Approaches to the gene therapeutic correction of the pathogenetic mechanisms of coronary insufficiency
Автор: Makeev Oleg G., Shuman Evgeny A., Korotkov Arteom V., Kostyukova Svetlana V.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 17, 2020 года.
Бесплатный доступ
The absence of neoangiogenesis in the ischemic myocardium is the reason for the widespread prevalence of cardiovascular diseases. The mechanism is the age-dependent epigenetic blockade of the corresponding genes. In model experiments, it was shown that transfection of ischemic myocardium with plasmid vectors carrying the HIF1a, HIF1b, VEGF165, VEGF225 genes in a stoichiometric ratio of 1: 0.2: 0.5: 0.3, at a concentration of 400 μg / ml of physiological solution at a rate of 200 μg DNA per cm2 of ischemia zone and a step over an area of 2-10 mm, with the addition of an adjuvant2-dimethylaminoethanol at a concentration of 2.5 mmol / L, restores full neoangiogenesis. For the first time, it was shown that as a result of gene therapy of myocardial ischemia using four genes (HIF-1α, HIF-1β, VEGF 225, VEGF 165).In contrast to the known monotherapy methods, a complete vascular network is formed in the ischemic zone, having anatamoses with intact vessels. The scientific novelty of the study lies in the fact that the obtained data open the possibility of correction of epigenetic blocking of gene expression by transfecting cells with copies of these genes without epigenetic labels.
Coronary insufficiency, therapeutic angiogenesis, growth factors, gene plasmids
Короткий адрес: https://sciup.org/148311478
IDR: 148311478 | DOI: 10.12710/cardiometry.2020.17.6675
Текст научной статьи Approaches to the gene therapeutic correction of the pathogenetic mechanisms of coronary insufficiency
of the pathogenetic mechanisms of coronary insufficiency. Cardi-ometry; Issue 17; November 2020; p.66-75; DOI: 10.12710/cardi-ometry.2020.17.6675; Available from: issues/no17-november-2020/gene-therapeutic-correction
Currently, the main approach to the treatment of patients with coronary heart disease is based on the use of either pharmacological preparations, mainly with the aim to eliminate the symptoms of the disease, as well as preventing “formidable” complications (myocardial infarction), or combining pharmacological with surgical methods (stenting and / or coronary artery bypass graft) [1].
However, the use of surgical methods is not always possible (there are a number of absolute contraindications for coronary artery bypass grafting and / or the use of interventional technologies) or is limited, for example, by the small diameter of the main coronary arteries or their angulation, and vascular stenting is associated with a high risk of thrombosis (with multi-vascular damage) and the formation of restenosis.
It is also known that the development of heart disease is associated with a genetically determined deficiency of HIF production by heart tissues. As a result of the deficiency of HIF, which is responsible for initiating transcription of a wide range of factors (over 30), which are responsible for the restoration of p02 in tissues and adaptation of cells to hypoxia, necrobiotic processes develop in the heart muscle in response to hypoxia, followed by replacement of the defect with connective tissue and the formation of coronary heart disease. In this case, the defining moment is the absence of neoangiogenesis. For complete angiogenesis, expression of the whole complex of genes is necessary, and the expression of VEGF 225 is of decisive importance. In a simplified form, the sequence of events is as follows: under conditions of hypoxia, HIF-1α expression occurs; and HIF-1β, which, in turn, combine into a single peptide and cause expression of a gene battery. However, another feature of heart tissue is a decreased expression of the VEGF 225 gene. In other tissues, expression of VEGF 225 leads to the accumulation of VEGF peptides resulting from alternative splicing, angiopoietins, and growth factors. The latter ensure the survival and mobilization of endotheliocytes, their migration to the ischemic zone, proliferation and the formation of vascular ischemia in the zone [2]. So, in 75 percent of patients with stenotic atherosclerosis of the coronary arteries, collateral vessels do not develop with occlusion, which is apparently due to genetic factors [3].Patients suffering from coronary artery disease with well-developed collateral vessels are characterized by a certain haptoglobin phenotype and have a low level of endostatin in the pericardial fluid. This indicates an individual genetically determined variability of the state of natural mechanisms of neovascularization [4].
It is known that the development of heart disease is associated with a genetically determined deficiency of HIF production by heart tissues. As a result of the deficiency of HIF, which is responsible for initiating transcription of a wide range of factors (over 30), which are responsible for the restoration of p02 in tissues and adaptation of cells to hypoxia, necrobiotic processes develop in the heart muscle in response to hypoxia, followed by replacement of the defect with connective tissue and the formation of IHD. In this case, the defining moment is the absence of neoangiogenesis. For complete angiogenesis, expression of the whole complex of genes is necessary, and the expression of VEGF 225 is of decisive importance.In a simplified form, the sequence of events is as follows: under conditions of hypoxia, HIF-1α expression occurs; and HIF-1β: which, in turn, combine into a single peptide and cause expression of a gene battery. However, another feature of heart tissue is a decreased expression of the VEGF 225 gene. In other tissues, expression of VEGF 225 leads to the accumulation of VEGF peptides resulting from alternative splicing, angiopoietins, and growth factors. The latter ensure the survival and mobilization of endotheliocytes, their migration to the ischemic zone, proliferation and the formation in the zone of vascular ischemia [5].
However, only every fourth patient with stenotic atherosclerosis of the coronary arteries develops collateral vessels with occlusion, which is apparently due to genetic factors [3].
