Arrangement of chemical elements in the three-dimensional matrix
Автор: Boris V. Gusev, Samuel Y-L. Yin, Anatoly A. Speransky
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: The results of the specialists’ and scientists’ researches
Статья в выпуске: 5 Vol.13, 2021 года.
Бесплатный доступ
It is emphasized in the article that thanks to the discovery of D.I. Mendeleev and the Periodic Table of chemical elements existing for more than 150 years, as well as the international table IUPAC, chemistry has been actively developing and keeps developing. A new model for arranging chemical elements in the form of a three-dimensional matrix has been proposed. This makes it possible to predict new elements with the designation of nuclear masses and the electronic structure of shells. There have been developed new patterns according to the cyclicity (block structure) of horizontal rows and the structure of vertical groups and their physical conception have been specified.
Cyclicity and block structure, three-dimensional matrix of chemical elements, nuclear masses, proton-neutron structure of nuclei, electronic levels, electronic characteristics of levels and sublevels (orbitals), prediction of new elements
Короткий адрес: https://sciup.org/142228306
IDR: 142228306 | DOI: 10.15828/2075-8545-2021-13-5-282-292
Текст научной статьи Arrangement of chemical elements in the three-dimensional matrix
Original article
T he periodic law and its presentation in the form of tables of chemical elements by D.I. Mendeleev and the international IUPAC is an outstanding discovery of mankind. At the end of 2019, the entire world scientific community under the auspices of the UN celebrated the 150th anniversary of this event. Currently, the tables contain 118 elements, but not all of them are found in natural form, since some are obtained artificially. The authors have written a new work on the Three-dimensional matrix of chemical elements [3].
There have been considered the concepts of the Universe and a physical model of the Big Bang and the expanding Universe is adopted to describe the emergence of chemical elements, the task is set to describe them in the form of a three-dimensional expanding matrix of chemical elements. At the top of the matrix are hydrogen ( H ) and helium ( He ), being the basis for the creation of all subsequent elements, and further other elements are arranged along the expanding spiral and group principles of valences serve as the boundaries for such structure.
The second most important consideration is the origin and evolution of the Universe, all living and non-living in it in a spiral [10, 11]. Being obvious for the living nature, for non-living nature it is assumed that chemical elements might serve as the building blocks for this spiral, which will form molecules in the future when interacting with each other. The entire material inorganic world is formed from molecules. And then natural processes take place i.e. the creation of living matter: plants, animals and people. Thus, the entire development of the mineral and living world proceeds in spirals.
Presented generalisations show that when structuring a system of chemical elements , in addition to the periodicity of the formation of valence groups of outer orbitals, the concept of cyclicity and block structuring of elements horizontally is preferable .
MAIN PART
1. Cyclicity and block structure of chemical elements
The modern periodic system is based on the nuclear charge (Z), which determines the place of an element in
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES the system [1, 13, 14]. Nuclear periodicity is expressed in a periodic change in the properties of atomic nuclei. The main parameter that determines this periodicity is the number of protons (P) and neutrons (N) in the nucleus. Figure 1 shows the dependence of the mass of nuclei of elements (protons and neutrons) and the mass of neutrons for the discovered 118 chemical elements.
The authors have calculated the neutron-proton ratio in the nuclei of chemical elements. In the short-period table 1, a, the averaged indicators of these ratios for the blocks are presented. It should be noted that these averaged values for blocks are equal both in the short-period table and in the IUPAC table (Table 1, b). Therefore, the block structure , in our opinion, corresponds to the concept of cyclicity i.e. the completeness of the system of periodicity of two periods, including the families of lanthanides and actinides.
The study of the three-dimensional periodic matrix of chemical elements based on a multiparameter coordinate system clearly demonstrates the stable formation of block patterns in the cyclic periodicity in the properties of chemical elements in periods and blocks with an increase in their ordinal numbers and unites all the previously described periodicity options [4, 7]. It should be noted that the concepts of “period” of the two types of tables are identical (there are 7 of them each), but an important circumstance that requires special attention is the obvious discrepancy between the «rows» and the group valence principle of periodicity in a long-period table.
In [3, 4], it was concluded that the 3D-spirally diverging system of the matrix of chemical elements has 4 periodicity blocks and 7 periods. Analysis of the structure of period formation confirms that periods can be divided into simple ones, in which one element is formed in each group (2 elements with an external signal orbital and 6 elements with an external valence orbital, 8 in total) and more complex ones containing grouped “families” elements within one group ( III or VIII ). Thus, in blocks, everything can be represented as follows (Table 1).
– The first block A corresponds to short single-element periods (and rows 0-1 ) of the matrix, where the first elements are hydrogen and helium ( H 1 -hydrogen and He 2 -helium). For the first block, the ratio of neutrons (N) to protons (P) can be taken equal to 1.
– The second block B structurally positions the completeness of the cyclic eight-element periodicity, which corresponds to simple periods (and rows) 2 (includes 8 elements from Li 3 to Ne 10 ) and 3 matrices (includes 8 elements from Na 11 to Ar 18 ). For the second block, N:P is less than 1.1.
