Blood mononuclear cell molecular landscape associated with tumor progression in triple-negative breast cancer
Автор: Patysheva M.R., Frolova A.A., Bragina O.D., Fedorov A.A., Vostrikova M.A., Garbukov E.Yu., Iamshchikov P.S., Vashisth M., Cherdyntseva N.V., Gerashchenko T.S.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Случай из клинической практики
Статья в выпуске: 5 т.22, 2023 года.
Бесплатный доступ
Introduction. Triple negative breast cancer is an aggressive clinical phenotype characterized by poor prognosis. Immune system plays an important role in the development, treatment response, and progression of solid tumor. The search for immune-related markers associated with the prediction of treatment efficacy and disease prognosis, and based on the use of high-resolution molecular techniques, is a promising area of research, the results of which can be translated into clinical practice. Case description. The molecular profile of blood mononuclear cells in a 48-year-old female patient with histologically proven triple negative breast cancer (Estrogen Receptor - 0; Progesteron Receptor - 0; Her2/neu - 0; GATA-3 - 0, Androgen Receptor - 0 and Ki67 - 70 %) was described. The patient did not response to neoadjuvant chemotherapy with 4 cycles of paclitaxel + carboplatin followed by 2 cycles of adriamycin + cyclophosphamide. The patient underwent surgery. Disease progression (pelvic bone metastases) occurred 2 months after surgery. The features of blood lymphocytes and monocytes associated with a lack of response to neoadjuvant chemotherapy and disease progression were described.
Triple-negative breast cancer, immune system, single cell sequencing, chemotherapy, progression
Короткий адрес: https://sciup.org/140303540
IDR: 140303540 | УДК: 618.19-006.6-08-036.17:577.21 | DOI: 10.21294/1814-4861-2023-22-5-197-204
Текст научной статьи Blood mononuclear cell molecular landscape associated with tumor progression in triple-negative breast cancer
Triple-negative breast cancer (TNBC) is diagnosed in 15–20 % of patients with newly diagnosed breast cancer. Unlike luminal and HER2-positive subtypes, TNBC is characterized by a lack of access points for currently available systemic treatment, aggressive disease course and unfavorable prognosis. In this regard, the main type of specialized treatment for TNBC is cytostatic therapy, which can be used in both neoadjuvant and adjuvant regimens [1]. The use of neoadjuvant chemotherapy (NACT) allows for the evaluation of tumor sensitivity for the ongoing treatment in vivo based on the degree of residual cancer burden along with an increased number of organ-preserving surgeries. To date, achievement of pathological complete response (pCR) is associated with a significant improvement in long-term treatment outcomes [2]. Despite the wide range of drugs used, it is not always possible to predict NACT response and pCR achievement [3].
The level of proliferative activity, the cancer grade, and the presence of mutations in the BRCA1 gene are expected to be used as predictive criteria [2]. Due to insufficient prognostic significance, it is necessary to search for additional informative parameters to predict the treatment efficacy and disease outcome.
The immune system is a direct participant in tumor growth. Normally, the immune system works to recognize and eliminate transformed cells, but when tumor growth occurs, a pathological cycle that ultimately causes immune suppression is formed. Tumor cells escape immunological surveillance and form their own microenvironment in the process of interaction with the body. Immune cells of the tumor microenvironment and circulating immune cells are known to be linked. Circulating mononuclear cells, which include populations of lymphocytes and monocytes, can be used as markers for predicting treatment efficacy or disease prognosis [4–6]. In this paper, we present our
-5

-
• CD4 Naive T cells
-
• CD14 Monocytes
-
• CD8 Naive T cells
-
• В cells
-
• CD16 Monocytes
-
• Erythroid cells
-
• CD8 T cells
-
• NK cells
-
• CD4 Memory T cells
-
• CD4 Cytotoxic T cells
-
• Treg cells
-10
-15 -10 -5 0 5 10
UMAP 1
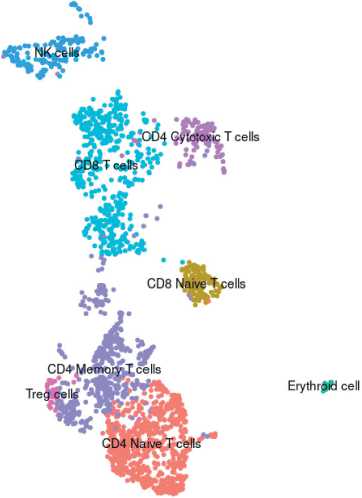
Fig. 1. Clusters (populations) of mononuclear blood cells in the patient with TNBC before treatment. Note: created by the authors Рис. 1. Распределение монону-клеарных клеток крови по кластерам (популяциям) у пациентки с ТНРМЖ до начала лечения. Примечание: рисунок выполнен авторами
experience describing the mononuclear cell phenotype using single cell resolution in a patient with TNBC which demonstrated poor response to NACT and the evidence of distant metastases within the first year of treatment.