The degree of development of collateral vessels in patients with coronary heart disease is closely correlated with an increase in the expression of HIF-1alpha [6] and CD44 [7] by monocytes.
The beginning of an intensive study of the mechanisms of angiogenesis was laid more than 30 years ago by Judah Folkman, who suggested, that the progression of malignant tumors is determined by their vascularization [8].
Soon, the tumor production of vascular growth factor was confirmed, which served as the rationale for using the isolated factor for the revascularization of ischemic myocardium [9].
The processes of neovascularization in the postnatal period of the development of the body are carried out through three main and interrelated mechanisms: angiogenesis, arteriogenesis and vasculogenesis. Angiogenesis is the formation of new capillaries from postcapillary venules, which is carried out through the activation of endothelial cells, the expression of proteases in them, the degradation of the extracellular matrix, the proliferation and migration of these cells, their formation of primary highly permeable vascular structures, the subsequent stabilization and "growing up" of these structures by attracting pericytes and smooth muscle cells with their subsequent organization into a complex three-dimensional vascular network [10].
Hypoxia acts as an incentive for angiogenesis, which, through hypoxia-induced factor-1 (HIF-1), activates the expression of many angiogenic factors and, above all, the main regulator, vascular endothelial growth factor (VEGF) and its receptors. VEGF selectively stimulates the proliferation and migration of endothelial cells, their precursors and monocytes expressing receptors for it, increases vascular permeability, promotes the penetration of plasma proteins into the perivascular space, and also induces the formation of endothelial NO synthase and NO, endothelial cells promoting vasodilation and expression destructive bonds between endothelial to and extracellular matrix.
In the process of stabilization and “growing up” of the newly formed immature vascular network, the following are involved:
-
1) angiopoietin-1, which inhibits EC proliferation and reduces vascular permeability, but helps to attract pericytes;
-
2) platelet RF (PDGF), attracting pericytes and MMC;
-
3) transforming FR-beta 1 (TGF-beta 1), stimulating the synthesis of matrix proteins.
In the postnatal period, a stable state of blood vessels is maintained by a balance between activators of angiogenesis (mainly FR and cytokines) and its inhibitors (matrix metalloproteases, thrombospondin, plasminogen inducers, endostatin, etc.). A shift in the balance towards the predominance of activators leads to short-term activation of angiogenesis during inflam mation, wound healing, and ischemia. In turn, un-in-duced physiological angiogenesis is characterized by low activity, which may be due to both insufficient production of RF or expression of their receptors, and an excess of their inhibitors. The latter contributes to an increase in the severity of ischemic diseases (cardiac morbidity, chronic lower limb ischemia).
Induced angiogenesis is accompanied by an increase in the density of the capillary network in ischemic tissues and a decrease in peripheral vascular resistance, which increases tissue perfusion. However, without arteriogenesis, angiogenesis does not provide complete revascularization.
Arteriogenesis forms collateral vessels from non-functioning arteriolar compounds through which blood flow is bypassed at the site of occlusion. The most important stimulator of arteriogenesis is an increase in shear stress above the site of occlusion, which promotes the expression of adhesion molecules by endothelial cells, followed by the accumulation of monocytes in the vessel wall. The latter secrete active growth factors, of which fibroblast growth factors (FGF), as well as PDGF, VEGF and CXC chemokines are the main regulators of arteriogenesis [11].
Asculogenesis is the formation of in-situ blood vessels from progenitor embryonic cells. Previously believed that true vasculogenesis occurs only in the embryonic period.
The goal of therapeutic angiogenesis is to ensure the revascularization of ischemic tissues by stimulating the natural processes of vascular formation and growth.
In this case, there are three main approaches to solving this problem:
-
1. The use of exogenous growth factors.
-
2. The introduction of stem or progenitor cells.
-
3. Gene therapy - transfection of cells with genetic constructs containing genes for growth factors.
Despite the fact that a large number of various factors are involved in the regulation of angiogenesis, the bulk of clinical research is devoted to the use of VEGF and fibroblast growth factor (FGF), which are representatives of large families of RF. So, VEGF contains six factors: VEGF-A (VEGF-1), VEGF-B (VEGF-3), VEGF-C (VEGF-2), VEGF-D, VEGF-E and PIGF, which are secreted proteins and bind to three types of receptors: Flt-1 (VEGFR-1), Flk-2 (VEGFR-2), Flt-4 (VEGFR-3). VEGF-A, represented by 5 isoforms depending on the number of amino acids (121, 145, 165, 68 | Cardiometry | Issue 17. November 2020
189 and 206), was identified first. Isoforms of VEGF-121 and VEGF-165 are most fully studied in both experimental and clinical studies [12, 13].
The fibroblast growth factors represented by a family of 20 proteins, FGF-1 (aFGF), FGF-2 (bFGF), and FGF-4 accumulated in the extracellular matrix are most often used for therapeutic angiogenesis. FGFs stimulate the proliferation and migration of many cells, including vascular smooth muscle cells, and are considered the most powerful inducers of arteriogen-esis. In experimental studies with the introduction of FGF, their cardioprotective effect was shown, associated with both the suppression of apoptosis of cardiomyocytes and the ability to initiate vascular remodeling and cardiac hypertrophy [14].
The possibility of neovascularization of ischemic tissues using recombinant VEGF and FGF has been proven in numerous experimental studies on models of myocardial and skeletal muscle in rodents (mice, rats, rabbits), dogs, pigs, and sheep [15, 16].