– The third block C structurally represents the completeness of the cyclic 10- and 8-element periodicity in the block, two additional “families” have appeared: iron
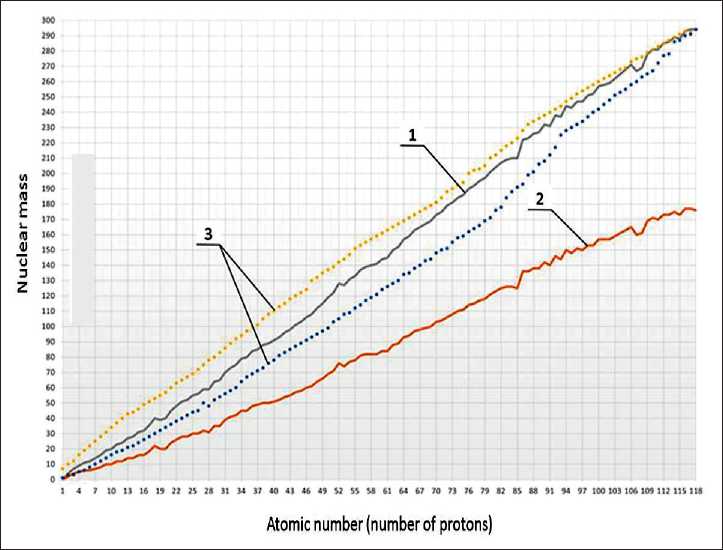
Fig. 1. Dependences of nuclear masses (protons and neutrons) and mass neutrons (neutrons) for 118 chemical elements: 1 – the number of protons and neutrons;
2 – the number of neutrons; 3 – isotopes
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Table 1
The ratio of neutrons and protons in the nuclei of atoms of chemical elements
a) Mendeleev Table
Ratio of neutrons(n)to / protons(p)
Blocks, periods / rows
I
II
Ш
IV
V
VI
VII
VIII
A
1
H 1
LOOS 1
2 2
He 4 1.0
Less than 1,1
В
2
3 4
Li 7 1,33
4 5
Be
9 1,25
5 6
В 11 1,20
6 6
c
12 1,0
7 7
N 14 1,0
8 8
О 16 1,0
9 10
F 19 1,11
10 10
Ne 20 1,0
В
3
1! 12
Na 23 1.09
12 12
Mr
24 1,0
13 14
Al 27 1.08
14 14
Si
28 1,0
15 16
P 3! 1.07
16 16
s
32 1,0
17 18
Cl 35 1.09
18 22
Ar
40 1.22
Less than
1,3
С 4/4-5
19 20
К
39 1,05
20 20
Ca 40 1,0
21 24
Sc 45 1,14
22 26
Ti
48 1,18
23 28
V
St 1.22
24 28
Cr 52 1,17
25 30
Mn
55 1.20
26 30
Fc 56 1,15
27 32
Co 59 1.19
28 31
Ni 59 1,11
29 35
Cu 63 1.21
30 35
Zn
65 1,17
31 39
Ga 70 1,26
32 41
Ge 73 1,28
33 42
As 75 1,27
34 45
Se 79 132
35 45
Br 80 1,29
36 48
Kr
84 133
С 5/6-7
37 49
Rb
85 132
38 50
Sr
88 1,32
39 50
Y
89 1.28
40 51
Zr
91 1,28
41 52
Nb
93 1.27
42 54
Mo
96 1.29
43 55
Tc
96 1,28
44 57
Ru
101 1.30
45 58
Rh
103 1.29
46 60
Pd 106 130
47 61
Ar 108 130
48 64
Cd 112 133
49 66
In 115 135
50 69
Sn 119 138
5! 71
Sb 122 139
52 76
Те 128 1.46
53 74
I 127 1.40
54 77
Xc 131 1.43
Less than 1,55
D 6/8-9
55 78
Cs 133 1,42
56 81
Ba 137 1,45
57 82
La 139 1,44
72 106
Hf 179 1,49
73 108
Ta 181 1,48
74 110
w 184 1,49
75 til
Re 186 1,48
76 114
Os 190 1,50
77 115
lr 192 1.49
78 117
Pt 195 1,50
79 118
Au
197 1.49
80 121
Hr
201 1.50
81 123
TI 204 1.52
82 125
Pb
207 1.52
83 126
Bi
209 1.52
84 126
Po 210 1.50
85 125
At
210 1.47
86 136
Rn 222 158
D 7/9-11
87 136
Fr 223 1.56
88 138
Ra
226 1,57
89 138
Ac 227 1.55
104 161
Rf 265 1.55
105 163
Db 268 1.55
106 165
Sr 271 1.56
107 160
Bh
267 1.50
108 161
Xs 269 1.49
109 169
Mt 278 1,55
110 171
Ds
281 1.55
111 170
Rg
281 1.53
112 173
Cn 285 1,54
113 173
Nh
286 1,53
114 175
Fl 289 134
115 173
Me
288 1.50
116 177
Lv
293 1.53
117 177
Ts 294 1,51
118 176
Or 294 1,49
58 82
Ce 140 1,41
59 82
Pr 141 1,39
60 84
Nd
144 1,40
61 84
Pm 145 1,38
62 88
Sin 150 1.42
63 89
Eu 152 1,41
64 93
Gd 157 1,45
65 94
Tb
159 1.45
66 97
Dy 163 1,47
67 98
Ho 165 1,46
68 99
Er
167 1,46
69 100
Tin 169 1,45
70 103
Yb
173 1,47
71 104
Lu 175 1.46
90 142
.232 1,58
91 140
Pa 231 1,53
92 146
и 238 1,59
93 144
Np 237 1,55
94 150
Pu
244 1,60
95 148
Ain 243 1,56
96 151
Cm
247 1,57
97 150
Bk
247 1,55
98 153
Cf
251 1,56
99 153
Es 252 1,55
100 157
Fm 257 1,57
101 157
Md 258 1,55
102 157
No 259 1,54
103 159
Lr 262 1,54
b) IUPAC Table
ratio
Block, period
1
II
III
IV
V
VI
VII
VIII
IX
X
XI
XI!
