Assocation of genotype and allelotype frequance of interleukine 13 with most viralance gene of staphlococcus aureus
Автор: Al-khafaji R.J., Al-fatlawi B.A.Z., Al-mamory A.A.J.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 31, 2024 года.
Бесплатный доступ
The current study was done to isolation and indentification of the Staphlococcus aureus isolates associated with polycystic ovarian syndrome patients and determine the molecular status for these patients and bacteria. The study included 155 clinical samples, which were collected from November 2022 to June 2023. The samples included blood samples - vaginal swabs. About 75 samples were collected from women with polycystic ovarian syndrome (PCOS) and 30 samples were collected from women with polycystic ovarian disease ( PCOD). They visit Imam Al-Sadiq Hospital and Babylon Teaching Hospital for Maternity and Children in Babylon Governorate. While 50samples were collected from women without polycystic ovaries as a healthy group. All samples were cultured on different media for full bacteriological identification . In addition, blood samples were taken in order to studying molecular status. The results of this study on the polymerase chain reaction revealed a number of genes related to Staphylococcus aureus isolates and some of its virulence factors, including genes (mec A, spa, luks). The study revealed the presence of the mec A gene in 5 out of 38 isolates. And by (13.15 %). While the spa gene was present in 9 out of 38 isolates, with a rate of (23.68 %). While the luks gene was present in 7 out of 38 isolates, with a rate of (18.42 %). while the study included the genes of sick people by detecting some genes, including the study of genetic polymorphisms of the gene for cytokine IL13 in patient samples.
Interleukine 13, staphlococcus aureus, viralance gene
Короткий адрес: https://sciup.org/148328856
IDR: 148328856 | DOI: 10.18137/cardiometry.2024.31.6371
Текст научной статьи Assocation of genotype and allelotype frequance of interleukine 13 with most viralance gene of staphlococcus aureus
Rana jaleil AL-Khafaji, Basaad Abed zaid Al-Fatlawi, AL-mamory Abdulnabi jwaid AL-mamory. Assocation of genotype and al-lelotype frequance of interleukine 13 with most viralance gene of Staphlococcus aureus. Cardiometry; Issue 31; May 2024; p. 63-71; DOI: 10.18137/cardiometry.2024.31.6371; Available from:
Polycystic ovary syndrome (PCOS) is characterized by the unregulated menstrual cycle (oligomenorrhea or amenorrhea), elevated androgenic hormones levels (hyperandrogenism) and many cysts of the ovaries (polycystic ovaries). Other features include male pattern hirsutism, acne, increased skin pigmentation with tags sometimes, and obesity (Zhang et al., 2020). PCOS comorbid with insulin resistance, dyslipidemia, and obesity, it also carries significant risk for the development of cardiovascular and metabolic sequelae, including diabetes and metabolic syndrome (Cincione et al., 2021) . The existence of a fully developed hypothalamic – pituitary – ovarian axis and highly harmonized hormonal feedback circles are essential for the normal ovulatory menstrual cycle. Which consisting of three phases (follicular phase, ovulatory phase, and luteal phase), the normal menstrual cycle leads to the format of a mature follicle and liberate of an oocyte during each cycle, without fertilization menses would occur (Itriyeva, 2022). Every woman in reproductive age exhibit an FSH level rise at the luteal – follicular transition, stimulating a group of follicular growth in the initial follicular phase. The dominant follicle is specified in the mid – follicular phase, and as this dominant follicle grows it progressively secretes in-hibin A and oestradiol for a week prior to ovulation and a later LH surge and oestradiol rise. The progesterone, oestradiol and inhibin A secreted by corpus luteum in response to LH pulses, and arrive at its peak in term of size, vascularization, and secretions (Mihm et al., 2011). Study found the most common causative organism in Polycystic ovary syndrome (PCOS) is Staphylococcus aureus but Escherichia coli, Pseudomonas aeruginosa and Staphylococcus epidermidis may also cause this infection, other gram-negative rods including Klebsiella pneumoniae,Enterobacter spp and Candida spp. which also present in Polycystic ovary syndrome (PCOS) (Springhouse., 2005).The present of this organism with its virulence factors such as (pili, capsule, and toxins) due to hormonal disorders that make the walls of urinary tract drier and the mucous membrane less acidic, which reduces their ability to fight infection ( Graham et al., 2021 )Molecular study of bacteria showed that spa gene is most common in Staphylococcus aureus compared to other genes; The results of clinical isolates are differs comparable to that (Jowad and Yousif, 2013) that found all S. aureus isolates possess spa genes. While this finding comparable from that of (Rezashateri et al ., 2021) that showed the spa gene distribution was present in (82%) of the samples and (Kareem et al ., 2020 ; Ali,2020 ) who showed that spa gene variation was detected in (63.5 %) .The spa is one of the surface proteins of S. aureus. In addition to being a virulence factor of the bacterium, it is used to determine the specific identity of S. aureus. With the molecular typing of this protein, it is possible to prevent epidemics, reduce the number of infections, and reduce the cost of nosocomial infections (Foster et al ., 2014).
