Avian IgY antibodies for the immunoprophylaxis and therapy of experimentally infected chicken by Salmonella enterica, Serovar enteritidis
Автор: Hajiyev I., Ali M., Dilbazi G., Hajiyeva I., Sizov A., Kuliyev N., Gasimova M.
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Сельскохозяйственные науки
Статья в выпуске: 11 т.10, 2024 года.
Бесплатный доступ
The possibility of preventing salmonellosis in chicken (pathogen agent - Salmonella enterica , serovar Enteritidis (SE)) by using specific yolk IgY was discussed in presented article. There has been raising the number of reports in the scientific periodicals about increased antibiotic resistance of Salmonella . SE is one of the most common Salmonella serovars worldwide that causes food poisoning in humans. At the same time, infected chicken eggs and meat serve as the main source of infection. For extraction of the IgY, 10 laying hens were used. All layers were hyperimmunized by a vaccine containing live attenuated strains of Salmonella enterica , ser. Enteritidis and Salmonella gallinarum Pullorum. The control group with the same number of layers was not vaccinated. A feed additive for newly hatched chicken and solution with purified and isolated IgY were prepared from egg yolks of immunized and non-immunized birds. During oral administration as a feed additive in per os and intraperitoneal experimental infection by a lethal dose of SE the examination of the efficiency of the obtained product on six-day-old chickens were conducted. Thereby, conducted examinations have shown the high efficiency of the feed additive and preparation with purified immunoglobulins prepared from the yolks of eggs of immunized chicken in experimental infection with a lethal dose of Salmonella Enteritidis.
Salmonella enteritidis, chickens, yolk immunoglobulins, antibiotics
Короткий адрес: https://sciup.org/14131591
IDR: 14131591 | УДК: 636.5.034 | DOI: 10.33619/2414-2948/108/40
Текст научной статьи Avian IgY antibodies for the immunoprophylaxis and therapy of experimentally infected chicken by Salmonella enterica, Serovar enteritidis
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 636.5.034
Enteritidis is the most common serovar associated with acute salmonellosis [1].
It has specific protective features, provided persistence in external environment, in organism of mammals and birds, as well as in bird eggs. These pathogens are difficult to control due to above-mentioned features. It is thought that the SE have a unique combination of genes encoding prevention of cell wall damage [2].
Also, various geographical variations of this strain are distinguished by the widest genotypic and phenotypic antimicrobial resistance profiles, phylogenic relatedness, plasmid and virulence composition [3].
Due to the rapid emergence of resistant Salmonella species is occurring worldwide, the efficacy of the many groups of antibiotics is endangered. Thus, Mølbak K. et al (2002) report that resistance to quinolones in S. E. increased from 0.8% in 1995 to 8.5% in 2000 and this trend continues [4].
The best results in the antibiotic prophylaxis of SE were obtained by the use of enrofloxacin and ofloxacin [5].
However, according to Morales-Barrera et. al (2016) the prophylactic use of enrofloxacin in poultry from the first to the fifth day of life increases further susceptibility to Salmonella infections and reduces the proportion of beneficial intestinal microflora [6].
The negative impact of antibiotics on the gut microbiome and the discovery of a large number of antibiotic-resistant SE isolates in food (particularly in chicken meat and eggs), requires finding a more effective way to control this pathogen. In recent years, egg yolk (IgY) antibodies have been proposed as an alternative to antibiotics. For the first time, the protective properties of these antibodies in experimental mice were described by Klemperer F. (1893). Thereby, it was demonstrated that the egg yolks from immunized hens by Clostridium tetani toxin contain antibodies that neutralize the effect of tetanus toxin [7].
Over the past twenty years, several experimental investigations and reviews have been devoted to the unique properties of egg yolk immunoglobulins [8-13].
