Биокаталитический синтез оптически чистых сульфоксидов
Автор: Кылосова Татьяна Ивановна
Журнал: Вестник Пермского университета. Серия: Биология @vestnik-psu-bio
Рубрика: Биотехнология
Статья в выпуске: 3, 2014 года.
Бесплатный доступ
Рассмотрены основные биокаталитические способы синтеза энантиомерно чистых сульфок-сидов, включающие использование индивидуальных ферментов и целых клеток микроорганизмов, принадлежащих к родам Acinetobacter, Aspergillus, Pseudomonas, Rhodococcus.
Оптически активные сульфоксиды, биокатализаторы, биотрансформация органических сульфидов
Короткий адрес: https://sciup.org/147204686
IDR: 147204686 | УДК: 579.66+547.544
Текст научной статьи Биокаталитический синтез оптически чистых сульфоксидов
Оптически активные (энантиомерно однородные, хиральные) сульфоксиды находят широкое применение в химической и фармацевтической практике. Получение оптически чистых сульфоксидов осуществляется в основном методами химического синтеза [Mata, 1996]. При использовании химических методов для получения хиральных сульфоксидов не всегда достигается высокая энан-тиоселективность реакций [Толстиков, Гришко, Ившина, 2003].
Альтернативой многостадийному химическому синтезу оптически активных соединений выступают биологические технологии, позволяющие существенно повысить уровень регио- и стереоселективности реакций. В качестве эффективных биокатализаторов процесса окисления прохиральных органических сульфидов используются ферментные препараты (окисигеназы, пероксидазы) [Zhang, Li, Xu, 2010; Ceccoli, Bianchi, Rial, 2014] и целые микробные клетки [Biotransformation ..., 2003; Isolation ..., 2009; Enantioselective ..., 2013; Highly ...,2013].
Оптически активные сульфоксиды
Оптически чистые сульфоксиды — соединения, обладающие широким спектром биологических свойств, начиная от вкусовых и ароматических предшественников до проявления антимикробной, противогрибковой активности [Fernandez, Khiar, 2003; Bently, 2005]. Биологически активные сульфоксиды обнаружены в составе экстрактов лука, чеснока, растений сем. Крестоцветные (горчица, редис, двояко плодник). Хиральную сульфоксидную группу содержит полифункциональный антибиотик спарсомицин - продукт жизнедеятельности стрептомицетов Streptomyces sparsogenes [Толстиков, Гришко, Ившина, 2003].
Энантиомерно чистые сульфоксиды широко используются в качестве высокоэффективных фармаконов и экологически безвредных инсектицидов. Среди современных лекарственных средств, действующим началом которых являются хиральные сульфинилсодержащие соединения, можно отметить противоязвенные (пантопразол, рабепра-зол, лансопразол, омепразол, тенатопразол) и ноотропные (армодафинил) препараты, эффективность которых обусловлена различием в скорости метаболизма (S)- и (/^-сульфоксидов [Drug interaction ..., 2001; McConathy, Owens, 2003; Armodafinil ...,2011].
В настоящее время хиральные органические соединения находят свое применение в качестве стереонаправляющих групп или хиральных строительных блоков для синтеза сложных по структуре фармацевтических препаратов [Synthesis ..., 2011; Cationic ..., 2013]. В асимметрическом синтезе сульфоксидная группа служит в качестве активного центра, способного с высокой стереоселективностью контролировать реакции алкилирования или конденсации карбанионов с последующим восстановительным удалением RS(O)-rpynnbi при синтезе насыщенных соединений; введения, а затем удаления RS(O)-rpynnbi в виде сульфеновой кислоты при синтезе олефинов; циклоприсоединения и альдольной конденсации [Сагеио, 1995; Прилежаева, 1998].
Биокаталитический синтез оптически активных сульфоксидов с использованием целых клеток микроорганизмов
Биологический синтез оптически активных сульфоксидов может проводиться путем асимметрического окисления прохирального сульфида с образованием оптически чистого (8)- или (7?)-сульфоксида и кинетического разделения рацемической смеси (5)- и (7?)-изомеров [Synthesis ..., 2011] (рис. 1).