MATERIALS AND METHODS
Collection of specimens
The study included 155 clinical specimens, which were collected from November 2022 to June 2023. The specimens included blood specimens – vaginal swabs. About 75 specimens were collected from women with polycystic ovarian syndrome (PCOS) and 30 specimens were collected from women with polycystic ovarian disease ( PCOD). They visit Imam Al-Sadiq Hospital and Babylon Teaching Hospital for Maternity and Children in Babylon Governorate. While 50 specimens were collected from women without polycystic ovaries as a healthy group.
vaginal swaps and blood specimens
The specimens were transported quickly to the bacteriology laboratory in public health laboratory and each specimen was inoculated using direct method of inoculation on different media such as MacCon-key agar and Blood agar, incubated at 37°C for 24-48 hours,(Cheesbrough,2010). While The blood was collected by using sterile syringe 5ml, then transfer to tube without anticoagulant .The serum was separated by centrifugation at about 3000 rpm for 5min within 2-3 hours after collection (Lewis and Bain., 2001).
Laboratory Diagnosis, Bacterial Identification Assays
According to the diagnostic procedures recommended by (MacFaddin, 2000), (Benson,2001) and (Forbes et al ., 2007) the isolation and identification of Gram-Negative Bacteria and Gram-Positive Bacteria that associated with patients under study were performed as follow:
Microscopic Examination and Colonial Morphology
A single colony was taken from each primary positive culture and its identification depended on the morphology properties (Colony size, shape, color,-type of pigments, translucency, edge, elevation,and texture). After staining the bacteria by gram stains, specific biochemical tests were done to reach the final identification include (Catalase Test, Oxidase Test,-Coagulase Test, Indol Test, Vogus-Proskaur Test, Simmons’ Citrate Test, Urease Test, Motility Test, capsule Test) (Bailey and Scott’s, 2017).
Vitek 2 system for Identification
The Vitek-2 system was used to confirm the biochemical test according to the manufacturer’s instruc-tions.( bioMérieux)
Genetics Assay for bacteria
Isolating Genomic DNA from Staphylococcus aureus
DNA Extraction
DNA was extracted by using a Favorgen DNA purification kit and in accordance with the manufacturer’s protocols , .
Polymerase Chain Reaction (PCR) Assay
PCR assay was performed in monoplex patterns in order to amplify different fragments of genes under study for detecting S. aureus virulence factors genes of 5 genes to be amplified separately in PCR technique used in this study Table (1).
Table (1)
Determining Virulence Genes and Encoding Properties
Genes Encoding protein mecA Resistance to Methicillin luks Encoding Panton-Valentine leukocidin toxin spa Encoding Staphylococcal protein A
Table (2)
Primers Used in the Study.
|
Primer Type |
Primer Sequence (5’-3’) |
Amplicon size (bp) |
Reference |
|
|
mecA |
F |
TAGAAATGACTGACGTCCG |
154 bp |
(Santos et al ., 1999) |
|
R |
TTGCGATCAATGTTACCGTAG |
|||
|
R |
ATC GAA CTT TGG CCC ATACTT T |
|||
|
luks |
F |
CAGGAGGTAATGGTTCATTT |
151 bp |
(Al-Talib et al .,2009) |
|
R |
ATGTCCAGACATTTTACCTAA |
|||
|
spa |
F |
ATCTGGTGGCGTAACACCTG |
350 bp |
(Fatemeh Shakeri et al ., 2010) |
|
R |
C GCTGCACCTAACGCTAATG |
|||
|
R |
ACTTCGAATATAAACTTGAATCAATGTTATACAG |
|||
F: Forward Primer *R: Reverse Primer
PCR Cycling Conditions
The PCR mixture had a total capacity of 25 μL, with 12 μL of PCR premix, 2 μL of each primer, and 5 μL of extracted DNA. Table of contents (3) The remaining volume was reduced to 25 μL of Nuclease-free water, followed by vortexes. The negative control comprised all of the materials except template DNA, which was replaced with distilled water. PCR – reaction tubes were briefly centrifuged to mix and bring the contents to the bottom of the tubes before being inserted into a thermal cycler PCR set to Table (4).