İn comparison with isolation of the immunoglobulins from mammals extracting IgY is easy and economically beneficial. High specificity and affinity, thermal resistance and stability over a wide pH range, hypoallergenicity and low immunogenicity, the use of non-invasive physiological and bioethical methods for isolation of yolk antibodies make them very attractive in passive immunization as a preventive and therapeutic agent. Improvements in laboratory methods and the adoption of new technologies make it possible to organize the commercial production of avian antibodies [14].
The companies produce yolk antibodies against widespread pathogens of human and animal diseases that have already been created in some countries. K. Horie et al. (2004) report regarding the preparation of special antigastritis yogurt with chicken antibodies [15].
Yolk immunoglobulins do not activate the mammalian immune system or interact with rheumatoid factor and Fc receptors. All above-mentioned features of IgY mean that they do not activate cascades of inflammatory reactions and do not lead to systemic complications. The lack of interaction with Fc receptors of effector cells makes IgY antibodies indispensable for diseases with etiology relating to antibody-dependent causative agents. The quantity of the protective antibodies is important factor in the production of effective preparation. Immunized hens produce high titers of IgY. At the time of laying, up to 200 mg of IgY can accumulate in the egg yolk [16].
Particular interest attributes towards effectiveness of avian antibodies against pathogens of diarrheal diseases. These infections are considered one of the leading causes of neonatal mortality in humans and animals. Diarrheal diseases caused by a mixed infection with various dangerous viruses, pathogenic serotypes of salmonella and colibacillus, are difficult to treat. The high resistance of yolk antibodies to the aggressive intestinal environment, especially in the presence of protectors (ovalbumin and ovomucoid of egg white or milk), can have a positive therapeutic effect in intestinal infections during the neonatal period of mammalian development [17].
Salmonella enterica subsp. enterica ser. Enteritidis (SE) is an intestinal zoonotic pathogen that is the most common cause of diarrhea in humans. The chickens serving as the main asymptomatic vectors for this disease [18].
The most common cause of food poisoning in humans is the consumption of contaminated meat or eggs produced by infected laying hens [2].
For all serotype of the pathogen, vaccination and the use of antibiotics are not sufficient to prevent and control salmonellosis in poultry [19]. In this regard, it is very promising to consider the use of specific yolk IgY antibodies for control of salmonellosis. Chalghoumi R. et al (2009) report that specific IgY has an inhibitory effect on the growth of SE and Salmonella typhimurium in vitro [20].
The researchers conclude that passive immunization with Salmonella-specific IgY may be useful in preventing colonization by this pathogen of broiler chickens and subsequent contamination of trunks during meat processing.
Considering the relevance of the problem of salmonellosis in chickens, a series of experiments to obtain a preventive anti-salmonella feed additive and a product with purified IgY immunoglobulins from the egg yolks of chickens, hyperimmunized with the anti-salmonella vaccine was conducted. As investigations have shown, the created feed additive and purified immunoglobulins had a preventive effect during experimental infection of chickens with SE.
Material and Methods
Hyperimmunization of laying hens. 10 one-year-old outbred chickens were selected for hyperimmunization. The newly hatched chickens were kept in one of the buildings of the Veterinary Research Institute. Contacts with other birds were excluded, and maintenance was carried out by special personnel. The AviPro Salmonella Vac E vaccine against avian salmonellosis for 1000 doses for oral administration was used as antigen. This vaccine contains lyophilized cultures of bacterial cells of the attenuated strain S. Enteritidis Sm24/Rif12/Ssq, with a total volume of 10 cm3.
The vaccine was previously diluted in 800 ml of physiological solution. The first two doses of 1 ml were administered orally with a one-week interval. Subsequently, the diluted vaccine was administered weekly to each chicken, 1 ml orally and 1 ml into the pectoral muscle. A total of 5 injections were made. For B and T memory cells activation, the last injection of the vaccine was carried out 30 days after the previous one.
