Биологическое тестирование никелидтитановых имплантатов с покрытиями из благородных металлов в эксперименте in vivo
Автор: Жеравин А.А., Доровских С.И., Викулова Е.С., Басова Т.В., Васильева М.Б., Русакова Я.Л., Морозова Н.Б.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 5 т.23, 2024 года.
Бесплатный доступ
Цель исследования - провести биологическое in vivo тестирование образцов никелида титана, модифицированных пленочными гетероструктурами Ag/Pt или AuAg/Pt, в сравнении с исходным носителем. Материал и методы. Объекты исследования - пластины из никелида титана, модифицированные пленочными гетероструктурами из благородных металлов, в качестве тест-системы для in vivo тестирования использовали лабораторную мини-свинью (мини-пиг). Для формирования пленочных структур на образцах никелида титана использованы физические методы газофазного осаждения: ионно-плазменное (IPD) и термическое (PVD) напыление. Гетероструктуры Ag/Pt и AuAg/Pt охарактеризованы методами рентгеновской дифракции и сканирующей микроскопии.
Имплантат, антибактериальный агент, покрытие, платина, серебро, золото, имплантация, рентгенография, гистология
Короткий адрес: https://sciup.org/140307924
IDR: 140307924 | УДК: 616.71-089.844:546.3]-092.9 | DOI: 10.21294/1814-4861-2024-23-5-73-84
Текст научной статьи Биологическое тестирование никелидтитановых имплантатов с покрытиями из благородных металлов в эксперименте in vivo
Эндопротезирование является «золотым стандартом» в лечении пациентов с первичными и метастатическими опухолями костей конечностей и таза. При костных саркомах выполнение радикальной резекции с сохранением конечности возможно более чем в 90 % случаев. Использование современных модульных мегапротезов позволяет решать вопросы замещения крупных суставов, суставных сегментов длинных костей одномоментно после удаления опухоли, обеспечивает раннюю и полноценную функциональную реабилитацию. Глобальной проблемой в онкоортопедической практике являются осложнения, ассоциированные с мегаконструкциями, обусловливающие необходимость ревизий, заканчивающихся в ряде случаев потерей конечности. Наиболее серьезными являются инфекционные осложнения, риск развития которых существует как в раннем, так и в отдаленном послеоперационном периоде. Частота инфекционных осложнений при эндопротезировании верхней конечности варьирует от 3 до 19 %, нижней конечности – от 6 до 41 %. Наибольшая частота осложнений отмечается при реконструкциях тазового кольца, при эндопротезировании таза, где периимплантные инфекции встречаются более чем в 50 % случаев [1]. Основными факторами, способствующими развитию инфекционных осложнений у онкологических пациентов, являются: наличие протяженного мертвого пространства на границе имплантата с мягкими тканями, дефицит мягких тканей в месте реконструкции, иммуносупрессия на фоне полихимиотерапии, постлучевые изменения в области оперативного вмешательства. Важными аспектами, существенно ограничивающими возможности лечебных мероприятий по борьбе с инфекционным процессом, являются наличие антибактериальной резистентности патогенных штаммов и способность бактерий формировать защитную биопленку на поверхности имплантатов. В этой связи исследования, направленные на поиск новых антибактериальных агентов, способов их доставки к очагу воспаления, а также совершенствование поверхностных биологических свойств имплантируемых материалов являются, несомненно, актуальными.
Среди металлических материалов имплантатов перспективны никелиды титана, т.е. практически эквиатомные сплавы титана с никелем, которые сочетают высокую прочность и ударную вязкость (предотвращение деформации или хрупкого разрушения) с относительно низкой жесткостью
(биомеханическая совместимость с костью) и особыми механическими свойствами (например, эффект памяти формы) [2]. Применение различных изделий из таких сплавов в реконструктивной хирургии подтвердило их высокую биосовместимость по сравнению с другими материалами [3–6], хотя остаются некоторые опасения относительного возможности выделения никеля вследствие коррозии в биологической среде и соответствующих неблагоприятных эффектов [2, 7, 8]. Однако при требуемой для остеоинтеграции шероховатости TiNi благоприятствует адгезии бактерий и образованию бактериальных биопленок [9]. В таком случае стандартная обработка антибиотиками может быть неэффективна [10].
Антимикробная активность материалов может быть обеспечена присутствием на поверхности компонентов, подавляющих рост бактериальных колоний, не оказывая при этом токсического действия на окружающие ткани. В связи с этим функционализация поверхности биоматериалов структурами, способными не только улучшить биоинтегративные свойства, но и обеспечить пролонгированный антибактериальный эффект, является одним из методов повышения эффективности современных имплантатов. Согласно литературным данным, такую функцию могут выполнять наночастицы и покрытия на основе Ag [11–13] и Au [14, 15]. Исследования антимикробных свойств наночастиц Ag, нанесенных на поверхность титана и его сплавов, подтвердили высокую биоцидную активность модифицированных материалов по сравнению с исходными имплантатами [16–18]. Также антибактериальную активность могут проявлять наночастицы золота [19, 20]. Усилить их действие можно при создании пленочных гетероструктур, где такие наночастицы будут находиться на поверхности подслоя из платинового металла (Pt, Ir) – за счет гальванического (для Ag) или синергетического (для Au) эффекта [21, 22].
Ранее проведенные нами исследования [23, 24] показали высокую антибактериальную активность пленочных гетероструктур Ag/Ir, Au/Ir, Ag/Pt, Au/ Pt, Au/Ag на титан-содержащих материалах: установлено полное уничтожение колоний штаммов двух бактериальных культур (грамположительная – S. aureus и грамотрицательная – P. aeruginosa ) уже через 24 ч культивации, тогда как на поверхности исходного носителя процесс ингибирования не зафиксирован. Более того, модифицированные таким образом имплантаты проявили заметно лучшую биосовместимость in vivo в экспериментах на крысах, по сравнению со стандартным TiAlV-сплавом, особенно на более поздних сроках (после 3 мес подкожной имплантации) [23]. В довершение при достаточной компактности сплошной подслой может также эффективно выполнять защитную и антикоррозионную функцию, препятствуя выделению никеля, что было показано на примере Ir/ TiNi и Au/TiNi [25].