Table (3): PCR Mixture
|
Materials |
Volume μL |
|
DNA |
5 μL |
|
Master mix |
12.5 μL |
|
Nuclease free Water |
3.5 μL |
|
Forward gene primer |
2 μL |
|
Reverse gene primer |
2 μL |
|
Total |
25 μL |
Table (4): PCR program that apply in the thermo-cycler.
|
Gene |
Temperature(°C )/Time |
Cycles No. |
||||
|
Initial Denaturation |
Cycling condition |
Final Extension |
||||
|
De-natur-ation |
anneal-ing |
Extension |
||||
|
mecA |
95/5 min. |
95/30 sec. |
55/30 sec. |
72/30 sec. |
72/5 min. |
35 |
|
spa |
95/5 min. |
95/30 sec. |
59/30 sec. |
72/2 sec |
72/5 min. |
35 |
|
luks |
95/5 min. |
95/30 sec. |
52/30 sec. |
72/30 sec. |
72/5 min. |
35 |
Agarose Gel Preparation
and DNA Loading
The agarose gel was made by mixing 1 gm of agarose powder with 100 ml of TBE buffer that had already been prepared (90 ml D.W. were added to 10 ml TBE buffer l0X, the final concentration was 1 X and pH 8). The mixture was placed in a boiling water bath until it became transparent, then allowed to cool to 50°C before adding 0.5 mg/ml ethidium bromide (Al-Sehlawi, 2012(.
The agarose composition, as well as the dye solution, were emptied into the comb-holding tray of the gel electrophoresis device. Furthermore, the agarose was allowed to be sold for 20 minutes in laboratory weather. After that, a comb was gently pulled from the gel inside the tray. The comb created wells that were utilized to load DNA samples (Sambrook and Rusell, 2006).
The agarose gel wells were filled with five microliters of amplified PCR product, followed by a DNA marker (ladder) in one of the wells. The tray containing the gel was placed inside the electrophoresis device, and IX TBE buffer was added to the chamber until it covered the gel’s surface. The electric current was run for 1.5-2 hours at 70 volts (Al-Sehlawi, 2012).
Genotyping Studies of pateint
Genomic DNA Extraction
The samples of whole blood which were collected in EDTA-tubes, then by using a Favorgen DNA purification kit and in accordance with the manufacturer’s protocols ,
Preparation of Primers
The lyophilized oligonucleotide upstream and downstream primers were prepared according to the manufacturing company (Macrogen) and kept at -20ºC.
The reaction mixture
Polymerase Chain Reaction(PCR) were performed in a total volume of 25 μl containing 2μl of both the forward and the reverse of the primer, 12.5μl master mix, 3.5μl free water nuclease and 5 μl of the extracted DNA (as DNA template ), then DNA amplification was carried out with the thermal cycler.
Thermal Cycling Conditions
The reaction was performed in a PCR thermal cycler apparatus, and after several trials, and according to the manufacture’s troubleshooting guide the program was adopted as table(5).
Agarose Gel Electrophorsis
All requirements, technical and preparation of agarose gel electrophoresis for DNA detection and analysis were performed by (Al-Sehlawi, 2012),(Sambrook and Rusell, 2006).
Gel Documentation
The amplified PCR products were detected by agarose gel electrophoresis was visualized by staining with ethidium bromide. The electrophoresis results was detected by using gel documentation system.The positive results were distinguished when the DNA band base pairs of sample equal to the target product size (Bartlett and Stirling,1998). Finally, the gel was photographed using E-graph gel documentation system.
Statistical analysis
Number and percentage were used to express categorical variables. Parametric variables were given as mean ± standard deviation (SD) and significant differences were assessed using the least significant difference (LSD) test. Nonparametric variables were expressed as the median and interquartile range (IQR), and the Mann-Whitney U test (to compare two groups) and the Kruskal-walls test (to compare three or more groups) were used to assess significant differences between medians. Multinomial logistic regression analysis was applied to determine the odds ratio (OR) and 95% CI. The association between the polymorphism and susceptibility to poly cystic ovary syndrome and poly cystic ovary disease was expressed as OR and 96% CI. A probability (p) value ≤ 0.05 was considered significant. The statistical analysis was performed using GraphPad Prism version 9.5.0 (San Diego, California USA).
RESULTS AND DISCUSSION
Clinical study:
The study included 155 clinical specimens,that include age (20-45) years which were collected from November 2022 to June 2023. The specimens included blood specimens and vaginal swabs. They visit Imam Al-Sadiq Hospital and Babylon Teaching Hospital for Maternity and Children in Babylon Governorate.