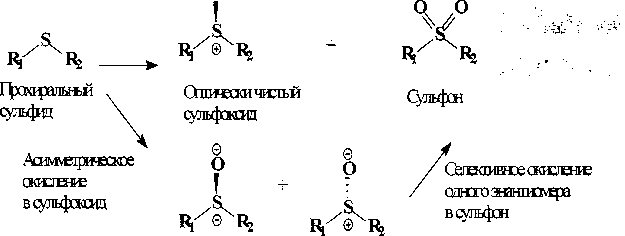
Эяшисмер! Э-внтимр2
Рис. 1. Способы получения оптически чистых сульфоксидов
Возможность практической эксплуатации биологических катализаторов с целью трансформации прохиральных сульфидов показана в многочисленных работах [Holland, 2001; Fernandez, Khiar, 2003; Biocatalytic ..., 2006; Isolation ..., 2009; Resolution ...,2011; Zhang, Li, Xu, 2010; Protein engineering of nitrobenzene ..., 2013]. В качестве катализаторов процесса сульфоксидирования используются как индивидуальные ферменты [Zhang, Li, Xu, 2010; Ceccoli, Bianchi., Rial, 2014], так и целые микробные клетки [Biotransformation ..., 2003; Isolation ..., 2009; Enantioselective ..., 2013].
Перспективность использования целых клеток микроорганизмов в качестве биокатализаторов процесса синтеза оптически чистых соединений обусловлена тем, что спектр метаболизируемых ими веществ намного шире естественных субстратов отдельных ферментов [Synthesis ...,2011]. Исследования по биологической трансформации органических сульфидов в сульфоксиды проводятся преимущественно с использованием мицелиальных грибов [Biotransformation ..., 2003; Pinedo-Rivilla, Aleu, Collado, 2007; Stereoselective biotransformations .... 2009; Aspergillus genus ..., 2013], дрожжей [Oxidative ..., 1995; Bakers' yeast ..., 1995], бактерий [Biotransformation ..., 2003; Opposite enantioselectivi-ties ..., 2005; Isolation ..., 2009; Enantioselective ..., 2013; Stereoselective oxidation ..., 2014], также описаны единичные примеры с использованием микроводорослей [Daligault, Niguer-Chauvin, Patin, 2006].
Способность к биотрансформации арилалкил-сульфидов и бензотиофена обнаружена у грамотри- цательных бактерий родов Acineiobacter, Pseudomo nas, Streptomyces. Так, мутант почвенных псевдо-
^^ад Pseudomonas putida UV4, содержащий активную толуолдиоксигеназу, катализирует окисление тиоанизола с образованием соответствующего (7?)-фенилметилсульфоксида с оптической чистотой >90% [Toluene ..., 1998; The oxidation ..., 2013]. Показано, что Р. montelii ТВ-1 катализирует образование (7?)-ар ил алкиловых сульфоксидов с химическим выходом 55-99% и энантиоселективностью 63-99% [Stereoselective oxidation ..., 2014]. В работе M.L. Mascotti с соавт. ^Aspergillus genus ..., 2013] впервые описаны эксперименты по биотрансформации алкил-и диалкилсульфидов с использованием Streptomyces phaeochromogenes NCIMB 11741, X flavogriseus ATCC33331, S. hiroshimensis ATCC 27429. Несмотря на то, что полученные сульфоксиды зачастую обладали высокой оптической чистотой, химический выход их был незначительным (до 50%).
Высокой сульфидокисляющей активностью характеризуются актинобактерии родов Gordonia и Rhodococcus. Клетки Gordonia terrae ИЭГМ 136 и
Rhodococcus rhodochrous ИЭГМ 66 катализируют образование (7?)- и (б)- арилалкилсульфоксидов с энантиоселективностью 85 и 95% соответственно
[Елькин, Гришко, Ившина, 2010; Enantioselective ..., 2013]. С использованием клеток Rhodococcus sp. ECU0066 детально исследован механизм образования (8)-энантиомернооднородного сульфоксида из метилфенилового сульфида путем селективного окисления (7?)-изомера в сульфон [Isolation ..., 2009] (рис. 2).
