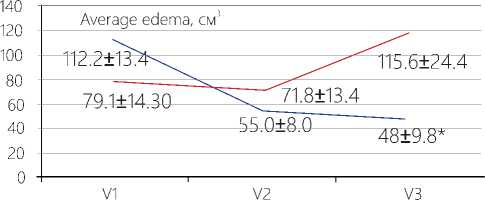
Change in the edema volume. Upon completion of the first course of TMT (before the radiotherapy), the absolute values of the edema volume have decreased in the average by 50% that is in agreement with the data demonstrating a decrease by 1,9 times in the ratio between the average volume value of the perifocal edema of the far tumor bed and its initial volume (V1/V2), as compared with the respective ratio recorded in the reference group (p= 0,0392). Judging from the absolute averaged values of the volume of the perifocal edema after RT with TMT, its value has become 2,4 times less than it is the case in the reference group. It has been also confirmed by the V1/V3 ratio, i.e. throughout the entire period of the dynamic observation, beginning with the moment of surgery and ending with the RT completion, when the difference in the edema ratio data between the main test group and the reference cohort has been found to be 3,6 higher (p=0,0180).
It is evident that an application of TMT in the early post-operative period, as well as in the course of the radiotherapy, has activated the membrane mechanisms of the K+-Na+ pump in the cells, promoting the transport of the Na+ cations from the interstitial space to the vascular bed. It is conceivable that due to an increase in the concentration gradient, “free” water also streams to the vascular bed, reducing in such a manner the edema in the interstitial space and the pressure-induced strains of the tissues that provokes pain.
The dynamics of the neurologic status in the MBT patients initially has shown practically no difference in each group. However on post-surgery day 7, upon completion of 5 sessions of TMT, a tendency could be identified that the patients in the main test group have experienced an earlier recovery without feeling pain. At the time of hospital discharge, upon completion of course one of TMT, recorded has been the absence of the neurological symptoms with complete pain relief in 84±7,3% of the patients in the main test group, while this sort of the recovering patients in the reference group has accounted for only 48±6,5% (p=0,0072*). Neurologic disorders of a mild to moderate degree (3-8 according to the NIHHS score) have been revealed in 12±6,5% of the patients in the main test group and in 40±9,8 % of the patients in the reference group (p=0,0240*). The majority of the patients in the main test group, who have received TMT, namely 72±9,0% against 48±10,0% in the reference cohort, even on post-surgery day seven have demonstrated that they are “able to care for self, but unable to carry on normal everyday activity” (Karnofsky grade 70-80).
Table 2. Relative expression of genetic loci
|
Experiment conditions |
Genetic loci |
|||||
|
Exposure |
Action |
BCL2 |
BAX |
P53 |
MDM2 |
CASP3 |
|
3 hours |
Reference |
220,3 |
6,5 |
1,5 |
5,9 |
5,6 |
|
15 mT |
188,0 |
7,1 |
1,6 |
5,9 |
5,4 |
|
|
300 mT |
235,2 |
7,8 |
1,8 |
6,0 |
6,3 |
|
|
24 hours |
Reference |
143,8 |
13,7 |
1,3 |
11,0 |
5,5 |
|
15 mT |
226,8* |
15,0 |
2,7* |
13,3 |
7,6* |
|
|
300 mT |
201,9* |
13,7 |
2,4 |
13,1 |
6,9* |
|
Note: р˂0,05*- statistically significant differences between experiment and reference.
Р<0,05* Р<0,05*
Main test ___ Reference ___ Main test ___ Reference p=0 0079*
group group group group
Figure 1. Changes in the tumor volumes at the stages of the complex treatment with an application of TMT. Notes: An averaged volume of tumor in absolute units: V1 – the volume before surgery; V2 - the volume before radiotherapy; V3 - the volume after radiotherapy. *The differences are significant for ratio V1/ V2 only, Р<0,05.
Figure 2. Data on the volume of the perifocal bed of the tumor in patients at the stages of complex treatment with use of TMT. Note: V1 – before surgery; V2 – before radiotherapy; V3 – after radiotherapy. *The differences are significant between the data recorded in the main test group and the reference cohort for V3 (р=0,0079) as well as for their ratios V1/ V2, V2/ V3, V1/ V3, р<0,05.