Настоящая работа продолжает исследования в данной области и представляет результаты тестирования in vivo образцов никелида титана, модифицированных пленочными гетероструктурами Ag/Pt или AuAg/Pt, в сравнении с исходным носителем. Для формирования пленочных структур использованы физические методы газофазного осаждения: ионно-плазменное (IPD) и термическое (PVD) напыление для получения комнатного слоя Pt и активного компонента (наночастиц) соответственно. Биологическое исследование для образцов NiTi с подобным образом модифицированной поверхностью, а также использование частиц AuAg, проявляющих улучшенные биологические свойства [26–30] в таких гетероструктурах, представлены впервые. Описывается предварительное единичное наблюдение, содержащее прецедент для продолжения поисков в изучаемом направлении.
Цель исследования – провести биологическое in vivo тестирование образцов никелида титана, модифицированных пленочными гетероструктурами Ag/Pt или AuAg/Pt, в сравнении с исходным носителем.
Материал и методы
Модельными объектами тестирования являлись пластины из никелида титана (TiNi, размер 60×10×1 мм, предизогнутые с кривизной диаметром 250 мм, имеющие сквозные отверстия), покрытые с обеих сторон пленочными гетероструктурами (далее образцы Ag/Pt/TiNi и AuAg/Pt/TiNi). Контрольный образец – аналогичная пластина из TiNi без покрытия. Образцы изготовлены в лаборатории медицинских сплавов и имплантатов с памятью формы Сибирского физико-технического института (г. Томск) из сплава c содержанием Ti=49,72 ± 0,18 ат.%, Ni=50,28 ± 0,18 ат. %, по разработанной технологии [31]. Дизайн пластин соответствует имплантатам, предназначенным для замещения дефектов реберного каркаса. Стерилизация всех образцов осуществлялась путем стандартной процедуры автоклавирования (при 1,1 атм, 45 мин, t=120 0С) в ЦСО ФГБУ «НМИЦ им. ак. Е.Н. Мешалкина» Минздрава России.
Образцы с пленочными гетероструктурами Ag/ Pt/TiNi и AuAg/Pt/TiNi характеризовали методами рентгеновской дифракции (РФА, прибор Shimadzu XRD-7000 (CuKα-излучение, линейный детектор OneSight, диапазон 10–60°, шаг 0,0143°, вращение образца 60 об/мин, накопление 5 с в точке), сканирующей электронной микроскопии (СЭМ, микроскоп JEOL-ISM 6700 F, детектор вторичных электронов, ускоряющее напряжение 15 кэВ) и энергодисперсионной спектроскопии (ЭДС, анализатор EX-2300BU, область сканирования 500×400 мкм2, усреднение по составу для образцов проводили по 3 точкам). Индицирование дифрак-тограмм проводили по данным картотеки PDF [32]. Размер частиц оценивали по данным СЭМ и обла-
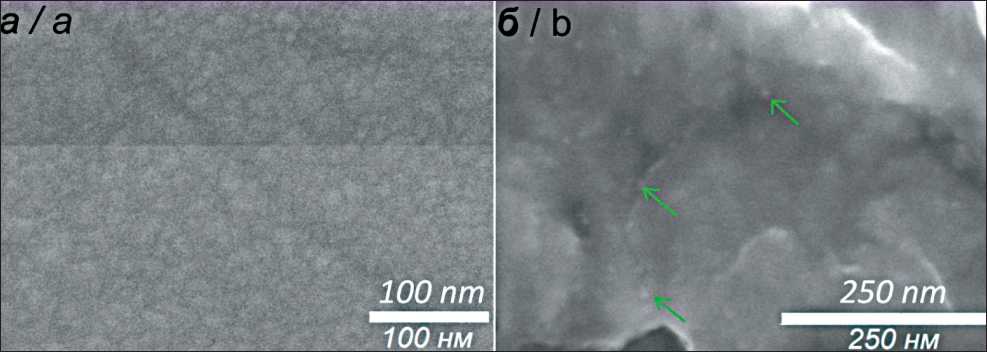
Рис. 1. СЭМ изображения Pt/TiNi (a) и Au/Pt/TiNi (зеленые стрелки указывают на наночастицы золота) (б). Примечание: рисунок выполнен авторами
Fig. 1. SEM images of Pt/TiNi (a) and Au/Pt/TiNi (green arrows point to gold nanoparticles) (b). Note: created by the authors
стям когерентного рассеяния (ОКР), рассчитанным по уравнению Шеррера из данных РФА [33].
Пленочные гетероструктуры Ag/Pt и AuAg/Pt наносили на TiNi при последовательном осаждении в несколько стадий. На 1-й стадии на поверхность пластин из NiTi методом IPD напыляли пленки из Pt в следующих условиях: установка УРМ.026, мишень из платины (Pt 99,99%, диск, диаметр 100 мм), напряжение мишени ( U =2500 B), ток мишени ( I =40 мA), давление в реакторе 10-7 мм рт. ст., температура подложек – 25–30 оС. Осаждение пленок проводили в течение 13 мин. По данным рентгенофазового анализа (РФА), полученные пленки Pt имеют выраженную (111) текстуру. Исследование методом сканирующей электронной микроскопии (СЭМ) показало, что пленки повторяют рельеф NiTi пластин и образованы зернами размером до 25 нм (рис. 1а).
На 2-й стадии на поверхность TiNi пластин с пленками Pt методом PVD напыляли наночастицы Au в следующих условиях: установка UVM.71-PVD ( I =500 мA, U =500 B), навеска порошкового золота 15 мг, температура испарения 1670 оС, давление в реакторе 8·10-7 мм рт. ст., температура осаждения 200 оС. По данным СЭМ, на поверхности пластин из TiNi, покрытых пленками Pt (образцы Au/Pt/TiNi), формируются наночастицы золота с размерами 8–15 нм (рис. 1б). По данным энергодисперсионной спектроскопии (ЭДС), содержание золота в образцах на поверхности составляет около 12 ат. %.