Manipulation with the eggs, obtaining feed additives, isolation and purification of immunoglobulins. After hyperimmunization was completed, the eggs were processed in a Delta MLD 120 microbiological laminar flow hood. The eggs were washed in a 0.5% chloramine solution to clean the shells of contamination. They were broken manually, separating the yolk from the white using a special cup. The yolks were stirred until they became mélange. The obtaining liquid was poured into food-grade PET bottles (GOST 33756) with a volume of 100 ml. Pasteurization of the mélange was carried out at a temperature of 60°C for 15 minutes in a Julabo SW22 water bath. IgY immunoglobulins do not lose their activity at this temperature and time exposure [21].
Preparation of feed additive. The prepared mélange was poured into Petri dishes and placed in a drying oven. The drying lasted 8 hours at a temperature of 38 °C. The obtaining dry, slightly sticky mass was crushed and collected in sterile vials. Further storage was carried out in a freezer at a temperature of ‒14°C.
Isolation and purification of IgY. The purified immunoglobulins were obtained by dilution of the egg yolks with distilled water in a ratio of 1:8 [22].
The mixture was homogenized on a magnetic stirrer (Magnetic Hot Plat model 78-1) at 500 rpm for 30 min. The obtaining material was stored in a Telstar Boreas freezer at –18°C for 12–14 hours. Defrosting was carried out at room temperature. To separate the supernatant from the sediment, it was centrifuged at 10,000 rpm for 15 min in refrigerated high speed centrifuge Celesta Centrifuger BLT. The immunoglobulins were precipitated using a saturated solution of ammonium sulfate (NH 4 ) 2 SO 4 ) in a 1:1 ratio to the supernatant at pH 7.0. After this, the supernatant was aspirated, the sediment was reached up to 10 ml with distilled water, and then resuspended.
Dialysis. To purify the solution from ammonium sulfate and other low-molecular compounds, the dialysis method was used. The MD25 dialysis bags with sediment were placed in a beaker containing 5 liters of 0.01 M phosphate buffer solution (pH 7.2). The buffer solution was changed three times within 24 hours.
Determination of the quantity of protein in the obtaining precipitate. Determination of concentration of the IgY was carried out according to the Bradford method [23] on Lambda Bio+ spectrophotometer.
The experiments to test prophylactic efficacy of feed additives. To conduct experiments to test the preventive effect of the feed additive against salmonellosis, 3 groups of newly hatched chicken (10 birds in each group) were formed. These chicken were fed the following diet. The control group received regular factory chicken feed: cereals (barley, corn and wheat), semolina, oatmeal and millet. The egg yolks from non-immunized chicken (first experimental group) were dried at a temperature of 37°C and added to the factory feed in 1:1 ratio. The egg yolks dried at the temperature of 37°C from chicken hyperimmunized with the AviPro Salmonella Vac E vaccine (second experimental group) were added in a 1:1 ratio to the factory feed of the third group. Experimental infection of chicken of all three groups with salmonellosis was carried out on the 6th day of life by oral administration of 0.5 ml (107 CFU) of a biosuspension of a daily SE culture.
The experiments to test prophylactic efficacy of product containing IgY immunoglobulins with intraperitoneal administration of a salmonella culture. Experiments to test the prevention of salmonellosis with a product containing IgY were carried out on six-day-old chicken, divided into three groups (ten birds each group). The newly hatched chicken were fed by a factory feed mixture with the addition of an equal proportion of dried egg yolks from hyperimmunized chicken. The experiments were conducted to the following scheme:
-
— The control group of chicken was intraperitoneally injected with a suspension of a daily SE culture at a dose of 0.5 ml (107 CFU/ml) with simultaneous administration of a sterile isotonic sodium chloride solution in the same dose;
-
— The second group was intraperitoneally injected with an SE culture at the same dose, with simultaneous administration of a drug in a dose of 0.5 ml obtained from the yolks of eggs of nonimmunized chickens after purification and dialysis (20 mg of nonspecific IgY);
— The third group was intraperitoneally injected with the SE culture in the same dose, with simultaneous administration of the drug in a dose of 0.5 ml, obtained after purification and dialysis from the yolks of hyperimmunized chicken eggs (20 mg of IgY).