The first clinical studies in patients with coronary heart disease and patients with critical lower limb ischemia, in which VEGF-165, FGF-1 and FGF-2 were used, showed encouraging results. However, data from double-blinded, placebo-controlled studies were less optimistic [17, 18].
So, in two large studies in which intracoronary administration of recombinant RF was tested (VEGF in the VIVA study in 178 IHD patients and FGF-2 in the FIRST study in 337 patients), no significant differences were found with the results in the placebo groups. The absence of differences is explained by a significant increase in exercise tolerance ("VIVA", "FIRST") and myocardial perfusion ("FIRST") both in the control and experimental groups. A similar situation was observed in the TRAFFIC study (two-time administration of FGF-2 into the femoral artery for patients with CINC), in which the greatest increase in painless walking time for those receiving FGF-2 in the first 3 months was offset by 6 months of increased painless walking in the group placebo. Nevertheless, the research results inspire some optimism regarding the possibility of using recombinant FGF-2 for lower limb ischemia.
In another double-blinded, placebo-controlled study, the intramyocardial administration of recombinant FGF-1 and FGF-2 was performed during coronary artery bypass grafting [20, 21].Although evaluating the effectiveness of therapeutic angiogenesis in combination with surgical revascularization seems problematic, the FGF-2 study deserves special attention. In this work, heparin alginate capsules containing recombinant FGF-2 or placebo were sutured under the epicardium during CABG along an artery that could not be bypassed. A more pronounced improvement in myocardial perfusion was recorded in patients who received capsules with FGF-2, and the positive effect of this procedure was recorded for 3 years [19].
At the same time, the method of therapeutic angiogenesis using recombinant growth factors has a number of drawbacks, which is confirmed by the negative results obtained in the course of experimental work and clinical observations [20].
Negative results are mainly associated with such features of recombinant proteins as a short half-life and low enigment in the myocardium (0.1% with intravenous administration and 5% with intracoronary) [21].
The use of recombinant growth factors is hindered by the need for their repeated introduction into the myocardium or skeletal muscle, which in a number of cases is unacceptable [22].
Gene therapy of cardiovascular diseases can dramatically change the treatment strategy for patients with coronary artery disease due to the correction of the genetic component of this disease, i.e. actually provide pathogenetic therapy. Unlike recombinant proteins, genetic constructs, when administered once, function in target tissues for several weeks and provide a smooth and continuous increase in the content of angiogenic factor.
Numerous experimental studies [23, 24] on the models of hind limb ischemia, chronic myocardial ischemia and brain ischemia, both in small (mouse, rat) and large (rabbit, dog, pig, sheep) animals, indisputable evidence of stimulation was obtained angio-and arteriogenesis, as well as improving the function and perfusion of muscle tissue using plasmid and adenovirus constructs containing the coding part of the genes of growth factors. In most studies, constructs with VEGF and FGF (secreted by FGF-4), regulated by the cytomegalovirus promoter, were tested.
Initial studies on the gene therapy of IHD and CINC have demonstrated two important results. First, good tolerance, feasibility, and safety of introducing genetic constructs encoding angiogenic factors into both the myocardium and skeletal muscle have been proven, both when using plasmid and adenoviral vec- tors. Secondly, it was possible to develop and evaluate various techniques for introducing genetic constructs into the heart:
-
1) intramyocardial, performed during coronary artery bypass grafting or through small thoracotomy;
-
2) transendocardial - using a catheter having a special device for injection and a sensor associated with the electromechanical mapping of the cavity of the left ventricle. The device provides detection of the zone of the hibernating myocardium and the introduction of the drug directly into the study area;
-
3) intracoronary.
Along with a demonstration of the good tolerance of gene therapy procedures, positive preliminary results of their effectiveness were obtained in these works. However, double-blind, placebo-controlled studies (phases II / III of a clinical study) on the use of gene therapy to stimulate angiogenesis have shown conflicting results [15, 16, 17, 18].Thus, in the KAT study (introducing VEGF-165 into the coronary artery in a plasmid and adenovirus vector using an infusion-perfusion catheter after angioplasty and stenting), a significant improvement in myocardial perfusion was noted only when using the VEGF gene in an adenovirus vector. In another EVROINJECT-1 study (a plasmid with VEGF-165 was introduced into the zone of the hibernating myocardium transendo-cardially using a NOGA catheter under the control of electromechanical mapping of the left ventricular cavity in 80 patients with IHD with III – IV angina class), after 3 months there were no differences with a placebo group in terms of perfusion defect area, against the background of a significant improvement in local contractility of the left ventricular wall in those receiving gene therapy. The effectiveness of the transendocardial method of introducing genetic constructs was tested in two more works - GENASIS and NOVA [17], which were stopped due to complications associated with intramyocardial injection. Although the transendocardial method is the most attractive in terms of the possibility of localized and repeated administration, it is associated with a high risk of complications and requires specially trained personnel.
In a randomized trial “REVASC” with intramyocardial administration via VEGF-121 via small thoracotomy in an adenoviral vector, load tolerance increased and the class of angina pectoris decreased in 67 inoperable patients with coronary heart disease [15]. These and other data allow us to hope for an im- provement in the results of treatment and to prognosis using gene constructs in seriously ill patients with coronary artery disease who are contraindicated in surgical revascularization.