На финальной стадии на поверхности пластин из TiNi, покрытых пленками Pt или гетероструктурами Au/Pt, методом PVD напыляли серебро, получая образцы Ag/Pt/TiNi или AuAg/Pt/TiNi соответственно. Эксперименты проводили в следующих условиях: установка UVM.71-PVD (I=500 мA, U=500 B), навеска порошкового серебра 5 мг, температура испарения 1300 оС, давление в реакторе 8·10-7 мм рт. ст., температура осаждения 150 оС. По данным СЭМ, серебро формирует нано- пленку, обволакивающую частицы Pt и/или Au/Pt (рис. 2а). Дифрактограммы образцов Ag/Pt/NiTi и AuAg/Pt/NiTi представлены на рис. 2б. По данным СЭМ, для образца Ag/Pt/NiTi размер кристаллитов Ag составляет менее 5 нм, для Pt – 17 нм. По данным ЭДС, содержание серебра в образцах составляет 1,5–1,7 ат. %.
В качестве тест-системы для экспериментальных исследований использовали лабораторную мини-свинью (мини-пиг), предоставленную ИЦиГ СО РАН. Пол – самка, возраст 2 года, вес 65 кг. На момент начала эксперимента животное не имело признаков соматической патологии, ветеринарный контроль проведен в полном объеме. На предварительном этапе за 12 ч до предполагаемого вмешательства животное переставали кормить и переводили на водную диету. Утром перед операцией ветеринарным врачом производился осмотр, которым подтверждали удовлетворительное состояние животного, о чем производили запись в экспериментальной истории болезни. Животное поступало из вивария в лабораторию в состоянии сна после седации препаратом Золетил-100 в дозе 50 мг/кг. Животное взвешивали на напольных весах, производили дополнительную санитарногигиеническую мойку, бритье и обработку кожных покровов. На операционном столе животное закрепляли в положении «на правом боку». В краевую вену уха устанавливали периферический катетер для инъекций подходящего размера, к грудной клетке прикрепляли ЭКГ-датчики. Плетизмографический датчик для контроля насыщения периферической крови кислородом крепили на хвост. Витальные параметры – пульс, температуру в глотке, ЭКГ, насыщение крови кислородом – фиксировали в режиме «реального времени» системой мониторинга V26E (Philips, Германия). Динамику данных периодически вносили в наркозную карту экспериментального животного. Затем внутривенно вводили миорелаксант (адруан, 4 мг) и выполняли интубацию трахеи трубкой диаметром 8–9 мм.
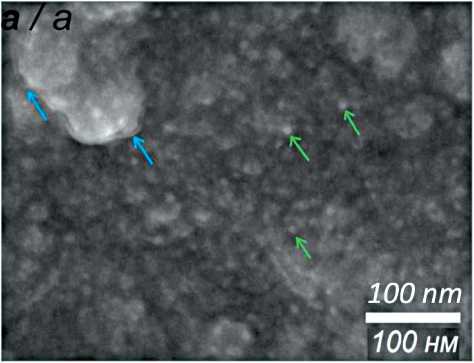
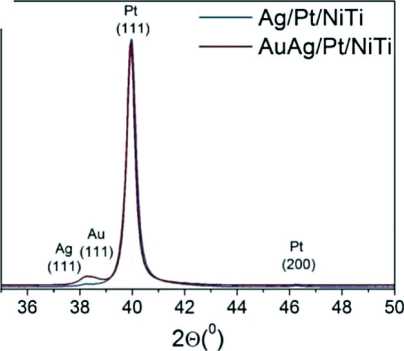
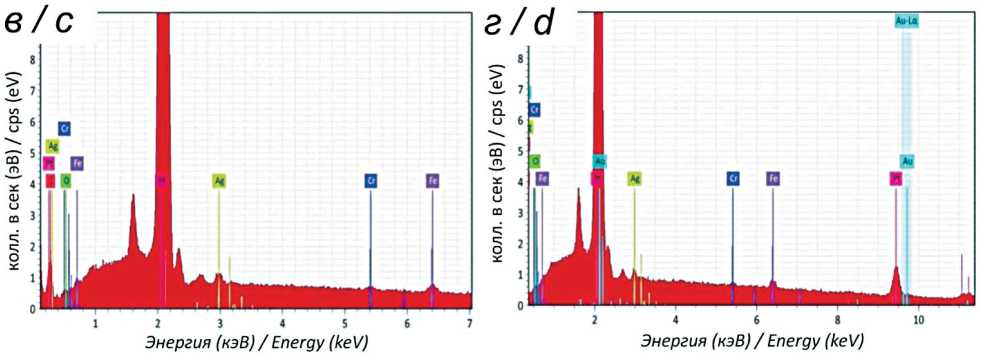
Рис. 2. СЭМ изображение AgAu/Pt/NiTi (зеленые стрелки указывают на наночастицы золота, синие – на нанопленку серебра) (а); дифрактограммы Ag/Pt/NiTi, AuAg/Pt/NiTi (б); ЭДС спектры образцов Ag/Pt/NiTi (в) и AuAg/Pt/NiTi (г).
Примечание: рисунок выполнен авторами
Fig. 2. SEM images of AgAu/Pt/NiTi (green arrows point to gold nanoparticles, blue arrows point to silver nanofilm) (а); XRD patterns of Ag/Pt/NiTi, AuAg/Pt/NiTi (b); EDS spectra of Ag/Pt/NiTi (c) and AuAg/Pt/NiTi (d). Note: created by the authors
Раздували обтурирующую манжету, подсоединяли аппарат искусственной вентиляции легких Julian (Draeger, Германия), начинали принудительную вентиляцию 60 % кислородом, содержащим 3–5 % севофлюрана (по объему) с частотой 12–14 циклов в минуту. Дыхательный объем подбирали индивидуально в зависимости от веса животного. Через установленный ранее периферический катетер проводили инфузионную поддержку кровообращения кристаллоидными (раствор Рингера, изотонический раствор) и/или коллоидными (гела-фузин, полиглюкин) растворами. Объем инфузии определялся врачом-анестезиологом согласно весоростовым показателям и данным объективного мониторинга витальных функций экспериментального животного. Обезболивание в ходе операции достигалось применением внутривенной инъекции фентанила.