ХШ
XIV
XV
XVI
XVII
XVIII
О
A
1
н 1 1,008 1
2 2
He 4 1,0
s «
z ^
J
В
2
3 4
Li
7 из
4 5
Be
9 1,25
5 6
В
11 1.20
6 6
C 12 1,0
7 7
N
14 1,0
8 8
0
16 1,0
9 10
F 19 1,11
10 10
Ne 20 1,0
В
3
II 1!
Na 23 1,09
12 12
Mg
24 1,0
13 14
Al
27 1,08
14 14
Si
28 1,0
15 16
P
31 1,07
16 16
S
32 1,0
17 18
Cl
35 1,09
18 22
Ar
40 1,22
C «
Г
с 4
19 2G
К
39 1,05
20 20
Ca
40 1.0
21 24 Sc
45 1,14
22 26
Ti
48 1,18
23 28
V
51 1,22
24 28
Cr
52 1.17
25 30
Mn
55 1,20
26 30
Fe
56 1,15
27 32
Co
59 1,19
28 31
Ni
59 1,11
29 35
Cu
64 1,21
30 35
Zn
65 1,17
31 39
Ga
70 1,26
32 41
Ge
73 1,28
33 42 As 75 137
34 45
Se
79 132
35 45
Br
80 139
36 48
Kr
84 133
С 5
37 49
Rb
85 132
38 50
Sr
88 132
39 50
Y
89 138
40 51
Zr
91 1,28
41 52
Nb
93 1,27
42 54
Mo
96 1.29
43 55
Tc
98 U3
44 57
Ru
10! 130
45 58
Rh
103 1,29
46 60
Pd
106 130
47 61
Ag
108 130
48 64
Cd
112 133
49 66
In
115 135
50 69
Sn
119 138
51 71
Sb
122 139
52 76
Те
128 1,46
53 74
I
127 1,40
54 77 Xe
131 1.43
=
j
D
6
55 78
Cs
133 1,42
56 81
Ba
137 1,45
57 82
La
139 1,44
72 106
Hf
179 1,49
73 108
Ta
181 1,48
74 110 w
184 1.49
75 111
Re
186 1,48
76 114
Os
190 1,50
77 115 lr
192 1,49
78 117
Pt
195 1,50
79 118
Au 197 1,49
80 121
Hg
201 130
81 123
Ti
204 132
82 125
Pb
207 1 32
83 126
Bi
209 1 32
84 126
Po 210 130
85 125
At
2101,47
86 136
Rn
222 158
D
7
87 136
Fr
223 1,56
88 138
Ra
226 1,57
89 138
Ac
227 135
104 161
Rf
265 1,51
105 163
Db
268 1,55
106 165 sg 271 136
107 160
Bh
267 130
108 161
Xs
269 1,49
109 169
Mt
278 135
110 171
Ds
281 135
111 170
Rg
281 133
112 173
Cn
285 1 34
113 173
Nh
286 1,53
114 175
Fl
289 1,54
115 173
Me
288 130
116 177
Lv
293 133
117 177
Ts
294 1,51
118 176
Og
294 1,49
2. Three-dimensional periodic matrix of chemical elements
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
(Fe) and platinum (Pt) – from ruthenium (Ru) to palladium (Pd).
The third block C presents the first short pair of rows 4 (includes 10 elements from K 19 to Ni 28 ) and 5 of the shortperiod table (includes 8 elements from Cu 29 to Kr 36 ) and the second short pair of rows 6 (includes 10 elements from Rb 37 to Pd 46 ) and 7 short-period tables (includes 8 elements from Ag 47 to Xe 54 ). In the long-period table, a pair of rows 4 and 5 corresponds to period 4 , and a pair of rows 6 and 7 corresponds to period 5 . For the third block, the N:P ratio is less than 1:1.3.
– The fourth block D structurally represents the completeness of the cyclic 24 and 8-element periodicity as a block of chemical elements D , which additionally included the “families” of lanthanides ( La ) and actinides ( Ac ), as well as two “families”: osmium ( Os ), iridium ( Ir ), platinum ( Pt ) and chassium ( Hs ), maitnerium ( Mt ), darmstadtium ( Ds ).