The effectiveness of intracoronary introduction of genetic material (an adenoviral construct with the FGF-4 gene) was tested in a series of controlled clinical trials “AGENT-1, 2, 3, and 4” [25, 26]. In the first works ("AGENT-1" - 79 patients, "AGENT-2" - 85 inoperable patients with coronary artery disease) when using a large dose of adenovirus construct, an increase in exercise tolerance and a decrease in perfusion defects were noted. In the next multinational multicenter study “AGENT-4” (116 patients), differences with the control group for increasing exercise tolerance were not found, and a similar study “AGENT-3” (416 patients) was stopped due to the wide variation in the values of tolerance to physical loads in groups, which did not allow to obtain significant differences. However, a subsequent analysis of the data of different subgroups of patients showed that gene therapy significantly increased exercise tolerance compared with controls in patients older than 55 years and with a more severe form of angina pectoris. Another approach to balanced stimulation of angiogenesis can be the creation of genetic constructs based on a hybrid of genomic DNA and cDNA forms of the VEGF gene that contain exons and introns in differently spliced regions, which ensures the expression of several VEGF isoforms by analogy with the natural process in tissues [27]. Further prospects for the gene therapy of ischemic disorders are also associated with the use of hepatocyte growth factor (HGF), which effectively stimulates angiogenesis and has cardioprotective properties due to its ability to activate the migration and mobilization of resident stem cells of the heart and inhibit the development of myocardial fibrosis [28]. Another strategy may be based on the use of genes encoding factors that activate the incorporation of endogenous angiogenic molecules. For example, the introduction of a genetic construct constitutively expressing HIF-1alpha, which activates the expression of angiogenic factors, effectively stimulated revascularization in the rabbit ischemic limb and in patients with CINC (WALK study) [16, 17].
In one of three completed randomized double-blind clinical trials of gene therapy in inoperable patients with CINCs [3, 25] (VEGF-165 was introduced in a plasmid or adenovirus vector into the femoral artery), a positive result was obtained (an increase in the num- 70 | Cardiometry | Issue 17. November 2020
ber of collateral vessels), in the second (VEGF-165 plasmid DNA was injected intramuscularly) - mixed (the number of amputations per 100 days remained unchanged, however, there was an improvement in the ankle-brachial index, healing of trophic ulcers and lessening pain at rest), and in the third (research Contents «RAVE»: VEGF121 in the adenoviral vector was administered intramuscularly into the affected limb) - negative on all endpoints. The introduction of VEGF did not lead to any serious complications, with the exception of transient edema, which was the result of the effect of VEGF on vascular permeability.
The discrepancy between the results of experimental and clinical studies may be based on the use of significantly lower doses of gene therapeutic drugs for people because of fear of possible complications. Thus, a rat plasmid with VEGF-165 was administered in rats at doses of 125–500 μg per animal weighing 250–300 g, and in humans at doses of 500–2000 μg with an average body weight of 75 kg. Caution in the use of large doses of VEGF in clinical studies is associated with the fear of possible stimulation of latent tumors or the development of angiomas, although no complications have been noted in any of the completed controlled studies. Therefore, genetic constructs in which the VEGF gene is regulated by a tissue-specific promoter that restricts the expression of growth factors only in the target tissue, or a promoter regulated by hypoxia and includes the expression of VEGF only in ischemic tissue, can solve this problem.
An important factor determining the effective stimulation of the growth of new vessels is the duration of the increase in the production of angiogenic factors in ischemic tissues. According to some data [15, 25], the duration of transgene expression of growth factors when using plasmid or adenoviral vectors in tissues usually does not exceed 2 weeks. In a model of a line of mice in which VEGF expression is regulated by tetracycline, it was shown that in order for the newly formed vessels to stabilize in the ischemic zone, in the absence of regression, an increased concentration of VEGF in the tissue is required for four weeks [29]. Such prolonged expression cannot be provided by plasmid or adenoviral vectors used in well-known clinical studies. A solution to this problem can be achieved by reintroduction of plasmid constructs or by the use of vectors based on adeno-associated viruses that efficiently transfer genetic material to muscles and provide longer expression of transgenes [25, 30].
In general, it should be noted that the expansion of the arsenal of angiogenic factors used for therapeutic angiogenesis, as well as the selection of optimal combinations and modes of their administration, can increase the effectiveness of therapeutic tactics.
Thus, in order to initiate and maintain proper neoangiogenesis in the heart of an adult, expression of HIF (two chains - HIF-1α; and HIF-1β;) and VEGF 225, which is absent or insufficient in 75% of the population, is necessary. The latter can be achieved by transfection of heart tissues with gene constructs carrying the corresponding genes:
HIF-1α;
HIF-1β;
VEGF 225 and
VEGF 165 (to enhance the effect).
Therefore, the treatment of patients with chronic coronary insufficiency, for whom traditional treatment is ineffective, requires the introduction of new therapeutic methods, which will be based on the correction of the pathogenetic mechanism - therapeutic angiogenesis.
Moreover, that gene therapy has its drawbacks associated with the introduction of foreign genetic material and the possibility of an immune response when using adenoviral vectors for gene delivery. Nevertheless, the majority of authors connect the further successes of this direction mainly with conducting extensive research under the conditions of a model experiment.