Подготовка операционного поля. Область грудной стенки в проекции II–V ребер слева, предварительно выбритая, 3-кратно обрабатывалась раствором бетадина. Операционное поле накрывали стерильной простыней с окном для доступа к коже. После достижения 3-го уровня стадии хирургического наркоза осуществляли разрез кожи в продольном направлении относительно оси тела, перпендикулярно направлению ребер. После выполнения кожного разреза подлежащие ткани разделяли ножницами тупым способом, визуализировали наружную поверхность ребер. Для достижения гемостаза использовали электронож-коагулятор Force-EZ (Valleylab, США). Образцы размещали субнадкостнично, параллельно в проекции III, IV и V ребер после формирования тоннеля над ребром путем разведения мягких тканей. Исследуемые образцы располагались вне контакта друг с другом. Для предотвращения миграции место введения имплантатов ушивали узловым швом. Далее область имплантации укрывалась мышечными тканями, производилось послойное ушивание. Ввиду адекватного гемостаза, отсутствия свободных полостей дренирование раны не осуществлялось. Кожные края соединялись внутрикожным швом. Линию доступа обрабатывали кожным антисептиком и изолирующим составом. В ходе операции выполняли рентгенографию зон имплантации образцов с помощью рентгеновской установки OEC9800 (GE HealthCare, США) до и после установки объектов исследования (рис. 3).
После окончания хирургического этапа выключали подачу севофлюрана, у животного периодически проверяли появление периферических
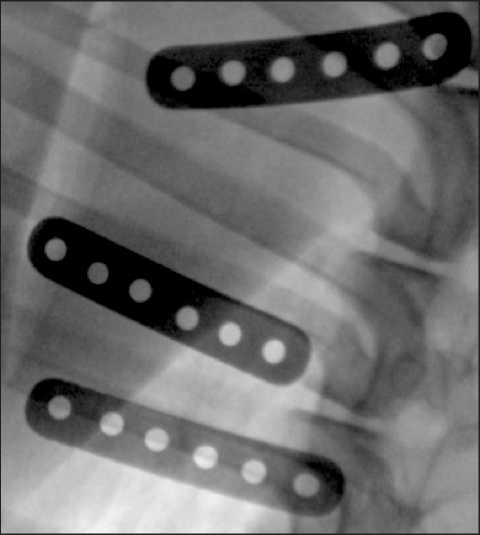
Рис. 3. Рентгенография области имплантации, выполненная на операционном столе.
Примечание: рисунок выполнен авторами
Fig. 3. X-ray of the implantation area performed on an operating table. Note: created by the authors рефлексов и спонтанного дыхания. При появлении дыхания аппарат ИВЛ переводили в режим поддерживающей вентиляции. После восстановления собственного дыхания у животного убирали эндотрахеальную трубку и перемещали в виварий под наблюдение ветеринара для окончательного восстановления.
Результаты
В первые 2 сут после операции животное получало кетопрофен – 50 мг 2 раза в день, внутривенно в качестве обезболивания, цефазолин – 1 г в сут, внутривенно для профилактики инфекционных осложнений и антисептическую обработку ран. В ходе дальнейшего наблюдения животное ежедневно осматривалось врачом-ветеринаром. Анализ объективного статуса животного, исследования цитологического (общий анализ крови), биохимического (маркеры повреждения печени, почек) и цитокинового состава крови (IL-1b, IL-6, TNFa), не показавшие никаких отклонений от нормы и дооперационных значений, позволяют сделать вывод об отсутствии общетоксических реакций организма на имплантаты.
Спустя 39 сут животное подвергли эвтаназии с помощью передозировки золетилом-100 (100 мг/кг) с последующим обескровливанием. Образцы забирались единым блоком с окружающими тканями. После проведения макроскопического осмотра и фотографирования производили забор фрагментов тканей, граничащих с имплантатами с последующим погружением в раствор забуференного формалина 10 % с маркировкой контейнеров. Материал фиксировали в течение 48 ч (смена фиксатора каж- дые 12 ч). Искомые образцы далее обезвоживали и заливали в парафин по стандартной методике. Гистологические срезы толщиной 5 мкм готовились на ротационном микротоме HM 340E (Thermo Fisher Scientific, USA). Полученные срезы окрашивались гематоксилином и эозином по общепринятой методике. Исследование гистопрепаратов выполняли методом светооптической микроскопии.
После извлечения исследуемых образцов с окружающими тканями последние располагали на препаровочном столе. Ткани, окружающие имплантат, аккуратно рассекались и отводились в сторону. При осмотре ни в одной локации признаков наличия гноя, избыточного количества жидкости не было выявлено (рис. 4). Определялась нежная соединительнотканая капсула с неярко выраженным коллатеральным сосудистым рисунком. Внутренняя поверхность капсулы – блестящая, гладкая, технологические отверстия в пластинах заполнены соединительноткаными мостиками. Признаков металлоза ни в одном случае выявлено не было. Отбор тканей для морфологического исследования осуществляли под визуальным контролем, в блок тканей включались как фрагменты капсулы, так и фрагменты мышц.
Для материала с хорошим уровнем биоинертности характерными признаками являются тонкая соединительнотканая капсула с преобладанием коллагеновых волокон, ориентированных параллельно поверхности имплантата. Соединительнотканая матрица заполнена преимущественно клетками фибробластного ряда, единичными диффузно расположенными микрососудами со слабо выраженной (в идеале отсутствием) лимфоцитарномакрофагальной инфильтрацией [34]. Основными параметрами оценки морфологических изменений являлись: тип соединительнотканой матрицы, клеточный состав (наличие клеток воспалительного ряда, соотношение фибробластов и фиброцитов), интенсивность васкуляризации капсулы.