Genetic study of isolates Staphylococcus aureus by PCR
A total of 105 vaginal swabs specimens were collected from women with polycystic ovarian syndrome (PCOS) and from women with polycystic ovarian disease ( PCOD)., Staphylococcus aureus were abundant isolates 38(40.86%) according to morphological characterization, biochemical tests and vitic technique of five gene were selected for molecular diagnosis of Staphylococcus aureus :
Detection of MecA gene methicillin resistant gene (mec A) was investigated through specific primer for 38 isolates that identified by biochemical test and vitic technique .It was found that 5 (13.15 %) isolates of Staphylococcus aureus gave
Table(5) The Cycling Conditions of the primers used in the study:
|
Markers |
Sequences 5’- 3’ |
Size of amplicon |
Thermal cycler conditions |
|
rs1295686 C/T for IL-13 |
F1 5’ ACATGAGTAGAACGCCAGAG3’ |
765 bp |
95 C°for 5min 95 C°for 30sec 56 C°for 45sec 72 C°for 55sec 72C°for5min |
|
F2 5’ ACGTGAGTAGAACGCCAGAG3’ |
|||
|
R 5’CTCTGCTCACTGTCACTTTGC 3’ |
positive results for this gene whereas 33 (86.84 %) isolates gave negative results. This result was shown in figure (1).
M 1 2 3 4 5 6 7 8 9 10
154bp
spa gene distribution was present in (82%) of the samples and (Kareem et al ., 2020 ; Ali,2020 ) who showed that spa gene variation was detected in (63.5 %) of the isolates.
Detection of luks gene
Leukocidins-component gene ( luks ) was investigated through specific primer for 38 isolates that identified by biochemical test and vitic technique .It was found that 7 (18.42 %) isolates of Staphlococcus aureus gave positive results for this gene whereas 31 (81.57%) isolates gave negative results . This result was shown in figure (3).
Figure (1) Agarose gel electrophoresis of PCR productes for deteciont of methicillin resistant gene ( mec A) gene amplicon product in Staphylococcus aureus . Lane 1-10 refer to isolates’ number. M DNA marker
These findings of isolates are incomparable to that of (Yurtsever et al ., 2020) and (Jowad and Yousif, 2013) they observed that mecA gene is present in all of the isolates (100%) however this result is compatible to that of (Alhamadani and Tuwaij, 2020) and ( Zarei Koosha et al ., 2016), who showed that the mecA gene ratio reached (80%), (87.3%) of S. aureus isolates included the mecA genes.
Detection of spa gene
Staphlococcus aureus protein A gene ( spa ) was investigated through specific primer for 38 isolates that identified by biochemical test and vitic technique .It was found that 9 (23.68 %) isolates of Staphlococcus aureus gave positive results for this gene whereas 29 (76.31 %) isolates gave negative results . This result was shown in figure (2).
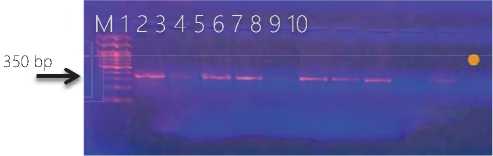
Figure (2) Agarose gel electrophoresis of PCR productes for deteciont of Staphlococcus aureus protein A gene( spa ) gene amplicon product in Staphlococcus aureus . Lane 1-10 refer to isolates’ number. M DNA marker.
The results of isolates are incomparable to that (Jowad and Yousif, 2013) that found all S. aureus isolates possess spa genes. While this finding comparable from that of (Rezashateri et al ., 2021) that showed the
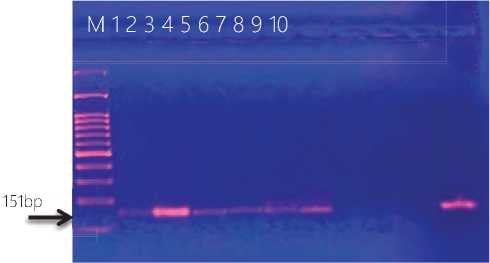
Figure (3) Agarose gel electrophoresis of PCR productes for deteciont of Leukocidins-component gene ( luks ) gene amplicon product in Staphlococcus aureus . Lane 1-10 refer to isolates’ number. M DNA marker.
These results this same results appeared in (Katsa-rou et al ., 2020) and (Neamah et al ., 2019) they found (21.5% ),(27.7%) S. aureus isolates carried luks , the finding did not agree with those of (Basanisi et al ., 2017), who found that 50% of isolated strains carried PVL-encoding genes.PVL may be produced by different strains of S. aureus , in particular both Methicillin-Sensitive S. aureus (MSSA) and MRSA (Shallcross et al ., 2010) .