Materials and Methods
The experiments were carried out on male Chinchilla rabbits weighing 2.8-3.2 kg and 1-1.2 years old. The animals, in order to ensure incomplete occlusion of the anterior descending artery of the heart, performed its ligation on the mandrel, which narrowed the lumen of the vessel by 80%. Immediately after ligation, two experimental animal groups No. 1 (n = 10) and No. 2 (n = 10) were injected intramiocardial-ly once with the vectors of growth factors VEGF165 (group No. 1) at a concentration of 100 μg / ml physiological saline at a rate of 50 μg / cm2 of the ischemic zone with a step over the area of the zone of 2-10 mm uniformly throughout the ischemic zone or in the second group at the same time a single complex of four genes HIF1a, HIF1b, VEGF165, VEGF225 in a stoichiometric ratio of 1: 0.2: 0.5: 0, 3, group No. 2 at a concentration of 400 μg / ml of physiological saline based on 200 μg of DNA per cm2 of ischemic zone and a step over an area of 2-10 mm, with the addition of adjuvant. As an adjuvant, 2-dimethylaminoetha-nol at a concentration of 2.5 mmol / L was used. The control group of animals (n = 10) was injected with only the adjuvant solution in the corresponding volume of saline.
The level of angiogenesis was evaluated on the 30th day after surgery. Blood vessels and their relationships with cardiac muscle fibers were detected using the method of intravascular injection with contrasting suspensions followed by histological processing [1]. The study of pO2 in the open heart injury zone was carried out polarographic. The radioactivity of the tissue after a tight filling of the vascular bed with a solution of uranyl acetate-238 was determined on a GSU-1M scintillation counter and expressed in kBq per gram of tissue (dry weight). Additionally, after 30 days, the animals underwent a pharmacological stress test with dipyridamole in a total dose of 0.75 mg per 1 kg of body weight of a 0.5% solution and the electrocardiogram was recorded. The experiments were carried out on male chinchilla rabbits weighing 2.8-3.2 kg and 1-1.2 years old. The animals, in order to ensure incomplete occlusion of the anterior descending artery of the heart, performed its ligation on the mandra, which narrowed the lumen of the vessel by 80%. Immediately after ligating, the two experimental animal groups No. 1 (n = 10) and No. 2 (n = 10) were injected intramiocardially once with the vectors of growth factors VEGF165 (group No. 1) at a concentration of 100 μg / ml physiological saline at a rate of 50 μg / cm2 of the ischemic zone with a step over the area of the zone of 2-10 mm uniformly throughout the ischemic zone or in the second group at the same time a single complex of four genes HI-F1a, HIF1b, VEGF165, VEGF225 in a stoichiometric ratio of 1: 0.2: 0.5: 0, 3, group No. 2 at a concentration of 400 μg / ml of physiological saline based on 200 μg of DNA per cm2 of ischemic zone and a step over an area of 2-10 mm, with the addition of adjuvant. As an adjuvant, 2-dimethylaminoethanol at a concentration of 2.5 mmol / L was used. The control group of animals (n = 10) was injected with only the adjuvant solution in the corresponding volume of saline.
The level of angiogenesis was evaluated on the 30th day after surgery. Blood vessels and their relationships with cardiac muscle fibers were detected using the method of intravascular injection with contrasting suspensions followed by histological pro
Issue 17. November 2020 | Cardiometry | 71
Table 1
Parameters of the microvasculature of the ischemic myocardium
|
Control |
Introduction of VEGF-165 |
Introduction of HIF1a, HIF1b, VEGF165 and VEGF225 |
Significance of differences at p <0.05 |
|
|
Intact group |
Group of animals No. 1 |
Group of animals No. 2 |
||
|
The number of capillaries n (1 mm2 per cut) |
3661,0+-92,0 |
4180.0+-60.0 |
5154.0+-282.0 |
*, + |
|
Diameter of open capillaries d (microns) |
6,5+-0,3 |
6.80+-1.10 |
6.90+-0.80 |
|
|
Capillary length l (mm / mm3) |
2123,0+-80,0 |
2600.0+-76.0 |
4018.0+-201.0 |
*, + |
|
Capillary exchange surface area S (mm2/mm3) |
43,30+-0,94 |
55.50+-3.11 |
87.10+-4.20 |
*, + |
|
pO2 mmHg |
18,0+-4,8 |
32.3+-4.9 |
45.4+-5.8 |
*, + |
|
Radioactivity of uranyl acetate-238 per gram of tissue (dry weight), kBq |
0,79+-0,06 |
1.04+-0.10 |
1.35+-0.05 |
*, + |
Note: * - significance of differences from the control group, + - significance of differences from the group of animals No. 1
cessing [1]. The study of pO2 in the open heart injury zone was carried out polarographic. The radioactivity of the tissue after a tight filling of the vascular bed with a solution of uranyl acetate-238 was determined on a GSU-1M scintillation counter and expressed in kBq per gram of tissue (dry weight). Additionally, after 30 days, the animals underwent a pharmacological stress test with dipyridamole in a total dose of 0.75 mg per 1 kg of body weight of a 0.5% solution and the electrocardiogram was recorded.