Groups of control and experimental chicken were kept under identical conditions. To avoid the spread of the pathogen, experimental infection of chickens with salmonellosis was reproduced under strictly controlled habitat. The experimental chickens were observed for 14 days. The effectiveness of the prophylactic measurements was assessed by chicken survival and general clinical condition.
Results
The results obtained in the first experiment to determine the preventive effectiveness of the feed additive are shown in the diagram (Figure 1).
The mortality of chicken of the first control group had already observed on the first day after exposure. A quantity of 0.5 ml (107 cfu) of a biosuspension of the day-old SE culture by oral administration of suspension of the SE culture was found to be a 100% lethal dose for six-day-old chicken were receiving factory feed. Somewhat different situation was observed in the second control group, where dried yolks from eggs of non-immunized chicken were added to the feed. Three birds died on the first day (30%). Six more chicken died on the third day of observation. The last chicken died on the seventh day. The chicks of this group showed weakness and lethargy, and the feathers in the anus were dirty. Chicks that died late had convulsions before death. There was 100% survival rate of chicks that received a feed additive with dried yolks obtained from chicken hyperimmunized with Salmonella vaccine.
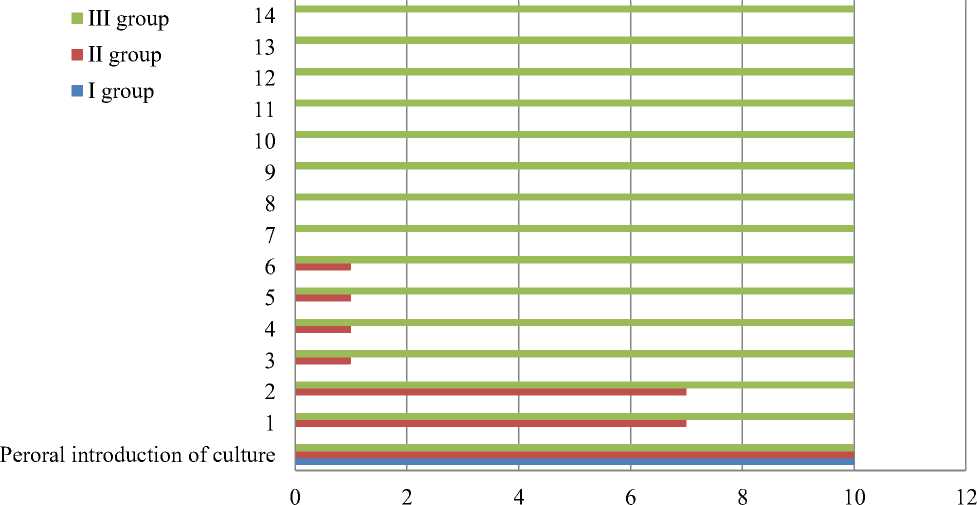
Figure 1. Preventive effectiveness of a feed additive against infection by Salmonella culture. The x-axis and y-axis are days and number of experimental birds, accordingly
The results of experiments for chicken intraperitoneally injected with Salmonella culture are shown in the second diagram (Figure 2).