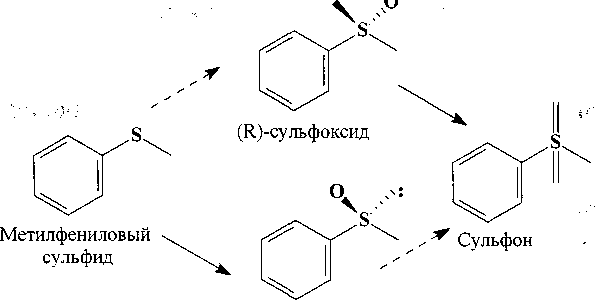
(S)-сульфоксид
Рис. 2. Схема биотрансформации метилфенилового сульфида штаммом Rhodococcus sp. ECU0066
Описаны примеры синтеза энантиомерно однородных сульфоксидов путем асимметрического окисления рацемического метилфенилового сульфоксида с использованием представителей рода Rhodococcus. Недостатком данной методики является низкий целевой выход образующихся сульфоксидов, так как при этом 50% сульфоксида трансформируется в сульфон. Сульфоксиды по сравнению с сульфидами обладают меньшим токсическим действием на живые клетки, вследствие чего становится возможным использование более высоких концентраций рацемического сульфоксидного субстрата [Isolation ..., 2009; Sequential racemization ...,2012].
При окислении сульфидов культурами мицел-лиальных грибов показано, что не существует строгой корреляции между видовой принадлежностью и их способностью к биотрансформации сульфидов. В работе H.L. Holland [2001] описан синтез (Ry сульфоксидов с высокой оптической чистотой с помощью коллекционной культуры мицелиального гриба Mortierella isabellina АТСС 42613. Грибы рода Aspergillus катализируют окисление различных арилалкиловых сульфидов с образованием (Ry сульфоксидов с высокой энантио-селективностью (ее 100%) (Aspergillus genus ..., 2013]. Y. Yamazaki с соавт. [Stereoselectivity of microbial ..., 1996] проведено стереоспецифическое окисление металлоценовых сульфидов культурой М. isabellina DSM 1414. При этом получены соответствующие сульфоксиды с оптической чистотой от 86.4 до 96.6% и химическим выходом 48-73%. Описаны единичные примеры успешного использования дрожжей Saccharomyces cerevisiae в реакциях окисления тиоанизола, его иора-замещенных производных и бензилалкилсульфидов [Oxidative ..., 1995; Bakers'yeast..., 1995].
Биокаталитический синтез оптически активных сульфоксидов с использованием индивидуальных ферментов
Известно, что для асимметрического окисления прохиральных сульфидов могут быть использованы ферменты, относящиеся к классам оксигеназ и пероксидаз. Диоксигеназные ферменты из почвенных бактерий Р. putida - толуолдиоксигеназы (ТДО) и нафталиндиоксигеназы (НДО) катализируют образование оптически активных алкиларил-и алкенарилсульфоксидов с ее 90%. Применение ТДО приводит преимущественно к образованию сульфоксидов с (/^-конфигурацией, в то время как при взаимодействии субстрата с НДО образуются исключительно (5)-энантиомеры [Lee, Brand, Gibson, 1995]. Описана возможность получения энантиомерно обогащенных (5)-энантиомерных фе-нилметил- и и-толилметилсульфоксидов (>99% ее) с помощью субстратспецифичной монооксигеназы из бактериальных клеток Р. fluorescens [Substrate ..., 2003]. ФАД-зависимая циклогексанонмонооксигеназа катализирует процесс асимметрического окисления диалкил-, фенилалкил-, бензилалкил- и замещенных бензилалкилсульфидных субстратов в энантиомерно обогащенные сульфоксиды с ее до 99% [A widely ..., 1981].