Genotype Study Results
Hereditary factor assumed to play a central role in the etiology of PCOS.
This study that investigate the relationship of IL-13 with PCOS and PCOD in genetical level by study ( rs1295686) SNP of IL-13 gene.
Polymerase chain reaction (PCR) for interleukin 13 Gene (rs1295686) Polymorphism
IL-13 Gene (rs1295686) SNPs, By using this study’s SNP, through specific primer for 90 sample out of 155 only gave 79 (87.77%) positive, while 11(12.22% ) ne- gitive results. results it was discovered (25.58% CC, 48.84% TT, and 25.58% CT) genotype carries in the PCOS patients, with an allele frequency of (0.38) for C-allele and (0.61) for T-allele. The genotype distribution in PCOD patients is as the following (30.00% CC, 35.00% TT, and 35.00% CT), The C-allele and T-allele frequencies are (0.48) and (0.53) in PCOD patients cases respectively and genotype distribution in the healthy controls are as the following (6.25% CC, 87.5% TT, 6.25% CT. The C-allele and T-allele frequencies are (0.9) and (0.91) in the healthy controls respectively. According to these findings, the frequency of the C allele was significantly higher in PCOS and PCOD compared to healthy controls (HC) (P ≤ 0.002). Such deviation scored an OR value of (6.79) (95% CI: 1.96
to 23.48). In the IL-13 rs1295686 Wild type, the CC genotype showed a significantly increased frequency in all cases compared to healthy control (HC) ( P-Val-ue ≤0.01; OR = 5.54 ; 95% CI =0.67 to 45.23). In the Mutant homozygous (TT), the frequency of all cases with the genotypes TT was significantly elevated in PCOS while decreased in PCOD compared to healthy control (HC) ( P-Value ≤0.006; OR = 0.11; 95% CI =0.023 to 0.54). In the Mutant heterozygous (CT), the frequency of all cases with the genotypes CT was significantly elevated compared to healthy control (HC) ( P-Value ≤0.09; OR = 6.00; 95% CI =0.73 to 48.83).This result show in Table (6) and Figure (4), (5).
In this study, a multiplex allele-specific polymerase chain reaction (ASPCR) protocol was developed to
Table (6): Distributions of genotypes and allele frequencies in IL-13 rs1295686 SNP in the Study population
|
Rs1295686 C\T Genotypes |
Study groups |
OR |
Cl 95% |
P-value |
||
|
PCOS patients N(%) |
PCOD patients N(%) |
Healthy controls N(%) |
References group |
|||
|
CC (wild type) |
11(25.58) |
6 (30.00) |
1 (6.25) |
5.54 |
0.67 to 45.23 |
0.01 |
|
TT (Mutant homozygous) |
21(48.84) |
7 (35.00) |
14(87.5) |
0.11 |
0.023 to 0.54 |
0.006 |
|
CT (Mutant heterozygous |
11(25.58) |
7 (35.00) |
1 (6.25) |
6.00 |
0.73 to 48.83 |
0.09 |
|
Total |
43(100) |
20 (100) |
16 (100) |
|||
|
Allele frequency (%) |
||||||
|
Allele |
PCOS patients |
PCOD patients |
Healthy controls |
OR |
Cl 95% |
P-value |
|
C |
33(0.38) |
19(0.48) |
3 (0.9) |
6.79 |
1.96 to 23.48 |
0.002 |
|
T |
53(0.61) |
21(0.53) |
29(0.91) |
|||
OR=Odd ratio, Cl (95%)=confidence interval, P-value ≤0.05 calculated for estimation of significant difference of the patient’s genotypes and alleles
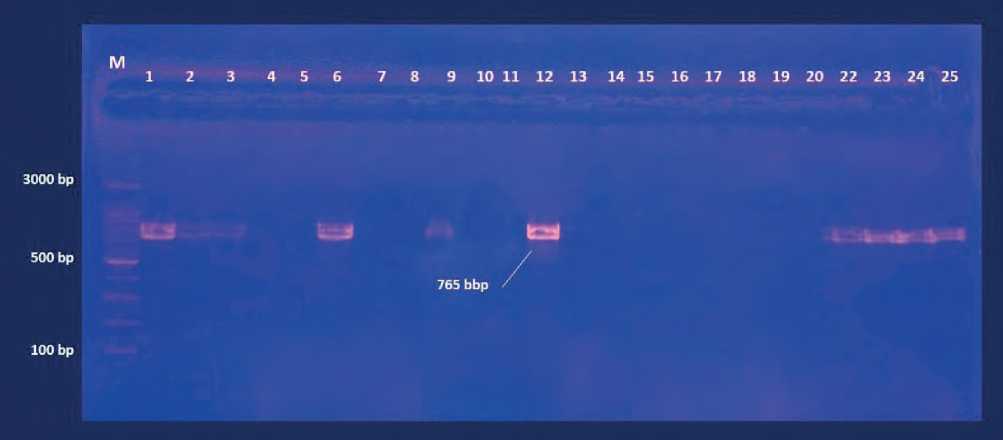
Figure (4) Agarose gel electrophoresis of PCR productes for deteciont of IL-13 (T allelic) polymorphism gene amplicon product in woman .Lane 1-25 refer to sample number. M DNA marker.