The fourth block corresponds to the first pair of the long row of lanthanides 8 (includes 24 elements from Cs 55 to Pt 78 ) and the short row of the short-period table (includes 8 elements from Au 79 to Rn 86 ) and the second pair of the long row of actinides 10 (includes 24 elements from Fr 87 to Ds 110 ) and the short row 11 of the short-period table (includes 8 elements from Rg 111 to Og 118 ). In the long-period table, a pair of rows 8 and 9 corresponds to period 6 , and a pair of rows 10 and 11 corresponds to period 7 . “Families” of lanthanides, actinides and some grouped metals require special study and attention of researchers. For block D , the N: P ratio is less than 1.55.
Thus, the block structure of the three-dimensional matrix of chemical elements using a three-dimensional coordinate system can provide a significant increase in information content (multidimensionality) compared to short-period and long-period tables of chemical elements.
Various literary sources draw attention to the contribution to the development of the periodic system of chemical elements by D.I. Mendeleev, Yu.-L. Mayer, A. Chancourtois, J. Newlands, W. Odling and G. Hiprix [6].
There may be highlighted the main prerequisites for the creation of the three-dimensional periodic matrix of chemical elements:
– based on the laws of the development of the Universe, the authors propose to consider the structure of the arrangement of chemical elements in the form of an expanding conical spiral. The main initial elements hydrogen and helium are at the beginning of the spiral.
– the spatial spiral provides, in comparison with the tabular form, a sequential continuous arrangement of elements with the possibility of including lanthanides and actinides and all discovered (discovered) and pre- dicted families. All known structures should find their reflection on the spiral on the basis of a deep study of the short-period and long-period tables, taken as a basis in different countries.
Thus, the three-dimensional matrix showcases:
– arrangement of all known elements along the coordinate axes which forms a planetary model of their structure. Atomic numbers n with the dimension of a continuous series of natural numbers from 1 to 118 (and more) are evenly arranged along a spiral from top to bottom.
– the ordinal number of the element coincides with the magnitude of the nuclear charge (Z) and the same total number of energetically balancing electrons in the orbitals of the shells. The approach is universal for both short-period tables and long-period tables.
– an exponential increase in the number of elements in periods from the first to the eleventh (and further) is shown, which forms a 3D-spiral spatial system of the framework of the matrix of chemical elements (Fig. 2) [4].
The proposed representation of the three-dimensional periodic matrix of chemical elements in the form of an expanding conical spiral is a universal tool that makes it possible to study a large variety of physical and chemical properties of already known and not yet discovered elements and their compounds [5]. The versatility of the three-dimensional matrix also lies in the fact that, in addition to the mandatory serial number and strict coordinate binding of chemical elements to groups, there appears a tremendous potential to structurally analyse physical and chemical properties of elements and the laws of their interaction.
-
3. Electron characteristics of elements and analysis of their cyclicity in the structure of the volumetric matrix of chemical elements