Образец капсулы имплантата TiNi без покрытия (контрольный образец) представлен плотной

Рис. 4. Вид тканей, окружающих исследуемые образцы на аутопсии. Примечание: рисунок выполнен авторами Fig. 4. View of the tissues surrounding the examined samples at autopsy. Note: created by the authors
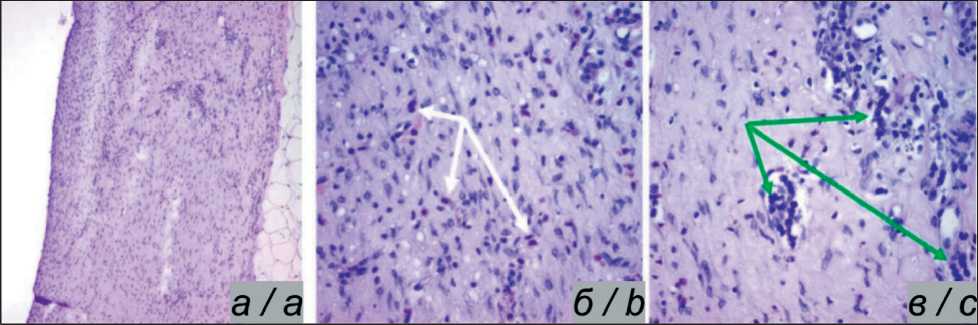
Рис. 5. Микрофото. Фрагмент ткани, прилегающей к образцу TiNi без покрытия. Окраска гематоксилином и эозином: а) общий вид образца. Плотная неоформленная волокнистая соединительная ткань, ×100; б) диффузная инфильтрация стромы эозинофильными клетками (белая стрелка), ×400; в) наличие отдельных микрозон скопления макрофагальных и лимфоцитарных клеток (зеленая стрелка), ×400. Примечание: рисунок выполнен авторами
Fig. 5. Microphoto. A fragment of tissue adjacent to the uncoated TiNi sample. Hematoxylin and eosin staining: a) general appearance of the sample. Dense unformed fibrous connective tissue, ×100; b) diffuse stromal eosinophilic infiltration (white arrow), ×400; c) the presence of separate microzones of macrophage and lymphocyte cells (green arrow), ×400. Note: created by the authors
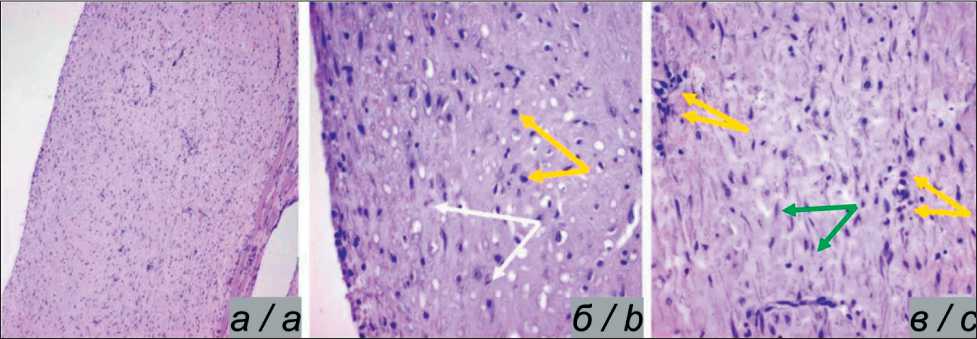
Рис. 6. Микрофото. Фрагмент ткани, прилегающей к имплантату Ag/Pt/TiNi. Окраска гематоксилином и эозином: а) общий вид стенки капсулы, ×100; б) ровный край из волокон соединительнотканого каркаса и отдельных фибробластных и лимфоцитарных клеток – желтая стрелка, единичных эозинофилов – белая стрелка, ×400; в) в строме среза преобладают клетки фиброци-тарного ряда – зеленая стрелка. Незначительное количество лимфоцитов – желтая стрелка – сосредоточено вокруг полостей кровеносных микрососудов, ×400. Примечание: рисунок выполнен авторами
Fig. 6. Microphoto. A fragment of tissue adjacent to the implant Ag/Pt/TiNi. Hematoxylin and eosin staining: а) general appearance of the capsule wall, ×100; b) smooth edge of the fibers of the connective tissue framework and individual fibroblast and lymphocyte cells (yellow arrow), single eosinophils (white arrow), ×400; c) fibrocyte cells predominate in the stroma of the slice (green arrow). A small number of lymphocytes (yellow arrow) are concentrated around the cavities of blood microvessels, ×400. Note: created by the authors
неоформленной волокнистой соединительной тканью (ПНВСТ). Зона прилегания ткани к имплантату имеет четкий ровный край, гомогенный по волокнистому и клеточному составу на протяжении всего среза. Клеточная компонента данной зоны представлена клетками фибробластного ряда (фиброцитов меньшее число) и лейкоцитарными клетками. На фоне умеренной диффузной лимфоцитарно-эозинофильной инфильтрации имелись дополнительные микроочаги, содержавшие макрофаги. В полях зрения обнаружены единичные кровеносные сосуды (рис. 5). Зона контакта с поверхностью имплантата гладкая, но содержит в себе преимущественно макрофагальнолимфоцитарный пул клеток.
Образец капсулы имплантата Ag/Pt/TiNi преимущественно представлен ПНВСТ. Клеточная компонента включала клетки фибробластного ряда с незначительным количеством лимфоцитов, диффузно расположенных по всей поверхности среза зоны ПНВСТ. В срезах встречались отдельные эозинофилы (рис. 6). Краевая зона капсулы гладкая, состояла главным образом из коллагеновых волокон, ориентированных вдоль поверхности имплантата, и клеток фибробластного ряда.
Образец капсулы имплантата AuAg/Pt/TiNi преимущественно представлен ПНВСТ (аналогично образцу Ag/Pt/TiNi). В толще среза ткани на фоне коллагеновых волокон стромы и преобладания фиброцитов над фибробластами отмечались выраженные периваскулярные скопления лимфоцитарных и эозинофильных клеток. Край образца исследуемой ткани, предлежавший к имплантату, был ровным, без зон детрита. Однако в краевой зоне выявлено большое количество лимфоцитов на фоне клеток фибробластного ряда.