At the time of hospital discharge, upon completion of the radiotherapy course, in the main test group, subjected to the 2nd TMT course, we have observed 92,0±5,4% of the cases of the successful rehabilitation of the patients versus 68,0±9,3% of those in the reference group (p=0,0391*). In this case, the number of patients in the main test group, who have demonstrated the absence of the cognitive disorders (MoCA scoring), have statistically significant differences from that of the patients free of the above disorders in the reference cohort (p=0,0099*) that supports the tendency noted before. Upon completion of the RT course, an assessment of the Barthel Index for Activities of Daily Living (ADL) has shown that the number of the functionally independent patients in the reference group has decreased to 44±9,9%, while in the main test group the functional independence has been recorded in 88±6,5% of the cases (p=0,0019*). Upon RT in combination with TMT, the restoration of the performance status with Karnofsky grade 90 has been reported for 60±9,8% of the patients in the test group. As to the reference group, on the contrary, the 90 Karnofsky index has been reached only in 28±9,0% of the cases (p=0,0271*), and the patients there have presented their complaints of feeling worse, appearance of the feeling of compression and headaches.
The immediate results from the completed complex therapy in the HGG patients in the examined groups bear witness to the fact that the median of the event-free survival (EFS) in the main test group accounts for 4,5 months that exceeds the respective parameter of 2 months in the reference group by 2,5 times (Log-Rank test). An overall six-month survival in the patients received TMT and RT has been recorded to be 84,2±10,8%, while the reference group has demonstrated this indicator at a level of 40,3±10,5% (р≤0,05) that is a confirmation of an increase in the efficacy of the adjuvant TMT.
Conclusion
The required expert appraisal of clinical safety and the necessity of the pathogenetic justification of the offered new technology has supported our conceptual idea that both a single-factor PMF and the RT-PMF combination are characterized by a considerable rise in the number of the dead cells of human malignant glioma T98G, a decline in their mythotic activity, a reliable response by the respective apoptosis genes and most pronounced expression of genetic locus Р53. The above data have demonstrated that the intimate mechanisms of the tumor growth are accessible to the targeted action produced by PMF.
The algorithm of TMT designed to be translated into clinical practice has implemented a compromise approach: the PMF impact on the tumor bed under the preliminary influence on the hypothalamus projection region by ELF MF in the double action mode of the activation therapy [30-33]. That has resulted first in an initiation of the operation of the mechanisms responsible for an integral response, and, second, upon expiration of a certain time, in the production of a local response by the cells in the residual tumor and the perifocal edema, induced by the pulsed-type field. As is can be seen, the general and the local actions, which are addressed to different neuronal systems of the brain, including the malignant glial cells, due to synergism of the major bio-parametric signals of PMF and ELF MF have generated their general output: the anti-tumor, anti-edematous and analgesic effects.
Considering important implications of the neuronal systems for the realization of the cortex activity and the formation of the psychosomatic status in patients with brain tumors of a high grade of malignancy, another suggestion along similar lines is that TMT has promoted a transition to the point of return (using the definition proposed by V.I.Orlov – please, refer to the previous paper) and favored the return to physiological region Smile-of-the-Life. To a certain extent, at the same time, we may say that the pathologically altered glial cells have reached their point of no return that has triggered an improvement of quality of life and contributed to the immediate beneficial treatment outcomes in the HGG patients.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Acknowledgments
The reported study was funded by RFBR, Project Number 19-315-90082\19.
Список литературы Anti-tumor, anti-edematous and analgesic effects of transcranial magnetic therapy in complex treatment of brain tumors of a high degree of malignancy
- Antonenkova NN, Yakimovich GV, Pashkevich AM, Rubtsov SI. malignant glial brain tumors in the Re-public of Belarus: morbidity and survival, 2000-2014. Oncological journal. 2016;10(4):58-68.
- Zhuykova LD, Choinzonov EL, Ananina OA, Odintsovo IN. Oncological morbidity in the Siberian and far Eastern Federal districts. Siberian journal of Oncology. 2019;18(6):5–11. doi: 10.21294/1814-4861-2019-18-6-5-11.