The spatial form of the Three-dimensional Periodic Matrix made it possible to structure the anomalous families of the III (lanthanides and actinides) and VIII (metalloids) groups of the third C and fourth D levels of block periodicity, and the level formulas of electron shells facilitate the study of systemic regularities of periodicity, including the mechanisms of saturation and transition electrons at different energy levels (orbital) of electron shells [2]. The consistency of the periodicity is relevant in the study of fine mechanisms of interlevel transitions and stable failures of the synthesis of electron orbitals (Table 2).
Distribution of electrons over energy levels (states) of shells K, L, M, N, O, P, Q, X (EL), consisting of sublevels (orbitals) s-, p-, d-, f-, g-, h - at each level, satisfies the principle of minimum potential energy. The maximum number of electrons at the energy level is calculated by the
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
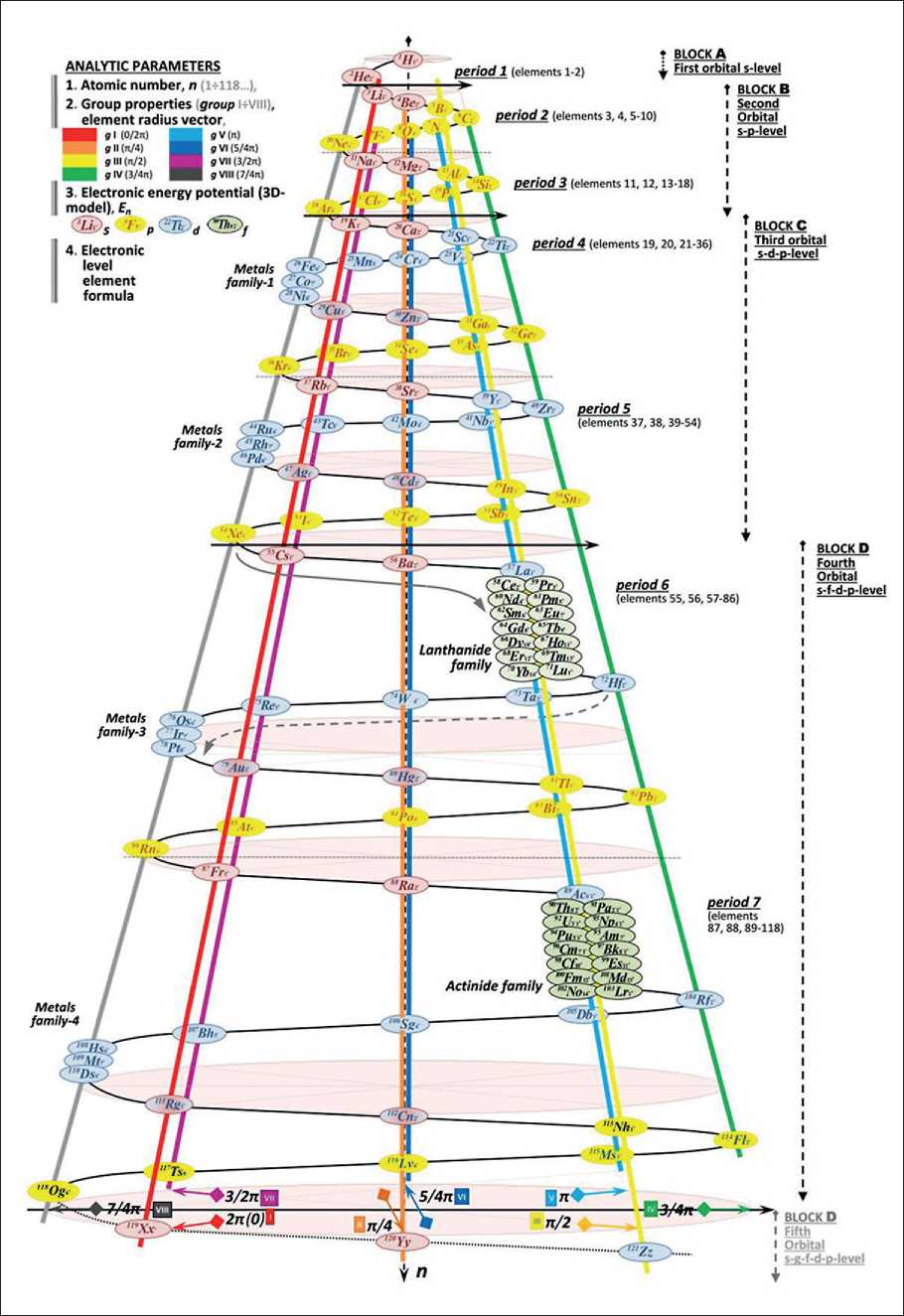
Fig. 2. Three-dimensional matrix of chemical elements with analytical parameters
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES formula R = 2n2, where n is the level number or the principal quantum number (1, 2, 3, etc.). Each filled level corresponds to a certain set of sublevels (orbitals) (Table 3).
The sequence of filling the orbitals with electrons is limited by the Madelung rule [12, 13]. Periodicity, as a cycle of valence transformation in a system of chemical elements, manifests itself in the formation of two successive orbitals – the initial s-orbital and the valence p-orbital – with the number of valence electrons continuously increasing within the periods [8].
Initial are two groups of elements. Group ∫ of chemical elements with one electron at the s 1 sublevel is Li 3 –
Table 2
The structure of energy levels and sublevels (orbitals) for blocks
Block А – 1 energy level ( valence orbital Кs ≡ 1 s2 ) ends with Helium He
Block В – 3 energy levels
КsLs-pMs-p- ( valence orbital M2-6- ≡ 3 s2 3 p6 ) ends with Argon Ar
Block C – 5 energy levels
КsLs-pMs-p-dNs-p-d-Os-p- ( valence orbital O2-6- ≡ 5 s2 5 p6 ) ends with Xenon Ксеноном Xe
Block D – 7 energy levels
К s L s-p M s-p-d N s-p-d-f O s-p-d-f- P s-p-d- Q s-p- ( valence orbital Q2-6- ≡ 7 s2 7 p6 ) ends with Oganesson Og
Predicted energy levels and electronic structure of the orbitals (sublevels) of Block E
Block E – 9 energy levels are predicted. from №119 to №218
К s L s-p M s-p-d N s-p-d-f O s-p-d-f-g P s-p-d-f-g- Q s-p-d-f- R s-p-d- X s-p- ( valence orbital Х2-6- ≡ 9 s2 9 p6 ) ends with element № 218
Table 3
Principal quantum number, types and number of orbitals, maximum number of electrons at sublevels and levels
|
Energy level (n) |
Number of sublevels (n) |
Orbital type |
Number of orbitals |
Maximum number of electrons |
||
|
In sublevel |
In level, equals to n2 |
In sublevel |
In level, equals to 2n2 |
|||
|
К (n = 1) |
1 |
1s |
1 |
1 |
2 |
2 |
|
L (n = 2) |
2 |
2s 2p |
1 3 |
4 |
2 6 |
8 |
|
M (n = 3) |
3 |
3s 3p 3d |
1 3 5 |
9 |
2 6 10 |
18 |
|
N (n = 4) |
4 |
4s 4p 4d 4f |
1 3 5 7 |
16 |
2 6 10 14 |
32 |
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Fr 87 , etc. The filling of the s 1 sublevel with the first electron indicates (signals) the completion of the filling of the valence shell of the previous period. The second group of chemical elements with two electrons at the s 2 – sublevel are Be 4 – Ra 88 , etc. The filling of the s 2 -sublevel with two electrons shows its saturation and the forthcoming formation of the p -valence shell sublevel, starting with the first electron of the p 1 – sublevel and the subsequent set of sublevels of the valence period. For clarity and convenience of analysis, electronic formulas of chemical elements were used.