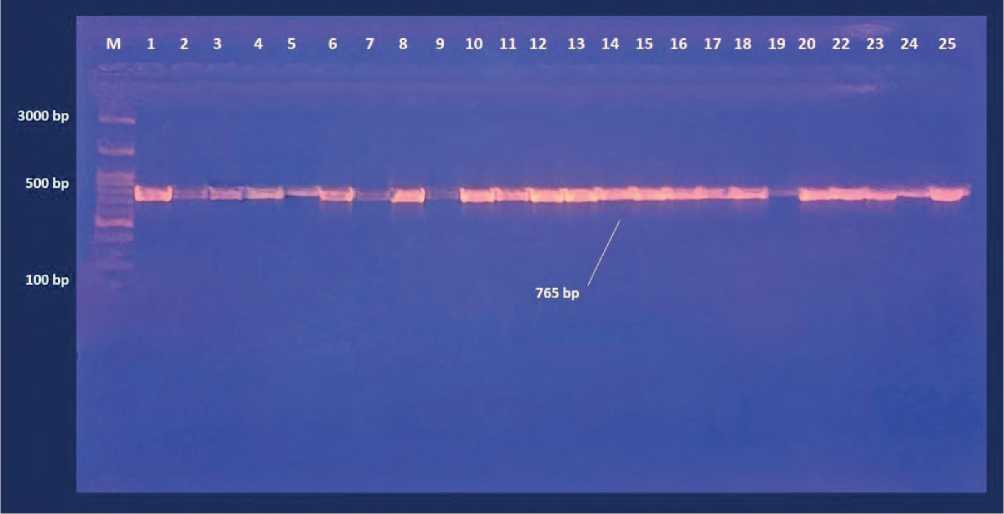
Figure (5) Agarose gel electrophoresis of PCR productes for deteciont of IL-13 (C allelic) polymorphism gene amplicon product in woman .Lane 1-25 refer to sample number. M DNA marker.
genotype people with and without PCOS from the at loci of the IL-13 gene: rs1295686. The aim was to calculate the genotype and allele frequencies of these variants, as well as to evaluate the association of allelic distribution in disease and control groups. A significant association was found between the allelic distribution of the rs1295686variant and PCOS,. The allele model (p = 0.007) indicated 4.125 times increased odds of being diagnosed with PCOS in individuals with the rs10986105 variant G allele. Analysis of phenotypic features showed a significant correlation of metabolic, reproductive, and endocrine anomalies with PCOS in the study population. An altered ratio of luteinizing hormone to follicle-stimulating hormone secretion, manifestations of acne, total testosterone, and higher than normal levels of blood glucose have a positive correlation with PCOS among women. Overall, this study introduces a rapid and straightforward method for simultaneously determining the genotypes at rs1295686 variants in the IL-13 gene. This approach provides a valuable means of predicting the risk of developing PCOS later in life. Several genetic variants have been linked to PCOS. However, such associations vary among populations. A study that looked at chromosomes 2p16.3, 2p21, and 9q33.3 in Han Chinese women found a strong link between PCOS and several loci in the LHCGR (rs13405728), THADA (rs12478601 and rs13429458), and DENN-D1A (rs2479106 and rs10818854) genes (Chen et al.,
2011). A follow-up genome-wide association study (GWAS) conducted on Han Chinese women discovered several more PCOS-associated variants in the FSHR (rs2268361andrs2349415), INSR (rs2059807), TOX3 (rs4784165), RAB5B (rs705702),and YAP1 (rs1894116) genes (Shi et al., 2012). In another study that involved Han Chinese women, a risk haplotype G_A composed of two polymorphisms at the rs6165 and rs6166 loci in the FSHR gene was found to be associated with PCOS, despite no significant individual association with the individual allelic or genotypic distributions of these variants in the FSHR gene (Du et al., 2010). Other studies on Han Chinese women found no association of the FSHR gene variants rs6165 and rs6166 with PCOS (Fu et al., 2013; Wu et al., 2014). No significant association of these variants with PCOS was found in Chinese Singaporean women with PCOS (Tong et al., 2001). However, significant associations have been found between these FSHR gene variants and PCOS in Korean, Japanese, and Caucasian women (Sudo et al., 2002; Valkenburg et al., 2009; Baek, 2010). Another study discovered a strong link between PCOS and the rs346795081, rs346803513, and rs346999236 loci on the THADA, DENND1A, and TOX3 genes, respectively (Chen et al., 2017). Upon studying women of European ancestry and comparing them with Han Chinese PCOS patients, it was discovered that two specific variants, rs10818854 and rs10986105, within the DENND1A gene exhibited a significant associa- tion with PCOS in the European population. (Welt et al., 2012). One study on Caucasian women with PCOS looked at three loci: rs13405728 on the LHCGR gene, rs13429458 on the THADA gene, and rs2479106 on the DENND1A gene, and found a significant