Results
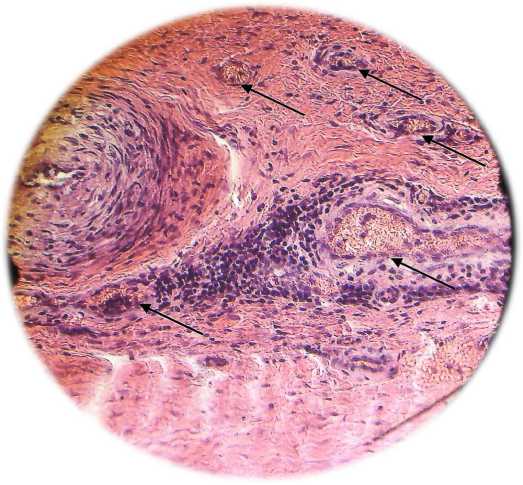
The results obtained (Table 1 and Fig. 1) indicate that transfection with the VEGF-165 gen alone is accompanied by the development of the microvasculature and an increase in pO2 in the ischemic zone compared with the control group of animals. At the same time (Fig. 1 B), pronounced perivascular edema is observed, accompanied by “collapse” of the vessel.
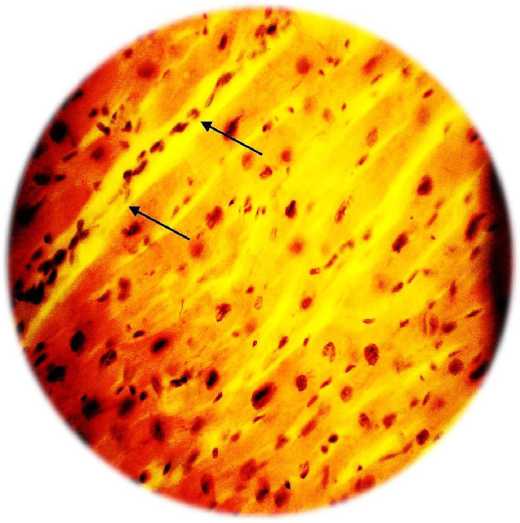
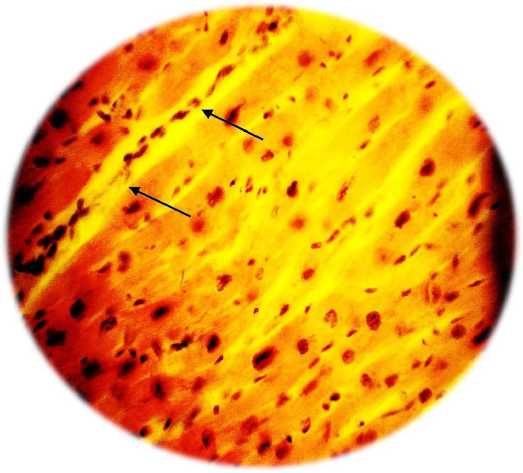
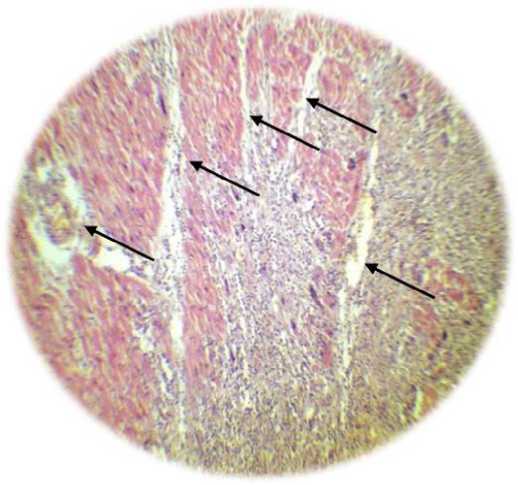
Transfection of cells with a combination of four genes of vascular growth factors has a more pronounced effect than transfection only with the VEGF-165 gene (Table 1 and Figure 2). In addition, along with the formation of the capillary network, transfection with a combination of vascular growth factors was accompanied by the formation of arterioles (Fig. 3). as well as the formation of anastomoses between the newly formed vessels and vessels of intact ischemia of the heart tissue.
In addition, along with the formation of the capillary network, transfection with a combination of vascular growth factors is accompanied by the formation of arterioles (Fig. 3). as well as the formation of anastomoses between the newly formed vessels and vessels of intact ischemia of the heart tissue.
72 | Cardiometry | Issue 17. November 2020
During the formation of a full-fledged vascular bed, normalization of metabolic processes in the area of the damaged myocardium is observed, as evidenced by the dynamics of changes in the electrocardiogram (ECG) during the performance of the load test. An ECG analysis after the introduction of a vector with the VEGF165 gene after 5 minutes of loading (test with dipyridamyl) indicates the appearance of a horizontal depression of the ST (V4) segment by 0.3 mV (3 mm, a sign of myocardial ischemia). With the introduction of a complex of vectors with the genes HIF1a, HIF1b, VEGF165, VEGF225, a negative ECG test is observed - the absence of ST segment depression.
Conclusion
From an overall prospective, the use of four genes integrated into the vectors of the complex allows more efficiently stimulating angiogenesis and remodeling the vasculature in an ischemic myocardium than the technical solution of the prototype. In turn, the epigenomic localization of gene constructs and the limited duration of stay in the cell are sufficient for the expression of the introduced genes and the formation of a complete vasculature.
The research was conducted in the frames of governmental assignment No. AAAA-A18-118041890076-1 of the Ministry of Health of the Russian Federation on the topic: "Genetic engineering appliance for the treatment of coronary insufficiency".