Thus, the unification of elements in new periods begins with a systemically bound pair of chemical elements of groups I and II , showing the completion of the filling of the valence electron shells of the atoms of the elements of the previous period and the readiness for the formation of the valence electron shell of the new period. In this case, in the chemical elements of group I , an act of formation of a new two-electron initial orbital of the ns sublevel of the corresponding energy level (from K, L, M, N, P, O, Q, X ) occurs by filling the formed energy shell with the first electron ( ns 1 , electronic formula designation K, L1, M1 , etc.), and in the subsequent chemical element of group II, the same atomic orbital is filled with a second saturating electron ( ns 2 , electronic formula designation K2, L2, M2 , etc.), forming a stable pair electrons of the outer atomic orbital. This initial pair completes the filling of the 1s 2 sublevel, after which the next layers of the corresponding energy level of the periodic sequence of chemical elements of the Threedimensional matrix are filled.
On the other hand, the filling of the outer atomic orbital with a stable pair of electrons always precedes the onset of the formation of a new layer of the valence shell of the atom. The initial elements of all periods of the matrix form new periods, on a new valence orbital ( np1 ), by filling the corresponding energy level with the first electron and reflect the periodic laws of the formation of the elements of the material world. The mechanism of block periodicity of filling atomic orbitals is present at all levels of the Three-dimensional periodic matrix of chemical elements.
According to the regularities of the periodicity of the formation of chemical elements, a special role is seen for the initial atomic orbitals of two successive elements. The first element of the pair states the completion of the stable state formation in the structure of the electronic layers of the previous, fully completed period with the limiting filling of the shells of all energy levels with electrons. All first elements are odd. Best practices confirm that the electronic layers of elements shells of a fully completed period have a high degree of resistance to external energy influences and a clear gravitation to stationary interaction with the energy field of the atomic nucleus [9, 12]. This regularity implies the indifference of the electronic layers of extremely filled atomic shells to the manifestation of valence (chemical interaction).
The second element is a harbinger of the formation of new electronic layers of elements in the subsequent period, consisting of electrons of energy levels new shells. All second elements are even. The electronic layers of the shells of the elements of the forming period do not have a high degree of resistance to external energy influences and have a lesser tendency to interact with the energy field of the atomic nucleus. This regularity implies the ability of the outer electronic layers of the unfilled shells of atoms to manifest polyvalence when combined into molecules.
The first elements of the valence p -orbitals of the new period in each energy level form elements with a p 1 -orbit-al with one valence electron ( B 5 – Nh 113 , etc.). All such elements are odd and belong to group III of chemical elements. The second element – with two valence electrons of the p2-orbital in each energy level ( C 6 – Fl 114 , etc.). All elements are even and belong to group IV of chemical elements. The subsequent elements with three valence electrons of the p3 orbital and a different number of electrons of the valence orbital in each energy level are structured similarly, belonging to groups V, VI, VII and VIII of chemical elements, respectively.
The regularities of the formation of models of electronic shells of atoms using electronic-level formulas allow, on the basis of the block approach and structural analysis, to predict chemical elements beyond the 118 th element, to form the structure of new periods, starting with the chemical elements of the 11th period of D.I. Mendeleev Table or the 8th period of the IUPAC table [15]. The electron-orbital formulas assume, based on the block approach, an extremely abbreviated description of the structure of chemical elements.
4. Prediction of new chemical elements
Of the known chemical elements in the short-period system, only 83 are found on Earth, the lightest of which is hydrogen (its atomic number is Z = 1), and the heaviest is uranium (Z = 92). Obviously, only those elements survived in the solar system and on the planet Earth, the lifetime of which is longer than the age of the Earth (4.5 billion years). Others broke up and did not survive to this day. Uranus, which has a half-life of about 4.5 billion years, is still decaying. It is a radioactive element [16]. In nature, stable formations (nuclei of elements, consisting of different numbers of protons and neutrons) exist only up to lead and bismuth, followed by a small area that includes thorium and uranium found on Earth. But as soon as the serial number of an element exceeds the number of uranium, its lifetime decreases sharply. For example, the nucleus of element 100 is 20 times less stable than the nucleus of uranium, and this instability only intensifies further due to the spontaneous nuclear
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES fission. With further attempts to obtain new elements, scientists around the world face the increasing difficulty of synthesis [18–20]. And only a small part of nuclear research ends with the successful synthesis of a new element. No laboratory can be compared with a neutron star, which can create other forms of matter. During the life of stars there take place nuclear reactions that are beyond the human’s power. Scientists are trying to find new types of elements, but experiments in search of “natural” superheavy elements still continue [17]. There arises a question: What is the practical use of such an expensive event to create new unstable elements? Despite this, the development of experimental methods for the transformation of elements led to the expansion of the periodic tables at the expense of transuranic elements, the problem of the table boundary remains one of the most fundamental in modern theoretical chemistry.