Таблица/table
Морфологические изменения в периимплантных тканях через 39 сут
Morphological changes in periimplant tissues after 39 days
|
Тип имплантата/ Type of the implant |
Тип каркаса/ Type of frame |
В строме доминируют фибробласты/ Fibroblasts dominate in the stroma |
В строме доминируют фиброциты/ Fibrocytes dominate in the stroma |
Лимфоциты (0–5)/ Lymphocytes (0–5) |
Эозинофилы (0–5)/ Eosinophils (0–5) |
Макрофаги/ Macrophages |
КИТ/ FBC |
|
TiNi без покрытия*/ TiNi without coating* |
ПНВСТ/ DIFCT |
+ |
- |
4 |
5 |
5 |
0 |
|
Ag/Pt/TiNi |
ПНВСТ/ DIFCT |
+ |
- |
1–2 |
0–1 |
0 |
0 |
|
AuAg/Pt/TiNi |
ПНВСТ/ DIFCT |
- |
+ |
3–4 |
2–3 |
0 |
0 |
Примечания: * – за «эталон» в данном исследовании принят TiNi без покрытия, т.к. этот материал имеет клинически доказанную биологическую совместимость; ПНВСТ – плотная неоформленная волокнистая соединительная ткань; КИТ – клетки инородного тела. Фибробласты/фиброциты – классические компоненты волокнистой соединительной ткани. Фибробласты активно продуцируют коллаген и могут делиться, имеют округлое или овальное ядро; фиброциты не способны делиться и продуцировать коллаген, имеют вытянутое веретенообразное ядро. Шкала от 0 до 5 – полуколичественный метод оценки числа искомых объектов в срезе: 0 – объект полностью отсутствует; 5 – максимальное содержание объекта в срезе (сравнение между всеми экспериментальными группами), наибольший показатель свидетельствует о более выраженной воспалительной реакции окружающих тканей; таблица составлена авторами.
Notes: * – uncoated TiNi was accepted as the “standard” in this study, since this material has clinically proven biological compatibility. DIFCT – Dense irregular fibrous connective tissue. FBC – foreign-body cell. Fibroblasts/fibrocytes are classic components of fibrous connective tissue. Fibroblasts actively produce collagen and can divide, have a rounded or oval nucleus; fibrocytes are unable to divide and produce collagen, have an elongated fusiform nucleus. The scale from 0 to 5 is a semi–quantitative method for estimating the number of desired objects in the slice: 0 – the object is completely absent; 5 – the maximum content of the object in the slice (comparison between all experimental groups). The highest indicator indicates a more pronounced inflammatory reaction of the surrounding tissues; created by the authors.
Во всех трех исследуемых типах образцов значимого различия по степени васкуляризации или преобладанию микрососудов какого-либо определенного диаметра не выявлено. Морфологические изменения в периимплантных тканях через 39 сут представлены в таблице.
Обсуждение
В целом, отмеченное улучшение in vivo биосовместимости для образцов из TiNi с пленочными гетероструктурами из комбинации благородных металлов соответствуют предыдущим исследованиям для образцов из Ti, проведенных на крысах [23, 24]. Это позволяет включить новые результаты в общую тенденцию. Вместе с тем, ранее нами было показано, что образцы с гетероструктурами Au/Pt, т. е. с наночастицами золота на платине [23], и Ag/Au, т. е. со сплошным золотым подслоем [24], проявляли лучшие характеристики приживаемости в сравнительных сериях. С учетом этого и литературных данных по свойствам AuAg пленок и наночастиц [13, 14] ожидалось дополнительное улучшение биосовместимости для гетероструктуры с биметаллическим активным компонентом. Однако для образца AuAg/Pt обнаружено увеличение количества лимфоцитов и эозинофилов по сравнению с Ag/Pt, хотя и не до показателей непокрытого TiNi. Вероятной причиной может быть усиленное биологическое действие наночастиц Au, покрытых тонкой пленкой Ag, например, за счет дополнительной активации выделения ионов серебра
[27], что приводит к их избыточному количеству. С другой стороны, непосредственно наночастицы Au также могут стимулировать иммунный ответ организма [35, 36]. Таким образом, для улучшения понимания и оптимизации результата необходимо расширение исследований гетероструктур с биметаллическими активными компонентами на уровнях in vitro и in vivo .
В заключение следует отметить, что материалы, содержащие только никелид титана и серебро, могут вызывать обильный фиброзный процесс [37], тогда как для представленных в настоящем исследовании образцов характерна обратная тенденция. Важно отметить, что в системе Ti-Ni никель, как более активный металл, является анодом, а введение благородного металла Ag увеличивает разность потенциалов, за счет чего процесс растворения никеля активизируется. Таким образом, фиброзный процесс для образцов Ag-Ti-Ni может быть воспалительной реакцией на усиленное выделение никеля в окружающие ткани. Количественно анодную активацию растворения никеля наблюдали при введении другого благородного металла, Ir, в виде пористого покрытия [25]. Это приводило к сильному цитотоксическому эффекту in vitro . Для предотвращения подобного эффекта в настоящей работе из благородного металла Pt формировали компактный слой. Таким образом, в образцах Ag/ Pt/TiNi и AgAu/Pt/TiNi поверхность TiNi была полностью закрыта, и роль растворимого анода выполняло серебро.
Заключение
Тестирование опытных образцов из сплава TiNi, модифицированных гетероструктурами из пленок Pt с антибактериальными агентами Ag и AuAg, в эксперименте in vivo продемонстрировало их лучшую биологическую совместимость по сравнению с аналогичным образцом без покрытия. Изменения, выявленные в периимплантных тканях на 39-е сут, в целом соответствуют репаративной фазе раневого процесса, протекающего по благоприятному сценарию. Об этом свидетельствуют небольшая толщина соединительнотканой капсулы, умеренная клеточность, преобладание стромальных элементов, относительно низкая васкуляризация (соответствует по типу васкуляризации неоформленной соединительной ткани). В соединительнотканой капсуле вокруг непокрытого образца TiNi выявлено наличие макрофагов, а показатели лимфоцитов
Список литературы Биологическое тестирование никелидтитановых имплантатов с покрытиями из благородных металлов в эксперименте in vivo
- Theil C., Schwarze J., Gosheger G., Moellenbeck B., Schneider K.N., Deventer N., Klingebiel S., Grammatopoulos G., Boettner F., SchmidtBraekling T. Implant Survival, Clinical Outcome and Complications of Megaprosthetic Reconstructions Following Sarcoma Resection. Cancers (Basel). 2022; 14(2): 351. https://doi.org/10.3390/cancers14020351.