Наряду с оксигеназами для биотрансформации органических сульфидов возможно использование пероксидаз. Показано, что полная конверсия тиоанизола в соответствующий (5)-сульфоксид при участии пероксидазы хрена достигается при усло вии постепенного введения Н2О2 в реакционную среду (60% ее) [Recent ..., 1999). Гемсодержащий фермент хлорпероксидаза способна катализировать Н2О2-зависимый процесс окисления органических сульфидов. В качестве продуктов данной реакции образуются (ЛЬ-сульфоксиды с химическим выходом от 33 до 100% и оптической чистотой 19-91%. Для получения оптически чистых сульфоксидов используются также ванадий-зависимые бромпероксидазы, которые катализируют окислительное галогенирование органических соединений через образование пероксида металла [Recent..., 1999].
В настоящее время ряд публикаций посвящен исследованиям по повышению эффективности и стабильности биокатализаторов, включающим применение иммобилизованных микробных клеток; введение дополнительных ростовых субстратов; культивирование бактерий в двухфазных системах; разработку генетически модифицированных биокатализаторов [Елькин, Гришко, Ившина, 2010; Protein engineering of toluene ..., 2008; The oxidation 2013; Protein engineering of nitrobenzene
2013; Development ..., 2013]. Так, J. Shainsky с соавт. [Protein engineering of nirobenzene ..., 2013] использовали нитробензолдиокисигеназу, выделенную из Comamonas sp., для окисления различных пара-замещенных арилалкиловых сульфидов. Методом сайт-направленного мутагенеза удалось повысить эффективность данного фермента для направленного окисления сульфидов более чем в 10 раз. J.-D. Zhang с соавт. [Sequence analysis ..., 2010] проводили успешные эксперименты по биотрансформации арилалкильных сульфидов с конверсией от 70 до 95% и энантиомерным избытком 80-100%, используя рекомбинантную Р450-зависимую монооксигеназу (таблица).
Окисление сульфидов (Ri-S-R2) в сульфоксиды индивидуальной Р450-зависимой монооксигеназой из Rhodococcus sp. ECU0066
|
Ri |
r2 |
Конверсия, (%) |
ее, (%)* |
Конфигурация |
|
СР. |
СНз |
17.8 |
86.5 |
|
|
Р- сн3-СбН5 |
СНз |
68.7 |
87.0 |
|
|
р- СН3О-СН. |
СНз |
83.5 |
63.5 |
|
|
р-р- GH. |
СНз |
37.4 |
90.0 |
|
|
/>С1-СбН5 |
СНз |
88.6 |
99.0 |
|
|
СИ. |
С2Н5 |
31.8 |
99.0 |
Примечание. *ее-энантиомерный избыток. Дит. по Sequence analysis ..., 2010.
Рекомбинантная 4-ацетофенон монооксигеназа, выделенная из Pseudomonas fluorescens АСВ, была использована G. de Gonzalo с соавт. [Biocatalytic .2006] для получения арилалкиловых сульфоксидов с высоким выходом и оптической чистотой. Так был получен сульфоксид тиоанизола с 99%-ной энантиоселективностью и степенью конверсии 96%. Показана возможность коэкспрессии генов формиатдегидрогеназы из Candida boidinii и циклогексанонмонооксигеназы из Acinetobacter cal-coaceticus NCIMB 9871 в Escherichia coli, что позволило сконструировать рекомбинантный штамм, способный синтезировать (Л)-сульфоксиды с высокой энантиоселективностью (ее 99%) [Development 2013].
Применение двухфазных систем для биотрансформации органических сульфидов позволяет использовать более высокие концентрации субстрата и обеспечивает защиту живых бактериальных клеток от его токсического влияния [Klibanov, 2003; Biocatalytic ..., 2006; Resolution ...,2011; Highly ..., 2013]. Y.-C. He с соавт. [Highly ..., 2013] путем биотрансформации метилфенилового сульфида в двухфазной системе октан-вода клетками Rhodococcus sp. CCZ10-1 удалось получить 118 ммоль целевого (8)-сульфоксида с высокой энантиоселективностью (ее 99.9%).
Заключение
Перспективность использования оптически активных сульфоксидов в химической и фармацевтической промышленности обусловливает поиск высокоэффективных биокатализаторов для стереосе-лективного окисления прохиральных сульфидов.