- Gafur-Akhunov M. A., et al. Dynamics indicators of morbidity and mortality of brain tumors in the Re-public of Uzbekistan. Theses of the XI Congress of oncologists and radiologists of the CIS and Eurasia. Eurasian cancer journal. 2020;8(2):763. [in Russian]
- Davis FG, Smith TR, Gittleman HR, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Glioblastoma in¬cidence rate trends in Canada and the United States compared with England, 1995–2015. Neuro-Oncol¬ogy. Febr. 2020;22(2):301–2. https://doi.org/10.1093/neuonc/noz203
- Jiang H, Cui Y, Wang J, Lin S. Impact of epide¬miological characteristics of supratentorial gliomas in adults brought about by the 2016 world health or¬ganization classification of tumors of the central ner¬vous system. Oncotarget. 2017;8(12):20354–61. doi: 10.18632/ oncotarget.13555;
- Dolma S, Selvadurai HJ, Lan X, et al. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29(6):859–73. DOI: 10.1016/j.ccell.2016.05.002;
- Tamimi AF, Juweid M. Epidemiology and Outcome of Glioblastoma. Glioblastoma. Codon Publications, Brisbane, Australia. 2017;143–153. doi: 10.15586/co¬don.glioblastoma.2017.ch8;
- Kaprin A.D., Mardynsky Yu. S. Therapeutic radiology, Moscow: GEOTAR-Media, 2018, 704 p.; [in Russian]
- Merabishvili V. M. medium-Term variant forecast of mortality of the Russian population from ma-lignant neoplasms. Siberian journal of Oncology. 2019;18(4):5–12. doi: 10.21294/1814-4861-2019-18-4-5-12; [in Russian]
- "Clinical recommendations for the diagnosis and treatment of patients with primary brain tumors", "All-Russian Union of public associations. Association of oncologists of Russia", Moscow, 2014, http://www.oncology.ru/association/clinical-guidelines/2014/32.pdf; [in Russian]
- Kit O. I., et al. The system of regulation of plasmin¬ogen in various brain tumors. Questions of neurosur-gery named after N. N. Burdenko. 2017;81(2): 22-27. DOI:10.17116/neiro201781222-27; [in Russian]
- Nikitin PV, et al. Distribution of the main pop¬ulations of tumor cells in glioblastoma cell clusters and their prognostic value // international journal of applied and fundamental research. 2019;10(1):90-7; URL: https://applied-research.ru/ru/article/view?id=12873. [in Russian]
- Li J, Liang R, Song C, Xiang Y, Liu Y. Prognos¬tic significance of epidermal growth factor receptor ex-pression in glioma patients. Onco Targets Ther. 2018 Feb 7;11: 731-742. doi: 10.2147 / OTT.S155160;
- Colwell N, et al. Hypoxia in the glioblastoma mi¬croenvironment: shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017;19(7):887-96. DOI: 10.1093/neuonc/now258;
- Gribanova TG, et al. Magnetic resonance perfu¬sion in the diagnosis of radiation necrosis in patients with high-grade gliomas after combined treatment. Russian neurosurgical journal named after Professor A. L. Polenov. 2017;9(1):25-9; [in Russian]
- Kartashev AV, et al. Radiotherapy of brain tumors. Practical Oncology. 2013;14(3):156-65; [in Russian]
- Rebrikova VA, et al. Possibilities of Mr perfusion in evaluating the effectiveness of treatment of malignant brain tumors. "Questions of neurosurgery" named af¬ter N. N. Burdenko. 2019;83(4):113-20; [in Russian]
- Barth RF, Zhang Z, Liu T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun (Lond). 2018 Jun 19; 38(1):36. doi: 10.1186/s40880-018-0280-5;
- Sun T, et al. Boron neutron capture therapy in-du¬ces cell cycle arrest and cell apoptosis of glioma stem/progenitor cells in vitro. Radiat Oncol. 2013 Aug 6;8(1):195. doi: 10.1186/1748-717X-8-195;