The orbital structure representation formulated by the authors makes it possible to predict the block pattern for the emergence of new elements, with the formation of families of lactanoids, actinides and other with d-, f-, g-, h-orbitals in the reverse order between s- and p-orbitals in the third group. There is a special prospect to predict chemical elements of the families of group III outside block D. The reason is that if the cyclic families of lanthanides and actinides of group III in the paired block D consist of 14 f-elements in each period, then the families of elements in the paired block E will consist of 32 g-f-elements in each period, and the families of elements in the paired block F will consist of 54 h-g-f-elements in each period, etc. [15]
The issues that determine the possibility of the predicted elements existence in special physical conditions have not been considered yet. However, taking into account the “theory of stability areas”, supported by the discoverer of the element Og 118 , Academician of the Russian Academy of Sciences Yu.Ts. Oganesyan, such existence of potential chemical elements is possible [16, 17], and the question of predicting new elements arises again. The structural analysis allows to predict the structure of so far unknown elements of the periodic system within 119–168 elements of the 8 th period and within 169–218 elements of the 9 th period of the block structure E , as well as within 219–290 elements of the 10 th period and
Table 4
Block structural analysis for D.I. Mendeleev Table with blocks E and F
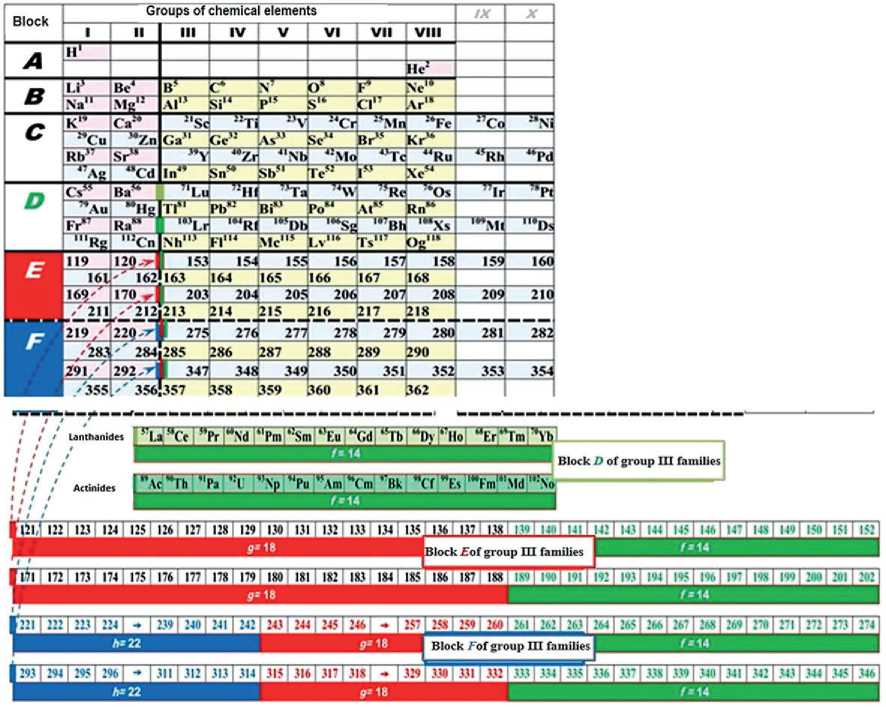
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES
Table 5
Comparison of Mendeleev’s Tables, IUPAC and VMCE
For the first time, a preliminary structural analysis of a short-period table is presented based on the forecast of four new periods of predicted chemical elements (Table 4). Thus, the idea of block structure made it possible to substantiate electronic-level formulas, including the alleged new chemical elements of the 5 th block E with numbers 119 to 218 and further.
Further the use of digital models for research in chemistry will be considered, which can significantly increase the effectiveness of computer modelling methods. Taking into account the crucial role of materials science, it is appropriate to conclude: “ whoever masters the digital system first can become a leader in many spheres of human life .”
CONCLUSION (MAIN CONCLUSIONS)
-
1. The periodic law and its representation in the form of tables of chemical elements is the greatest discovery in chemistry . DI Mendeleev’s two-dimensional tables of chemical elements and IUPAC table played a huge role in the development of chemistry. However, the fact that there are currently more than 500 options for their modernization, including the statements of N.N. Semyonov, indicate the need to continue wor k in this direction at a new level of comprehension.
-
2. First of all, it is the formation of a physical model for the probable origin of chemical elements . The concept of the Universe as an expanding system presented in the work of Stephen Hawking and Martin Rees is a compelling evidence for it. In our opinion, the physical interpretation (model) of the origin and development of the
-
3. Like the Universe, the proposed three-dimensional Periodic Matrix is an expanding system (spiral) and a continuous sequence in the arrangement of elements from hydrogen (1) and helium (2) to Oganesson (118) with the inclusion of lanthanides and actinides in it and possible inclusion of other information with the preservation of the periodicity for the groups of elements and the valence framework of the matrix, proposed by D.I. Mendeleev. Hydrogen and helium are obviously structural elements and all other elements were formed on their basis.
-
4. The authors formulated the concept of cyclicity in the arrangement of the horizontal levels of chemical elements in the blocks of the Three-dimensional matrix of chemical elements. Each of the blocks provides an approximate equality of the neutrons – protons mass ratio in the nuclei of chemical elements. There has been formulated the pattern of 4 levels of block cyclic structure in the existing system of chemical elements . The blocks additionally include all cluster formations, as well as the families of lanthanides and actinides. New regularities of periodicity in the block matrix structure of chemical elements from block A to block D were obtained, combining the periods presented in the tables of chemical elements by D.I. Mendeleev and IUPAC.