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
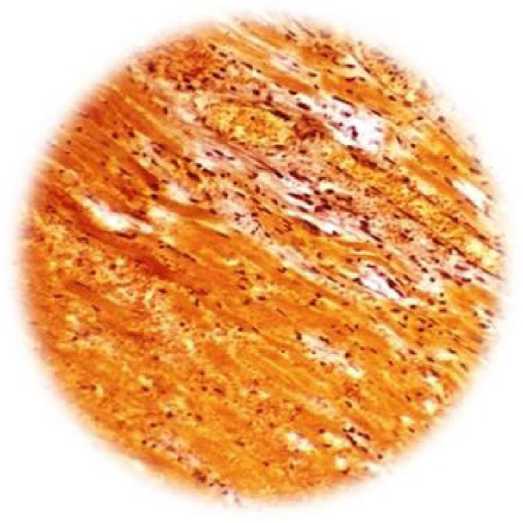
Figure 1. A - control group of animals. In the upper part, the ischemic zone is clearly visible, which developed as a result of occlusion of the anterior descending branch of the coronary artery (Van Giesz stain, x100). B - formation in the zone of vascular ischemia (indicated by arrows) under the influence of hyperexpression of the VEGF 165 gene. Pronouncedperivascularedema (hemotoxylin
A
B
/ eosin, x200).
A
B
Fig. 2. A - formation of vascular ischemia in the zone (indicated by arrows) under the influence of VEGF 165 overexpression. Severe perivascular edema (hemotoxylin / eosin, x200). B - vascular formation under the influence of overexpression of the HIF1a, HIF1a, VEGF 165, VEGF 225 genes. There is no edema, the capillaries are filled with red blood cells (hemotoxylin / eosin, x100)
A
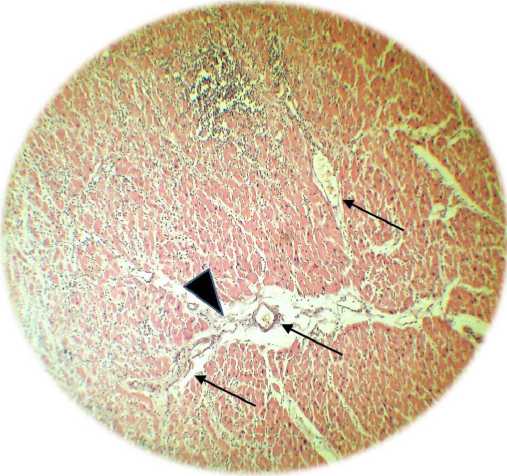
Fig. 2. A - formation of vascular ischemia in the zone (indicated by arrows) under the influence of VEGF 165 overexpression. Severe perivascular edema (hemotoxylin / eosin, x200). B - vascular formation under the influence of overexpression of the HIF1a, HIF1a, VEGF 165, VEGF 225 genes. There is no edema, the capillaries are filled with red blood cells (hemotoxylin / eosin, x100)
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Approaches to the gene therapeutic correction of the pathogenetic mechanisms of coronary insufficiency
- Deev RV, Mzhavanadze ND. Major trends in thera¬peutic neoangiogenesis in treatment of chronic lower limb ischemia (literature review) OJSC Human Stem Cells Institute, Moscow. Eruditio Juvenium.2013. p. 103-109. Retrieved from: http://paper.researchbib.com/view/paper/60629
- Jung TW, Yoo HJ, Choi KM. Implication of hepa¬tokines in metabolic disorders and cardiovascular diseases//BBA Clin. 2016 Mar 5;5:108-13. https://doi.org/10.1016/j.bbacli.2016.03.002.
- Bigler MR, Seiler C. The Human Coronary Col¬lateral Circulation, Its Extracardiac Anastomoses and Their Therapeutic Promotion. Int J Mol Sci. 2019 Jul 30;20(15). pii: E3726. doi: 10.3390/ijms20153726.
- Frangogiannis NG. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circu¬lation Research. 2019;125:117–146. doi: 10.1161/CIRCRESAHA.119.311148. https://doi.org/10.1161/CIRCRESAHA.119.3111.
- Zhou X, et al. Multifunctional Phosphorescent Conjugated Polymer Dots for Hypoxia Imaging and Photodynamic Therapy of Cancer Cells. Adv Sci (Weinh). 2015 Sep 10;3(2):1500155. https://doi.org/10.1002/advs.201500155.
- Avezov K, Aizenbud D, Lavie L. Intermittent Hy¬poxia Induced Formation of "Endothelial Cell-Col¬ony Forming Units (EC-CFUs)" Is Affected by ROS and Oxidative Stress. Front Neurol. 2018 Jun 14;9:447. eCollection 2018. doi: 10.3389/fneur.2018.00447.
- Simons M, Eichmann A. Molecular Controls of Arterial Morphogenesis. Circulation Research. 2015;116:1712–24. https://doi.org/10.1161/CIRCRE¬SAHA.116.302953.
- Folkman J. Tumor angiogenesis: therapeutic impli¬cations. N Engl J Med. 1971 Nov 18;285(21):1182-6. DOI: 10.1056/NEJM197111182852108.
- Guo M, Shi JH, Wang PL, Shi DZ. Angiogenic Growth Factors for Coronary Artery Disease: Current Status and Prospects. J Cardiovasc Pharmacol Ther. 2018 Mar;23(2):130-41. doi: 10.1177/1074248417735399. Epub 2017 Oct 12.
- Carmeliet P. Mechanisms of angiogenesis and ar¬teriogenesis. Nature Medicine. 2000;6:389–95. DOI: 10.1038/74651.