association between PCOS susceptibility and the rs2479106 variant (Lerchbaum et al., 2011). Other association studies in Chinese and European populations discovered more loci in diverse genes suchas SOD2 (rs17186366), ERBB4 (rs113168128), WWTR1 (rs144248326), PLGRKT (rs10739076), ZBTB16 (rs1784692),and MAPRE1 (rs853854) (Zhang et al., 2020; Day et al., 2018).The DENND1A gene is located on chromosome 9q22.32 and consists of 22 exons that span about 500,000 bases (McAllister et al., 2014). DENND1A is a single subunit protein that acts as a guanine nucleotide exchange factor (GEF) for RAB35, which is required for botbasal and gonadotropin-releasing hormone-induced gonadotropin release (Marat and McPherson, 2010; Marat et al., 2011; Allaire et al., 2006; Allaire et al., 2010). Variants in the DENND1A gene may influence gonadotropin exocytosis (Welt et al., 2012). This genetic variant has been linked to hyperandrogenism and irregular menstruation in women with PCOS of Han Chinese ancestry as well as European ancestry (Chen et al., 2011; Welt et al., 2012).In this study two DENND1A variants- rs10818854 and rs10986105, that have been found to be strongly correlated with PCOS in both Chinese and European populations (Chen et al., 2011; Welt et al., 2012), were investigated in Bangladeshi PCOS patients. Additionally, the study sought to investigate at the relationships between known phenotypic characteristics of PCOS and disease susceptibility in the Bangladeshi community.
CONCLUSION
Molecular study of bacteria showed that spa gene is the most present in Staphylococcus aureus compared to other genes. Also molecular study of blood showed that (IL-13) polymorphism gene is trace related to pathogenesis of pateints with Polycystic ovary syndrome and Polycystic ovary disease
Список литературы Assocation of genotype and allelotype frequance of interleukine 13 with most viralance gene of staphlococcus aureus
- Alhamadani, R . M . I . and Tuwaij, S.S.N. (2020) . Genetic Diversity of Vancomycin – Resistant Staphylococcus aureus Isolated From Different Clinical Specimens in Al-Najaf Province. M.Sc. Genetics Department / Faculty of Biology/ University of Sofia (2013), Ph.D. in Science/Biology/ Microbiology / University of Kufa (2020) .
- Al-Sehlawi, Z.S.R. (2012). Occurrence and Characterization of 216Ampc Beta-Lactamases in Klebsiella Pneumoniae Isolated from Najaf Hospitals. Ph.D. Thesis. Faculty of Science, University of Babylon Iraq.
- Bailey and Scott’s Diagnostic Microbiology (2017). P. 249, 219-225.
- Basanisi, M. G.; La Bella, G.; Nobili, G.; Franconieri, I. and La Salandra, G. (2017). Genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and dairy products in South Italy. Food Microbiology, 62, 141-146.
- Benson (2001). Microbial applications laboratory manual in 143 -General microbiology . 8th ed . The McGraw – Hill Companies . PP: 168,257 437-261,434.
- Chessbrough, M. (2010). District Laboratory Practice in Tropica Countries, Part-2, New York, USA, Cambridge University,: 184- 186.
- Cincione, R. I.; Losavio, F.; Ciolli, F.; Valenzano, A.; Cibelli, G.; Messina, G. and Polito, R. (2021). Effects of Mixed of a Ketogenic Diet in Overweight and References 106.
- Forbes, B.E .; Sahm, D.F. and Weissfeld, A.S. (2007). Bailey & Scott’s Diagnostic Microbiology.12 th ed. Mosby Elsevier. Texas, USA.
- Foster, T.J. (2017). Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 449_ 430, 007.