В последнее время исследования по биокатали-тическому синтезу энантиомерно чистых сульфоксидов развиваются довольно интенсивно [Stereoselective biotransformations ..., 2009; Aspergillus genus ..., 2013; Enantioselective ..., 2013; The oxidation ..., 2013; Protein engineering of nitrobenzene ..., 2013; Stereoselective oxidation ..., 2014]. Несмотря на высокую эффективность использования чистых ферментных препаратов в реакциях энантиоселективно-го сульфоксидирования, проблемы выделения и обеспечения стабильности ферментов ограничивают их использование в практике. Указанные недостатки позволяют не применять целые микробные клетки, многочисленные примеры успешного использования которых представлены в литературе [Holland, 2001, Biotransformation ..., 2003; Enantioselective ..., 2013; Highly ..., 2013; Development ..., 2013]. При этом биокатализаторы на основе целых клеток перспективны в синтезе более структурно сложных молекул, а именно таких фармакологически значимых сульфинилсодержащих соединений, как (8)-омепразол или (7?)-модафинил [Fernandez, Khiar, 2003; Olivo, Lozada, 2009; Whole-cell..., 2011].
Анализ литературных данных свидетельствует о возрастающем спросе современных методов генной инженерии для регуляции эффективности биоката-литических процессов синтеза оптически чистых сульфоксидов [Protein engineering of toluene ..., 2008; The oxidation ..., 2013; Protein engineering of nitrobenzene ..., 2013; Development..., 2013].
Список литературы Биокаталитический синтез оптически чистых сульфоксидов
- Гришко В.В., Ившина И.Б., Толстиков А.Г. Биотрансформация тиоанизола актинобактериями Rhodococcus sensu stricto//Биотехнология. 2004. № 5. С. 49-56
- Елькин А.А., Гришко В.В., Ившина И.Б. Окислительная биотрансформация тиоанизола свободными и иммобилизованными клетками Rhodococcus rhodocrhous ИЭГМ 66//Прикл. биохим. и микробиол. 2010. Т. 46, № 6. С. 637-643
- Прилежаева Е.Н. Химия сульфоксидов и сульфонов//Получение и свойства органических соединений серы/под ред. Л.И. Беленького. М.: Химия, 1998. С. 115-259
- Толстиков А.Г., Гришко В.В., Ившина И.Б. Энантиоселективное биокаталитическое окисление органических сульфидов в хиральные сульфоксиды//Современные проблемы асимметрического синтеза. Екатеринбург, 2003. С. 165-205
- Armodafinil versus Modafinil in patients of excessive sleepiness associated with shift work sleep disorder: a randomized double blind multicentric clinical Trial/D.V. Tembe et al.//Neurol. Res. Internat. Vol. 2011 DOI: 10.1155/2011/514351
- Aspergillus genus as a source of new catalysts for sulfide oxidation/M.L. Mascotti et al.//J. Mol. Catal. B: Enzymatic. 2013. Vol. 82. P. 32-36
- A widely useful chiral stationary phase for the high-performance liquid chromatography separation of enantiomers/W.H. Pirkle et al.//J. Am. Chem. Soc. 1981. Vol. 103. P. 3964-3966
- Bakers' yeast oxidation of methyl para-tolylsuifide: synthesis of a chiral intermediate in the preparation of the mevinic acid-type hypocholestemic agents/J. Tang et al.//Tetrahedron. 1995. Vol. 51(48). P. 13217-13238
- Bently R. Role of sulfur chirality in the chemical processes of biology//Chem. Soc. Rev. 2005. Vol. 34. P. 609-624
- Biocatalytic properties of Baeyer-Villiger monoox-ygenases in aqueous-organic media/G. de Gon-zalo et al.//J. Mol. Catal. B: Enzymatic. 2006. Vol. 39. P. 91-97