- Bansiddhi A., Sargeant T.D., Stupp S.I., Dunand D.C. Porous NiTi for bone implants: a review. Acta Biomater. 2008; 4(4): 773-82. https://doi.org/10.1016/j.actbio.2008.02.009.
- Topolnitskiy E., Chekalkin T., Marchenko E., Yasenchuk Y., Kang S.B., Kang J.H., Obrosov A. Evaluation of Clinical Performance of TiNi-Based Implants Used in Chest Wall Repair after Resection for Malignant Tumors. J Funct Biomater. 2021; 12(4): 60. https://doi.org/10.3390/jfb12040060.
- Topol'nitskii E.B., Shefer N.A., Marchenko E.S., Fomina T.I., Mikhed R.A., Tsydenova A.N., Garin A.S. Osobennosti integratsii dvukhsloinogo metallotrikotazha iz nikelida titana pri zameshchenii torakoabdominal'nogo defekta v eksperimente. Acta Biomedica Scientifca. 2023; 8(2): 244-53. https://doi.org/10.29413/ABS.2023-8.2.24.
- Topol'nitskii E.B., Shefer N.A., Yunusov A.N., Fomina T.I., Marchenko E.S. Morfologicheskoe obosnovanie primeneniya nikelida titana v rekonstruktivnoi khirurgii diafragmy. Voprosy rekonstruktivnoi i plasticheskoi khirurgii. 2023; 26(4): 13-23. https://doi.org/10.52581/1814-1471/87/02.
- Topol'nitskii E.B., Dambaev G.Ts., Gyunter V.E., Baikov A.N., Khodorenko V.N., Fomina T.I., Shefer N.A. Morfologicheskaya otsenka sposoba zameshcheniya postrezektsionnykh defektov perikarda tkanevym implantatom na osnove nanostrukturnoi nikelid-titanovoi niti. Byulleten' sibirskoi meditsiny. 2011; 10(3): 62-66.
- Srivastava A.K., Snapper D.M., Zheng J., Yildrim B.S., Srivastava S., Wood S.C. Examining the role of nickel and NiTi nanoparticles promoting infammation and angiogenesis. J Immunotoxicol. 2022; 19(1): 61-73. https://doi.org/10.1080/1547691X.2022.2080307.
- Marchenko E.S., Dubovikov K.M., Baigonakova G.A., Shishelova A.A., Topolnitskiy E.B., Chernyshova A.L. The infuence of hydroxyapatite coatings with diferent structure and crystallinity on osteogenesis stimulation. Ceramics International. 2024; 50(15): 27317-30. https://doi.org/10.1016/j.ceramint.2024.05.029.
- Jang S.R., Suh I.W., Heng L. Nanoscale Polishing Technique of Biomedical Grade NiTi Wire by Advanced MAF Process: Relationship between Surface Roughness and Bacterial Adhesion. J Funct Biomater. 2023; 14(4): 177. https://doi.org/10.3390/jfb14040177.
- Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial bioflms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004; 2(2): 95-108. https://doi.org/10.1038/nrmicro821.
- Scholz M.S., Blanchfeld J.P., Bloom L.D., Coburn B.H., Elkington M., Fuller J.D., Gilbert M.E., Mufahi S.A., Pernice M.F., Rae S.I., Trevarthen J.A., White S.C., Weaver P.M., Bond I.P. The use of composite materials in modern orthopaedic medicine and prosthetic devices: A review. Composit Sci Technol. 2011; 71(16): 1791-803. https://doi.org/10.1016/j.compscitech.2011.08.017.
- Chernousova S., Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013; 52(6): 1636-53. https://doi.org/10.1002/anie.201205923.
- Knetsch M.L.W., Koole L.H. New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers. 2011; 3(1): 340-66. https://doi.org/10.3390/polym3010340.
- Demann E.T., Stein P.S., Haubenreich J.E. Gold as an implant in medicine and dentistry. J Long Term Ef Med Implants. 2005; 15(6): 687-98. https://doi.org/10.1615/jlongtermefmedimplants.v15.i6.100.
- Miyazawa N., Hakamada M., Mabuchi M. Antimicrobial mechanisms due to hyperpolarisation induced by nanoporous Au. Sci Rep. 2018; 8(1): 3870. https://doi.org/10.1038/s41598-018-22261-5.
- Schmidt-Braekling T., Streitbuerger A., Gosheger G., Boettner F., Nottrott M., Ahrens H., Dieckmann R., Guder W., Andreou D., Hauschild G., Moellenbeck B., Waldstein W., Hardes J. Silver-coated megaprostheses: review of the literature. Eur J Orthop Surg Traumatol. 2017; 27(4): 483-9. https://doi.org/10.1007/s00590-017-1933-9.
- Aurore V., Caldana F., Blanchard M., Kharoubi Hess S., Lannes N., Mantel P.Y., Filgueira L., Walch M. Silver-nanoparticles increase bactericidal activity and radical oxygen responses against bacterial pathogens in human osteoclasts. Nanomedicine. 2018; 14(2): 601-7. https://doi.org/10.1016/j.nano.2017.11.006.
- Ferraris S., Spriano S. Antibacterial titanium surfaces for medical implants. Mater Sci Eng C Mater Biol Appl. 2016; 61: 965-78. https://doi.org/10.1016/j.msec.2015.12.062.
- Shamaila S., Zafar N., Riaz S., Sharif R., Nazir J., Naseem S. Gold Nanoparticles: An Efcient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials (Basel). 2016; 6(4): 71. https://doi.org/10.3390/ nano6040071.
- Tao C. Antimicrobial activity and toxicity of gold nanoparticles: research progress, challenges and prospects. Lett Appl Microbiol. 2018; 67(6): 537-43. https://doi.org/10.1111/lam.13082.