- Ghorbani Tajandareh, S. and Imani Fooladi, A. A. (2016). Distribution of tsst-1 and mecA Genes in Staphylococcus aureus Isolated From Clinical Specimens. Jundishapur journal of microbiology, 9(3), e29057.
- Graham ME, Herbert WG, Song SD, Raman HN, Zhu JE, Gonzalez PE, Walther-António MRS, Tetel MJ. Gut and vaginal microbiomes on steroids: implications for women’s health. Trends Endocrinol Metab. 2021;32(8):554–65.
- Itriyeva, K. (2022). The normal menstrual cycle. Current Problems in Pediatric and Adolescent Health Care, 6: 101183.
- Jowad,R.M. and Yousif G.M. (2013). Detection of some Virulence Factors and Antibiotic Resistance Genes in Staphylococcus aureus Isolated from Clinical Cases in Al-Diwanyia City. M.S.C. in biology \ College of Science, Al-Qadisiya University.
- Kareem, S. M.; Aljubori, S. S. and Ali, M. R. (2020). Novel determination of spa gene diversity and its molecular typing among Staphylococcus aureus Iraqi isolates obtained from different clinical samples. New Microbes and New Infections, 34, 100653.
- Katsarou, I.; Paraskevopoulou, N. M.; Papadimitriou- Olivgeris, M.; Giormezis, N.; Militsopoulou, M.; Kolonitsiou, F.; ... and Spiliopoulou, I. (2020). Fatality of Staphylococcus aureus infections in a Greek university hospital: role of inappropriate empiric treatment, methicillin resistance, and toxin genes’ presence. European Journal of Clinical Microbiology & Infectious Diseases, 39(3), 443-450.
- Lewis S.M. and B.J Bain. 2001. Dacie and Lewis, Practical Haematology. 9th ed, Churchill Livigstone: 447-477.
- MacFaddin, J.F. (2000). Biochemical Tests for Identification of Medical Bacteria. 3rd edition. Lippincott Williams and Wilkins, USA.
- Mihm, M.; Gangooly, S. And Muttukrishna, S. (2011). The normal menstrual cycle in women. Animal reproduction science, 124: 229-236.
- Neamah, A. J.; Ayyez, H. N.; Klaif, S. F.; Khudhair, Y. I. And Hussain, M. H. (2019). Molecular and phylogenetic study of Staphylococcus aureus isolated from human and cattle of Al-Qadisiyah Governorate, Iraq. Veterinary World, 12 (9), 1378.
- Rezashateri, M.; Ahrabi, M. and Salehi, M. (2021). Molecular Analysis of the Presence of pvl, spa, and mecA Genes and Their Correlation with a Range of Antibiotics in Staphylococcus aureus Collected from Burn Patients. Iranian Journal of Medical Microbiology, 15(6), 625-637.
- Sambrook, J. and Russell, D.W. (2001). Molecular cloning: a laboratory manual (3rd ed.), Cold Spring Harbor Laboratory Press, Cold Spring. Harbor, NY, USA
- Shallcross, L.J. ; Williams, K. ; Hopkins, S. ; Aldridge, R.W.; Johnson, A.M. and Hayward, A.C. (2010). Panton-valentine leukocidin associated staphylococcal disease: a cross-sectional study at a London hospital, England. Clin Microbiol Infect 2010; 16(11):1644–1648. PubMed PMID: 20969671.
- Springhouse .(2005). Professional Guide to Diseases. 8th ed, Lippincott Williams and Wilkins.Isban; 1:58255.370.
- Tong, S. Y.; Davis, J. S.; Eichenberger, E.; Holland, T. L. and Fowler,J. V. G. (2015). Staphylococcus aureus infections,epidemiology, pathophysiology, clinical manifestations, and Management. Clinical microbiology reviews, 28(3), 603-661.
- Yurtsever, S. G.; Aygül, A.; Öztürk, İ.; Nemli, S. A.; Kaya, S. and Ermertcan, Ş. (2020). Investigation of Various Virulence Factors and SCCmec Types in the Health care-associated and Community-associated Methicillin Resistance Staphylococcus aureus Strains. European Journal of Therapeutics, 26(2), 113-118.
- Zarei Koosha, R.; Mahmoodzadeh Hosseini, H.; Mehdizadeh Aghdam, E.;
- Zhang, Y.; Ho, K.; Keaton, J. M.; Hartzel, D. N.; Day, F.; Justice, A. E.; Josyula, N. S.; Pendergrass, S. A.; Davis, L. K. and Edwards, D. R. V. (2020). A genome- wide association study of polycystic ovary syndrome identified from electronic health records. American journal of obstetrics and gynecology, 223: 559. e1-559. e21.