-
5. On the basis of cyclicity , the structures of the electron shells for the known 118 elements in four blocks A, B, C, D are presented, which also makes it possible to obtain electron-orbital formulas, including for new elements (119–218) of block E of the periodic system and
-
6. It should be noted that a progressive amount of new elements is in group III of the three-dimensional matrix and special attention should be paid to the formation of chemical elements in this group for blocks C, D and subsequent ones, the number of which is progressively increasing for new blocks E and F . This circumstance 100 years ago made it necessary to move the groups of lanthanides and actinides outside the tables of D.I. Mendeleev and IUPAC.
-
7. Thus, the three-dimensional matrix of chemical elements is a more general structure to further generalize main features of chemical elements (their valence, polyvalence and valence abodes in the tables of D.I. Mendeleev and IUPAC). Its volume and the concept of cyclicity made it possible to make such a generalization, and the tabular forms of D.I. Mendeleev and IUPAC are presented in its composition (Table 5).
-
8. The use of the three-dimensional matrix of chemical elements allows to apply mathematical methods and create digital models for the interaction of chemical elements with each other, which will make it possible to obtain new types of molecules for new materials.
Universe makes it possible to formulate a more general concept of the structuring process of chemical elements and its representation in the form of an expanding conical spiral as well as to draw a number of new conclusions.
THE RESULTS OF THE SPECIALISTS’ AND SCIENTISTS’ RESEARCHES the subsequent block F. In block E, for 100 new elements their atomic numbers and nuclear masses are presented. Elements 119 and 218 could be named after N.N. Semenov Sm1 and Sm2.
Список литературы Arrangement of chemical elements in the three-dimensional matrix
- Mendeleev D.I. Fundamentals of Chemistry. Moscow: State Scientific and Technical Publishing House of Chemical Literature. In 2 volumes. 13th edition. 1947.
- Semyonov N.N. Chemistry and Electronic Phenomena. (UFN, 4 .1924). Selected Works, Vol.2. Moscow: Science; 2005.
- Gusev B.V., Ying Yen-Liang S., Speransky A.A. Three-Dimensional Matrix of Chemical Elements. Moscow: RAE; 2021.
- Gusev B.V., Speransky A.A. Regularities of the Block Approach for the Analysis of the Structure of Chemical Elements and the Problems of Materials Science. Nanotechnologies in Construction. 2019; 11(1): 76–88.
- Gusev B.V., Ying Yen-Liang S., Speransky A.A. Block Structure of Periodicity and Forecasting of New Chemical Elements. Industrial and Civil construction. 2021. Available from: doi: 10.33622/0869-7019.
- Gladyshev V.P. Current State and Methodological Significance of the Periodic Table of Chemical Elements. Tomsk State Pedagogical University. Bulletin of TSPU. 2000; 2 (18). Series: Natural Sciences (Special Issue): 28–32.
- General and inorganic chemistry. Vol. 1. Theoretical Foundations of Chemistry. Textbook for universities in 2 volumes. Ed. A.F. Vorobyov. Moscow: ICC “Akademkniga”; 2004.
- Coulson C. Valence. Moscow: 1965.
- Kuznetsov V.I. General chemistry. Development Trends [Scientific Population]. Moscow: Higher school; 1989.
- Rees M. Just Six Numbers: The Deep Forces That Shape the Universe. Moscow: Alpina non-fiction; 2018.
- Stephen Hawking. About the universe in a nutshell. OGIZ. Moscow: Publishing house AST.
- Dickerson R., Gray G., Hight J. Basic laws of chemistry. Moscow: Mir; 1993.
- Novoshinskiy I.I., Novoshinskaya N.S. Chemistry. Advanced level. Textbook. Russian word. Series: Innovation School. 2018.
- Korablev T.P., Korolkov D.V. Theory of the Periodic System. Saint-Petersburg: University Publishing House; 2005.
- Gusev B.V. New ideas on the three-dimensional matrix of chemical elements and the possibility of the existence of 100 additional new elements. In: Second International Kosygin Readings “Energy-Resource-Efficient Environmental Safe Technologies and Equipment”. Materials of the plenary session. Moscow: RSU; 2019.
- History of the synthesis of superheavy elements. The materials prepared on the basis of information from RAE. Available from: https://ria.ru/20111201/503670512.html
- Maksimchuk A. “The area of stability” among chemical elements. Especially for R&D. Available from: https://maxpark.com/community/6285/content/2676531.
- Hartree D. Calculations of atomic structures. Moscow: Publishing House of Foreign Literature; 1960.
- Zahed Allahyari, Arttem R.Oganov. Nonempirical Definition of the Mendeleev Numbers: Organizing the Chemical Space. Journal of Physical Chemistry C. 2020; 124 (43): 23867–23878.
- Audi G., Kondev F. G., Wang M., Huang W. J., Naimi S. The Nubase2016 evaluation of nuclear properties. Chinese Physics C. 2017; 41(3): 030001-1–030001-138.