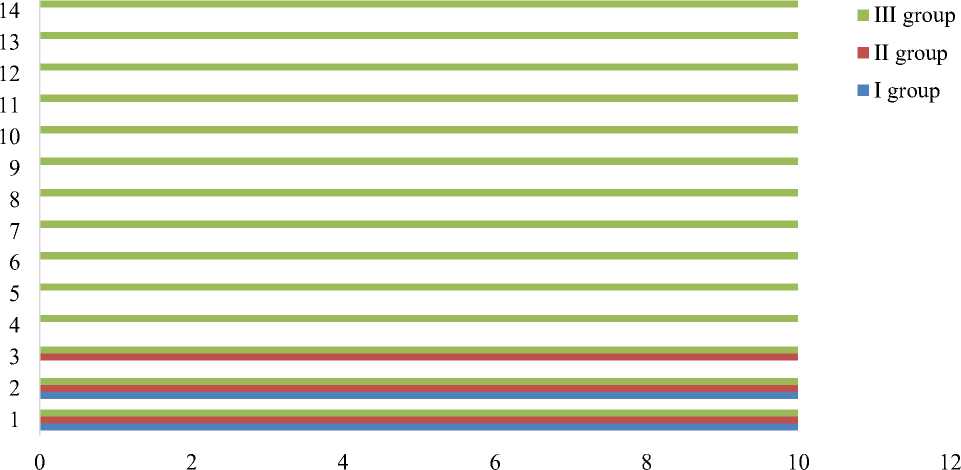
Figure 2. Results of intraperitoneal injection of Salmonella culture. The x-axis and y-axis are days and number of experimental birds, accordingly
For the first group, isotonic sodium chloride solution was injected intraperitoneally at the same time as the injection of Salmonella culture. During the first day, the chicken demonstrated a depressed state. On the third day, all the chicken died. In the second group situation was identical to the first one. On the fourth day, all the chicks in this group died. In the third group, the survival rate of chicks was 100%. However, no clinical changes in the condition of the chicks of this group were observed. During the entire observation period, the chicks remained active.
Discussion
The practical significance of research is the possibility of usage feed additives enriched with specific IgY antibodies against poultry diarrheal pathogens that are common in rural areas. Due to the mass death of chicks from diarrhea in villages in the first days of life, the population has no longer bred birds themselves, instead they purchase chicks from breeders (entrepreneurs who raise them to the age of one month and then sell them to the people). The most typical pathogens of newly hatched chicks are members of Enterobacteriaceae, such as Salmonella gallinarum-pullorum and Salmonella enteritidis [24]. Breeders use antibiotics from the birthday of chicks for prevention of diarrheal diseases [25, 26].
The result of this preventive approach is predictable. The formation of the intestinal microbial community, which plays a vital role in nutrient absorption, immunity and disease resistance, is inhibited. Such chicks develop poorly and often get sick, that makes their keeping economically unprofitable. The analysis of veterinary reports of the districts showed that up to a year the loss of poultry by the population reaches 30%. A feed additive with specific yolk antibodies against pathogenic Salmonella can successfully replace daily antibiotic doses to newly hatched chicks. The product with these antibodies showed a significant prophylactic effect even in the case of massive oral infection of chicks. In the group of chickens receiving egg yolks from unimmunized hens, the mortality of Salmonella infection was delayed compared to the control group. Explanation for this fact may be that immunoglobulins in egg yolks from unimmunized birds have some protective properties against salmonellae. The usage of yolk antibodies as an alternative to antibiotics does not impair the formation of the gut microbiota and immune system and ultimately the adaptive immunity of the whole young organism. Perspectivity of using yolk antibodies in diagnostics, treatment and prophylaxis of infectious diseases is proved by numerous experimental researches published in the last 20 years. However, they have not been widely applied in practice up to the present time [27].
To solve this problem, it is necessary to further popularization of IgY technologies, to reduce the cost of production and to develop a protocol for industrial production of additives with yolk antibodies as the active ingredient. IgY technology provides new opportunities in the creation of drugs used in various fields of biology and, most importantly, as an alternative to antibiotics.
Conclusion and recommendations
Chicken meat and eggs are the main source of the of the causative agent of zoonotic salmonellosis. Prophylaxis and monitoring of salmonellosis at the chick rearing period can significantly reduce contamination of eggs and chicken meat during slaughtering and processing. Due to the negative impact of antibiotics on the formation of the intestinal microbiota of chicks and the tightening of food hygiene laws, oral passive immunization with pathogen-specific antibodies of egg yolk (IgY) may be a useful and attractive method for the prevention of diarrheal diseases of chicken. Keeping chicken hyperimmunized with antigens of pathogens of gastrointestinal diseases of animals and birds in small enterprises of Republic will not be a difficult and costly.