- Biotransformation of sulfides by Rhodococcus eryt-hropolis/H.L. Holland et al.//J. Mol. Catal. B: Enzymatic. 2003. Vol. 22(3-4). P. 219-223
- Careno M.C. Applications of sulfoxides to asymmetric synthesis of biologically active compounds//Chem. Rev. 1995. Vol. 95(6). P. 1717-1760
- Cationic arene ruthenium (II) complexes with che-lating P-functionalized alkyl phenyl sulfide and sulfoxide ligands as potent anticancer agents/G. Ludwig et al.//Dalton Trans. 2013. Vol. 42. P. 3771-3744
- Ceccoli R.D., Bianchi D.A., Rial D.V. Flavoprotein monooxygenases for oxidative biocatalysis: re-combinant expression in microbial hosts and applications//Front. Microbiol. 2014. Vol. 5(25) DOI: 10.3389/fmicb.2014.00025
- Daligault F., Niguer-Chauvin C., Patin H. Microal-ga Chlorella sorokiniana: a new sulfoxidation biocatalyst//Org. Biomol. Chem. 2006. Vol. 4. P.1474-1477
- Development of a whole-cell biocatalyst with NADPH regeneration system for biosulfoxidation/X.-H. Zhai [et al.]//J. Ind. Microbiol. Biotechnol. 2013. Vol. 40. P. 797-803
- Drug interaction studies with esomeprazole, the (S)isomer of omeprazole/T. Andersson et al.//Clin. Pharmacokinet. 2001. Vol. 40(6). P. 411-426
- Enantioselective oxidation of sulfides to sulfoxides by Gordonia terrae IEGM 136 and Rhodococ-cus rhodochrous IEGM 66/A.A. Elkin [et al.]//J. Mol. Catal. B: Enzymatic. 2013. Vol. 89. P. 82-85
- Expanding the toolbox for enantioselective sulfide oxidations: Streptomyces strains as biocatalysts/M.L. Mascotti et al.//Biocatal. Agricul. Bio-technol. 2013. Vol. 2. P. 399-402
- Fernandez I., Khiar N. Recent developments in the synthesis and utilization of chiral sulfoxides//Chem. Rev. 2003. Vol. 103. P. 3651-3705
- Holland H.L. Biotransformation of organic sulfides//Nat. Prod. Rep. 2001. Vol. 18. P. 171-181
- Highly enantioselective oxidation of phenyl methyl sulfide and its derivatives into optically pure (S)-sulfoxides with Rhodococcus sp. CCZU10-1 in an n-octane-water biphasic system/Y.C. He et al.//Appl. Microbiol. Biotechnol. 2013. Vol. 97(24). P. 10329-37
- Isolation of Rhodococcus sp. strain ECU0066, a new sulfide monooxygenase-producing strain for asymmetric sulfoxidation/A.T Li et al.//Appl. Environ. Microbiol. 2009. Vol. 75. P. 551-556
- Klibanov A.M. Asymmetric enzymatic oxidoreduc-tions in organic solvents//Curr. Opin. Biotechnol. 2003. Vol. 14. P. 427-431
- Lee K., Brand J.M., Gibson D.T. Stereospecific sul-foxidation by toluene and naphthalene dioxyge-nases//Biochem. Biophys. Res. Commun. 1995. Vol. 212. P. 9-15
- Mata E. G. Recent advances in the synthesis of sul-foxides from sulfides//Phosphorus. 1996. Vol. 117. P. 231-286
- McConathy J., Owens M.J. Stereochemistry in Drug Action//J. Clin. Psychiatry. 2003. Vol. 5(2). Р. 70-73
- Olivo H.F., Lozada A.V.O. Microbial sulfoxidation and amidation of benzhdrylsulfanyl carboxylic acids and uses thereof//Patent US 07553646. 2009
- Opposite enantioselectivities of two phenotypically and genotypically similar strains of Pseudomo-nas frederiiksbergensis in bacterial whole-cell sulfoxidation/W. Adam et al.//Appl. Environ. Microbiol. 2005. Vol. 71(4). Р. 2199-2202
- Oxidative biotransformations by microorganisms: stereoselective sulfoxide formation by Saccha-romyces cerevisiae/I. Beecher et al.//Biotech-nol. Lett. 1995. Vol. 17. P. 1069-1074