- Choo GH. Collateral Circulation in Chronic To¬tal Occlusions – an interventional perspective. Curr Cardiol Rev. 2015 Nov; 11(4):277–84. DOI: 10.2174/1573403X11666150909112548.
- Parfenova EV, Tkachuk VA. Prospects of gene therapy of cardiovascular diseases. Vopr. med. chem. 2000;46:293–310. [in Russian]
- King C, Hristova K. Direct measurements of VEGF:VEGFR2 binding affinities reveal the cou¬pling between ligand binding and receptor dimeriza¬tion. J Biol Chem. 2019 Jun 7; 294(23): 9064–75. doi: 10.1074/jbc.RA119.007737
- Johnson T, et al. Approaches to therapeutic angio¬genesis for ischemic heart disease. JMolMed (Berl). 2019 Feb; 97(2):141-51. doi: 10.1007/s00109-018-1729-3. Epub 2018 Dec 15.
- Ziegler T, Abdel Rahman F, Jurisch V, Kupatt C. Atherosclerosis and the Capillary Network; Patho-physiology and Potential Therapeutic Strategies. Cells. 2019 Dec 24;9(1). pii: E50. doi: 10.3390/cells9010050.
- Makarevich PI, Parfyonova YV. Therapeutic An¬giogenesis: Foundations and Practical Application. Physiologic and Pathologic Angiogenesis - Signaling Mechanisms and Targeted Therapy. Intech Open, DOI: 10.5772/66411, 2017 Apr 5.
- Ylä-Herttuala S, Baker AH. Cardiovascular Gene Therapy: Past, Present, and Future. Mol Ther. 2017 May 3;25(5):1095-106. doi: 10.1016/j.ymthe.2017.03.027, Epub 2017 Apr 4.
- Jain A, et al. Advancements in pharmacotherapy for an¬gina. Expert Opin Pharmacother. 2017 Apr;18(5):457-69. doi: 10.1080/14656566.2017.1303483, Epub 2017 Mar 15.
- Selke F, Laham R, Edelman E. Therapeutic an¬giogenesis with basic fibroblast growth factor - Tech¬nique and early results. The Ann. Thorac. Surg. 1998;65:1540–54, doi:10.1016/S0003-4975(98)00340-3, 1998 June 01.
- de Leeuw K, Kusumanto Y, Smit AJ. Skin capil¬lary permeability in the diabetic foot with critical limb ischaemia: the effects of a phVEGF165 gene product. Diabet Med. 2008 Oct;25(10):1241-4. doi: 10.1111/j.1464-5491.2008.02557.x. PMID: 19046206
- Zhu H, et al. Intramyocardial delivery of bFGF with a biodegradable and thermosensitive hydrogel im-proves angiogenesis and cardio-protection in infarcted myocardium. Exp Ther Med. 2017 Oct;14(4):3609-15. doi: 10.3892/etm.2017.5015, PMCID: PMC5639332, PMID: 29042955. Epub 2017 Aug 24.
- Potz BA, et al. Novel Molecular Targets for Cor¬onary Angiogenesis and Ischemic Heart Disease. Coron Artery Dis. 2017 Nov; 28(7): 605–13. doi: 10.1097/MCA.0000000000000516, PMID: 28678145, PMCID: PMC5624824.
- Jazwa A, et al. Limb ischemia and vessel regen¬eration: Is there a role for VEGF? Vascul Pharmacol. 2016 Nov;86:18-30. doi: 10.1016/j.vph.2016.09.003, PMID: 27620809. Epub 2016 Sep 10.
- Zhang D, et al. Efficacy and safety of therapeutic angiogenesis from direct myocardial administration of an adenoviral vector expressing vascular endo¬thelial growth factor 165. Chin Med J (Engl). 2002 May;115(5):643-8.
- Samura M, et al. Therapeutic strategies for cell-based neovascularization in critical limb ischemia. J Transl Med. 2017;15:49. doi: 10.1186/s12967-017-1153-4
- Henning RJ. Therapeutic angiogenesis: angiogen¬ic growth factors for ischemic heart disease. Future Cardiol. 2016 Sep;12(5):585-99. doi: 10.2217/fca-2016-0006. Epub 2016 Jul 15.
- Kaminsky SM, et al. Safety of Direct Cardiac Ad¬ministration of AdVEGF-All6A+, a Replication-De¬ficient Adenovirus Vector cDNA/Genomic Hybrid Expressing All Three Major Isoforms of Human Vascular Endothelial Growth Factor, to the Ischemic Myocardium of Rats. Hum Gene Ther Clin Dev. 2013 Mar;24(1):38-46. doi: 10.1089/humc.2013.054.
- Sanada F, et al. Gene-Therapeutic Strategies Tar¬geting Angiogenesis in Peripheral Artery Disease. Medicines (Basel). 2018 Jun; 5(2): 31. doi: 10.3390/medicines5020031.
- Gianni-Barrera R, et al. Therapeutic vasculariza¬tion in regenerative med.//Stem Cells Transl Med. 2020 Apr;9(4):433-44. doi: 10.1002/sctm.19-0319. Epub 2020 Jan 10.
- Liew LC, Ho BX, Soh BS. Mending a broken heart: current strategies and limitations of cell-based ther-apy. Stem Cell Res Ther. 2020 Mar 26;11(1):138. doi: 10.1186/s13287-020-01648-0.