At the general meeting of Barda district of Azerbaijan Republic, organized by the veterinary administration, veterinary and zoological specialists discussed the results and perspectives of the application of yolk immunoglobulins in the control of infectious diseases.
Список литературы Avian IgY antibodies for the immunoprophylaxis and therapy of experimentally infected chicken by Salmonella enterica, Serovar enteritidis
- Ferrari, R. G., Rosario, D. K., Cunha-Neto, A., Mano, S. B., Figueiredo, E. E., & Conte-Junior, C. A. (2019). Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Applied and environmental microbiology, 85(14), e00591-19. https://doi.org/10.1128/AEM.00591-19
- Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., Gast, R., Humphrey, T. J., & Van Immerseel, F. (2009). Mechanisms of egg contamination by Salmonella Enteritidis. FEMS microbiology reviews, 33(4), 718-738. https://doi.org/10.1111/j.1574-6976.2008.00161.x
- Alzahrani, K. O., Al-Reshoodi, F. M., Alshdokhi, E. A., Alhamed, A. S., Al Hadlaq, M. A., Mujallad, M. I., ... & Alajel, S. M. (2023). Antimicrobial resistance and genomic characterization of Salmonella enterica isolates from chicken meat. Frontiers in Microbiology, 14, 1104164. https://doi.org/10.3389/fmicb.2023.1104164
- Mølbak, K., Gerner-Smidt, P., & Wegener, H. C. (2002). Increasing quinolone resistance in Salmonella enterica serotype Enteritidis. Emerging infectious diseases, 8(5), 514. https://doi.org/10.3201/eid0805.010288
- Skvortsov, V. N., Yurin, D. V., Nevzorova, V. V., & Mazur, A. D. (2020). Terapiya eksperimental'nogo sal'monelleza tsyplyat antimikrobnymi preparatami gruppy ftorkhinolonov. Mezhdunarodnyi vestnik veterinarii, (2), 104-107. (in Russian).
- Morales-Barrera, E., Calhoun, N., Lobato-Tapia, J. L., Lucca, V., Prado-Rebolledo, O., Hernandez-Velasco, X., ... & Tellez, G. (2016). Risks involved in the use of enrofloxacin for Salmonella Enteritidis or Salmonella Heidelberg in commercial poultry. Frontiers in veterinary science, 3, 72. https://doi.org/10.3389/fvets.2016.00072
- Klemperer, F. (1893). Ueber natürliche Immunität und ihre Verwerthung für die Immunisirungstherapie. Archiv für experimentelle pathologie und pharmakologie, 31, 356-382. https://doi.org/10.1007/BF01832882
- Kaplin, V. S., & Kaplina, O. N. (2023). The use of avian yolk antibodies in biomedical research. Laboratory Animals for Science, 3. https://doi.org/10.57034/2618723X-2023-03-04
- Hatta, H., Tsuda, K., Akachi, S., Kim, M., Yamamoto, T., & Ebina, T. (1993). Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Bioscience, biotechnology, and biochemistry, 57(7), 1077-1081. https://doi.org/10.1271/bbb.57.1077
- Lee, L., Samardzic, K., Wallach, M., Frumkin, L. R., & Mochly-Rosen, D. (2021). Immunoglobulin Y for potential diagnostic and therapeutic applications in infectious diseases. Frontiers in Immunology, 12, 696003. https://doi.org/10.3389/fimmu.2021.696003
- Rahimi, S., Shiraz, Z. M., Salehi, T. Z., Torshizi, M. K., & Grimes, J. L. (2007). Prevention of Salmonella infection in poultry by specific egg-derived antibody. Int J Poult Sci, 6(4), 230-5.