- Köller M., Sengstock C., Motemani Y., Khare C., Buenconsejo P.J., Geukes J., Schildhauer T.A., Ludwig A. Antibacterial activity of microstructured Ag/Au sacrifcial anode thin flms. Mater Sci Eng C Mater Biol Appl. 2015; 46: 276-80. https://doi.org/10.1016/j.msec.2014.10.058.
- Li T., Albee B., Alemayehu M., Diaz R., Ingham L., Kamal S., Rodriguez M., Bishnoi S.W. Comparative toxicity study of Ag, Au, and Ag-Au bimetallic nanoparticles on Daphnia magna. Anal Bioanal Chem. 2010; 398(2): 689-700. https://doi.org/10.1007/s00216-010-3915-1.
- Dorovskikh S.I., Vikulova E.S., Sergeevichev D.S., Guselnikova T.Y., Korolkov I.V., Fedorenko A.D., Nasimov D.A., Vasilieva M.B., Chepeleva E.V., Zherikova K.V., Basova T.V., Morozova N.B. Heterostructures Based on Noble Metal Films with Ag and Au Nanoparticles: Fabrication, Study of In Vivo Biocompatibility and Antibacterial Activity. Coatings. 2023; 13(7): 1269. https://doi.org/10.3390/coatings13071269.
- Sergeevichev D.S., Dorovskikh S.I., Vikulova E.S., Chepeleva E.V., Vasiliyeva M.B., Koretskaya T.P., Fedorenko A.D., Nasimov D.A., Guselnikova T.Y., Popovetsky P.S., Morozova N.B., Basova T.V. Vapor-PhaseDeposited Ag/Ir and Ag/Au Film Heterostructures for Implant Materials: Cytotoxic, Antibacterial and Histological Studies. Int J Mol Sci. 2024; 25(2): 1100. https://doi.org/10.3390/ijms25021100.
- Vikulova E.S., Karakovskaya K.I., Korolkov I.V., Koretskaya T.P., Chepeleva E.V., Kuzmin N.B., Fedorenko A.D., Pischur D.P., Guselnikova T.Y., Maksimovskii E.A., Marchenko E.S., Zheravin A.A., Morozova N.B. Application of biocompatible noble metal flm materials to medical implants: TiNi surface modifcation. Coatings. 2023; 13(2): 222. https://doi.org/10.3390/coatings13020222.
- Gao L., Wang Y., Li Y., Xu M., Sun G., Zou T., Wang F., Xu S., Da J., Wang L. Biomimetic biodegradable Ag@Au nanoparticle-embedded ureteral stent with a constantly renewable contact-killing antimicrobial surface and antibioflm and extraction-free properties. Acta Biomater. 2020; 114: 117-32. https://doi.org/10.1016/j.actbio.2020.07.025.
- Geissel F.J., Platania V., Tsikourkitoudi V., Larsson J.V., Thersleff T., Chatzinikolaidou M., Sotiriou G.A. Silver/gold nanoalloy implant coatings with antibioflm activity via pH-triggered silver ion release. Chem Commun (Camb). 2024; 60(60): 7729-32. https://doi.org/10.1039/d4cc01168f.
- Gunther V., Marchenko E., Chekalkin T., Baigonakova G., Kang J.H., Kim J.S., Klopotov A. Study of structural phase transitions in quinary TiNi (MoFeAg)-based alloys. Materials Research Express. 2017; 4(10). https://doi.org/10.1088/2053-1591/aa9087.
- Betts A.J., Dowling D.P., McConnell M.L., Pope C. The infuence of platinum on the performance of silver-platinum anti-bacterial coatings. Materials & Design. 2005; 26(3): 217-22. https://doi.org/10.1016/j.matdes.2004.02.006.
- Köller M., Bellova P., Javid S.M., Motemani Y., Khare C., Sengstock C., Tschulik K., Schildhauer T.A., Ludwig A. Antibacterial activity of microstructured sacrifcial anode thin flms by combination of silver with platinum group elements (platinum, palladium, iridium). Mater Sci Eng C Mater Biol Appl. 2017; 74: 536-41. https://doi.org/10.1016/j.msec.2016.12.075.
- Yang L., Yan W., Wang H., Zhuang H., Zhang J. Shell thicknessdependent antibacterial activity and biocompatibility of gold@silver core-shell nanoparticles. RSC advances. 2017; 7(19): 11355-61. https://doi.org/10.1039/c7ra00485k.
- Cullity B.D. Elements of X-ray Difraction. Massachusetts, ed. Cohen M.: Addison-Wesley Publishing Company Reading, 1978. 350 p.
- Kraus W., Nolze G. POWDER CELL - a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J Appl Crystallograph. 1996; 29(3): 301-3. https://doi.org/10.1107/S0021889895014920.
- Phillips E.A., Klein G.R., Cates H.E., Kurtz S.M., Steinbeck M. Histological characterization of periprosthetic tissue responses for metalon-metal hip replacement. J Long Term Ef Med Implants. 2014; 24(1): 13-23. https://doi.org/10.1615/jlongtermefmedimplants.2014010275.
- Mehanna E.T., Kamel B.S.A., Abo-Elmatty D.M., Elnabtity S.M., Mahmoud M.B., Abdelhafeez M.M., Abdoon A.S.S. Efect of gold nanoparticles shape and dose on immunological, hematological, infammatory, and antioxidants parameters in male rabbit. Vet World. 2022; 15(1): 65-75. https://doi.org/10.14202/vetworld.2022.65-75.
- Vanharen M., Girard D. Impact of gold nanoparticles (AuNPs) on eosinophils isolated from male and female individuals. Immunobiology. 2023; 228(6). https://doi.org/10.1016/j.imbio.2023.152762.
- Topol'nitskii E.B., Shefer N.A., Marchenko E.S., Larikov V.A. Sposob plevrodeza poroshkom nikelida titana, legirovannogo serebrom. Patent RF № 2810367. Zayavl. 02.05.2023; Opubl. 27.12.2023.