- Pinedo-Rivilla C., Aleu J., Collado I.G. Enantiomer-ic oxidation of organic sulfides by the filamentous fungi Botrytis cinerea, Eutypa lata and Tri-choderma viride//J. Mol. Catal. B: Enzym. 2007. Vol. 49. P. 18-23
- Preparation of pharmaceutically active compounds by biooxidation/R. Holt et al.//Patent US 5840552. 1998
- Protein engineering of nirobenzene dioxygenase for enantioselective synthesis of chiral sulfoxides/J. Shainsky et al.//Protein Eng. Des. Sel. 2013. Vol. 26(5). Р. 335-345
- Protein engineering of toluene monooxygenases for synthesis of chiral sulfoxides/R. Feingersch et al.//Appl. Environ. Microbiol. 2008. Vol. 75(5). P. 1555-1566
- Recent biotechnological developments in the use of peroxidases/S. Colonna et al.//Trends Bio-technol. 1999. Vol. 17(4). P. 163-168
- Resolution of racemic sulfoxides with high productivity and enantioselectivity by a Rhodococcus sp. strain as an alternative to biooxidation of prochiral sulfides for efficient production of enantiopure sulfoxides/A.T. Li et al.//Biore-sour. Technol. 2011. Vol. 102. P. 1537-1542
- Sequence analysis and heterologous expression of a new сугоспготе P450 monooxygenase from Rhodococcus sp. for asymmetric sulfoxidation/J.-D. Zhang et al.//Appl. Microbiol. Biotechnol. 2010. Vol. 85. P. 615-624
- Sequential deracemization of sulfoxides via whole-cell resolution and heterogenous oxidation/M. Tudorache et al.//Appl. Catal. A: General. 2012. Vol. 441-442. P. 42-46
- Significantly improved asymmetric oxidation of sul-fide with resting cells of Rhodococcus sp. in bi-phasic system/A.T. Li et al.//Process Bio-chem. 2011. Vol. 46. P. 689-694
- Stereoselective biotransformations using fungi as biocatalysts/K.B. Borges et al.//Tetrahedron: Asymmetr. 2009. Vol. 20. P. 385-397
- Stereoselective oxidation of sulfides to optically active sulfoxides with resting cells of Pseudomonas monteilii CCTCC M2013683/Y. Chen et al.//J. Mol. Catal. B: Enzymatic. 2014. Vol. 106. P. 100-104
- Stereoselectivity of microbial oxygenation of metal-locene sulphides with different substituent size and central atom/Y. Yamazaki et al.//Appl. Microbiol. Biotechnol. 1996. Vol. 45. P. 595-599
- Substrate Specificity and Enantioselectivity of 4-Hydroxyacetophenone Monooxygenase/N.M. Kamerbeek et al.//Appl. Environ. Microbiol. 2003. Vol. 69(1). P. 419-426
- Synthesis of enantioenriched sulfoxides/G.E O'ma-hony et al.//ARKIVOC. 2011. P. 1-110
- The oxidation of alkylaryl sulfides and ben-zo[b]thiophenes by Escherichia coli cells expressing wild-type and engineered styrene mo-nooxygenase from Pseudomonas putida CA-3/J. Nikodinovic-Runic et al.//Appl. Microbiol. Biotechnol. 2013. Vol. 97. P. 4849-5858
- Toluene and naphthalene dioxygenase-catalysed sul-foxidation of alkyl aryl sulfides/D.R. Boyd et al.//J. Chem. Soc. Perkin Trans. 1998. Vol. 12. P. 1929-1933
- Whole-cell oxidation of omeprazole sulfide to enan-tiopure esomeprazole with Lysinibacillus sp. B71/P. Babiak et al.//Bioresour. Technol. 2011. Vol. 102. P. 7621-7626
- Zhang J-D., Li A.-T., Xu J.-H. Improved expression of recombinant cytochrome P450 monooxygenase in Escherichia coli for asymmetric oxidation of sulfides//Bioprocess Biosyst. Eng. 2010. Vol. 33. P. 1043-1049