- Spillner, E., Braren, I., Greunke, K., Seismann, H., Blank, S., & du Plessis, D. (2012). Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals, 40(5), 313-322. https://doi.org/10.1016/j.biologicals.2012.05.003
- Angulo, C., Sanchez, V., Delgado, K., Monreal-Escalante, E., Hernández-Adame, L., Angulo, M., ... & Reyes-Becerril, M. (2022). Oral organic nanovaccines against bacterial and viral diseases. Microbial Pathogenesis, 169, 105648. https://doi.org/10.1016/j.micpath.2022.105648
- Svendsen, L., Crowley, A., Ostergaard, L. H., Stodulski, G., & Hau, J. (1995). Development and comparison of purification strategies for chicken antibodies from egg yolk. Laboratory animal science, 45(1), 89-93.
- Horie, K., Horie, N., Abdou, A. M., Yang, J. O., Yun, S. S., Chun, H. N., ... & Hatta, H. (2004). Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. Journal of dairy science, 87(12), 4073-4079. https://doi.org/10.3168/jds.S0022-0302(04)73549-3
- Lanzarini, N. M., Bentes, G. A., Volotão, E. D. M., & Pinto, M. A. (2018). Use of chicken immunoglobulin Y in general virology. Journal of Immunoassay and Immunochemistry, 39(3), 235-248. https://doi.org/10.1080/15321819.2018.1500375
- Li, X., Wang, L., Zhen, Y., Li, S., & Xu, Y. (2015). Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: a review. Journal of animal science and biotechnology, 6, 1-10. https://doi.org/10.1186/s40104-015-0038-8
- Awad, W. A., Aschenbach, J. R., Khayal, B., Hess, C., & Hess, M. (2012). Intestinal epithelial responses to Salmonella enterica serovar Enteritidis: effects on intestinal permeability and ion transport. Poultry science, 91(11), 2949-2957. https://doi.org/10.3382/ps.2012-02448
- Calenge, F., Kaiser, P., Vignal, A., & Beaumont, C. (2010). Genetic control of resistance to salmonellosis and to Salmonella carrier-state in fowl: a review. Genetics Selection Evolution, 42, 1-11. https://doi.org/10.1186/1297-9686-42-11
- Chalghoumi, R., Thewis, A., Beckers, Y., Marcq, C., Portetelle, D., & Schneider, Y. J. (2009). Adhesion and growth inhibitory effect of chicken egg yolk antibody (IgY) on Salmonella enterica serovars Enteritidis and Typhimurium in vitro. Foodborne Pathogens and Disease, 6(5), 593-604. https://doi.org/10.1089/fpd.2008.025
- Gandhi, S., & Alshehri, S. M. (2020). Molecular stability of the rabbit and chicken egg yolk immunoglobulins. Frontiers in Bioscience-Elite, 13(1), 185-194. https://doi.org/10.2741/877
- Yudina, A. N., & Krasnoshtanova, A. A. (2019). Sposoby vydeleniya immunoglobulinov iz zheltka yaits sel'skokhozyaistvennoi ptitsy. Uspekhi v khimii i khimicheskoi tekhnologii, 33(5 (215)), 49-50. (in Russian).
- Walker, J. M. (2002). The protein protocols handbook. Humana press. https://doi.org/10.1385/1592591698
- Shaji, S., Selvaraj, R. K., & Shanmugasundaram, R. (2023). Salmonella infection in poultry: a review on the pathogen and control strategies. Microorganisms, 11(11), 2814. https://doi.org/10.3390/microorganisms11112814
- Chalghoumi, R., Beckers, Y., Portetelle, D., & Théwis, A. (2009). Hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: a review. Biotechnologie, Agronomie, Société et Environnement, 13(3). https://hdl.handle.net/2268/117115
- Diraviyam, T., Zhao, B., Wang, Y., Schade, R., Michael, A., & Zhang, X. (2014). Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: a systematic review and meta-analysis. PloS one, 9(5), e97716. https://doi.org/10.1371/journal.pone.0097716
- Yakhkeshi, S., Wu, R., Chelliappan, B., & Zhang, X. (2022). Trends in industrialization and commercialization of IgY technology. Frontiers in Immunology, 13, 991931. https://doi.org/10.3389/fimmu.2022.991931