- Ryabova AI, et al. The value of the factors for conducting thermochemical therapy of glioblas¬toma of the brain. Siberian journal of Oncology. 2018;17(5):27–36. doi: 10.21294/1814-4861-2018-17-5-27-36; [in Russian]
- Dai X, et al. AHIF promotes glioblastoma pro¬gression and radioresistance via exosomes. Int J On¬col. 2019 Jan; 54(1):261270. https://doi.org/10.3892/ijo.2018.4621;
- Yunusova NV, Fedorov AA, Startseva ZA, Yeon JH. Abscopal effect of radiotherapy and hyperther-mia: role of exosomes. Siberian journal of oncology. 2020;19(2):108-115. https://doi.org/10.21294/1814-4861-2020-19-2-108-115;
- Abdullaev OA, et al. Initial results of treatment of recurrent glioblastomas of the brain using resection in combination with intraoperative balloon electron¬ic brachytherapy. Siberian scientific medical journal. 2019;39(4):99-109. DOI 10.15372/SSMJ20190413; [in Russian]
- Krivoshapkin AL, et al. Method of treatment of re¬lapse of malignant gliomas of the brain. Pat. 218110143 RF; Publ. March 22, 2018; [in Russian]
- Burkova EA, et al. Modern methods of rehabili¬tation of patients after removal of intramedullary tu-mors in the early postoperative period. “Fundamental and clinical neurology. transcranial magnetic stimu¬lation: achievements and prospects”. Materials of the first Moscow conference with international participa¬tion (ed. by M. A. Piradov), published in the FGBNU NCN Moscow 2015. p. 9-10; [in Russian]
- Garkavi LK, Ukolova MA, Kvakina EB. Regular¬ity of development of qualitatively different General non-specific adaptive reactions of the body. Available online: http://ross-nauka.narod.ru/03/03-158.html; [in Russian]
- Garkavi LK, Kvakina EB, Kuzmenko TS, Shikhl¬yarova AI. Antistress reactions and activation ther¬apy. Activation response as a path to health through self-organization processes. Yekaterinburg: Philan-throp, 2002. [in Russian]
- Atmachidi DP. Adjuvant chemoradiotherapy using a magnetic field in the complex treatment of malig-nant glial brain tumors. Dissertation. Rostov-on-Don, 2009. [in Russian]
- Chilingaryants SG. Improvement of some methods of postoperative therapy of lung cancer: Dissertation. Rostov-on-don, 2006. 51 p. [in Russian]
- Shikhlyarova AI. the role of biotropic parameters of electromagnetic fields in increasing non-specific antitumor resistance. Dissertation. Rostov-on-don, 2001. 50 p. [in Russian]
- Shikhlyarova AI., Zhukova GV, Atmachidi D, Babieva SM. Efficiency of the activation electromag-netometry in complex treatment of patients with malignant tumors of different localizations. The XIX Intern. Sc. Conf. "Oncology – XXI century", 2015, Svetlogorsk–Perm. P. 252-255. [in Russian]
- Alla I. Shikhlyarova, Galina Y. Maryanovskaya, Ly¬udmila P. Barsukova, Faina M. Zakharyuta, Galina V. Zhukova, Elena P. Korobeinikova, Tatiana P. Protaso¬va, Elena A. Sheiko, Olga F. Evstratova, Tatiana A. Bar¬teneva, Tatiana A. Kurkina, Natalia M. Maschenko. Methodological fundamentals of experimental mag¬netotherapy of tumors (historical essay). Cardiome¬try; No.7; November 2015; p.42-46; DOI:10.12710/cardiometry.2015.7.4246 Available from: http://www.cardiometry.net/issues/no7-november-2015/magne¬totherapy-of-tumors
- Alla I. Shikhlyarova, Galina V. Zhukova, Natalia M. Mashchenko. Some recommendations and exam-ples in the activation therapy practical implemen¬tation (according to L.K. Garkavi, E.B. Kvakina and M.A. Ukolova’s research studies). Cardiometry; No.7; November 2015; p.64-69; DOI:10.12710/cardiome¬try.2015.7.6469 Available from: http://www.cardiom¬etry.net/issues/no7-november-2015/activation-thera¬py-practical-implementation