Case presentation
Patient W., 48 years old, referred to the Department of General Oncology of the Cancer Research Institute in June 2022 with complaints of a mass in the left side of the chest wall. According to the results of histological examination, she was diagnosed with invasive breast carcinoma (сT3cN1cM0/pT3pN1cM0) IIIA.
Past medical history. The patient found a mass in December 2021 and consulted a physician. According to the results of an ultrasound scan dated April 13, 2022: 4.0×4.2 cm mass in the lining of the pectoralis major muscle. Results of spiral computer tomography from April 18, 2022 showed a mass of the pectoralis major muscle on the right 4.9×3.6×4.3 cm. She applied to the Cancer Research Institute for further examination and determination of treatment tactics.
Microscopic examination of the tissue revealed tumor cells forming small islands and clusters in the fibrous stroma. There were areas of necrosis. Immunohistochemical study of the tumor was performed using a Leica Bond Max immune-analyzer. The immunohistochemical analysis reveled: expression of Estrogen Receptor (clone 1D5) – 0; Progesteron receptor (clone PgR636, Dako) – 0; c-erB-2 (Her2/neu)(Poly-clonal Rabbit, Dako) – 0; GATA-3 (clone L50-823, Cell Marque) – 0, Androgen Receptor (clone AR441, Dako):0; Ki67 (clone SP6, Cell Marque) – 70 % of tumor cells. Based on the combined morphological data and immunohistochemical profile of the tumor cells, the diagnosis was: invasive breast carcinoma, NOS (8500/3) G2. Clinical blood test: white blood cells – 5.22 ×109/L; red blood cells – 4.44 ×1012/L; hemoglobin – 112 g/L; platelets – 366 × 109/L; seg- mented blood cells – 68 %; lymphocytes – 23 %; monocytes – 7 %; eosinophils – 2 %; hematocrit – 35 %;ESR – 39 mm/h.
Patient gave written informed consent in accordance with the Declaration of Helsinki on 29 June 2022 (the approval number is 25). The study was approved by the review board of the Cancer Research Institute, Tomsk NRMC on 29 August 2022 (the approval number is 16).
After diagnosis and before NACT, peripheral venous blood was taken from the patient, followed by molecular profiling of blood mononuclear cells. Molecular profiling was performed using single-cell RNA sequencing based on BD Rhapsody technology (BD Bioscience, USA). The obtained data were processed using Seurat 4.2.0 software package (https://satijalab. org/seurat/). The patient was typed into clusters corresponding to blood cell populations (Fig. 1). A total of 11 clusters were detected, including CD14 and CD16 monocytes, natural killer cells, naive CD4 and CD8 cells, CD4 memory and cytotoxic CD4 cells, cytotoxic CD8, T regulatory cells, B cells and a small admixture of erythroid cells (Fig. 1). A decrease in cytotoxic T cells was observed in the patient under study compared to that of healthy donors as described in the literature (Table 1). In addition, there was a decrease in the CD14+ and CD16+ monocyte populations among all blood mononuclear cells compared to the normal proportion of these cells (Table ). The classic CD14+ monocyte population was characterized by gene expression of both the standard monocyte marker CD14 and FCN1, and components of the histocompatibility complex HLA-DRA and CD74, monocyte extravasation factors S100A9 and S100A12, proinflammatory factors IL1B and galectin-1 (LGALS1), and transforming growth factor TGFBI (Log2FC≥1.3, FDR≤0.05) (Fig. 2). The minor nonclassical CD16+ monocyte population was characterized by expression of the major markers of nonclassical monocytes FCGR3A,
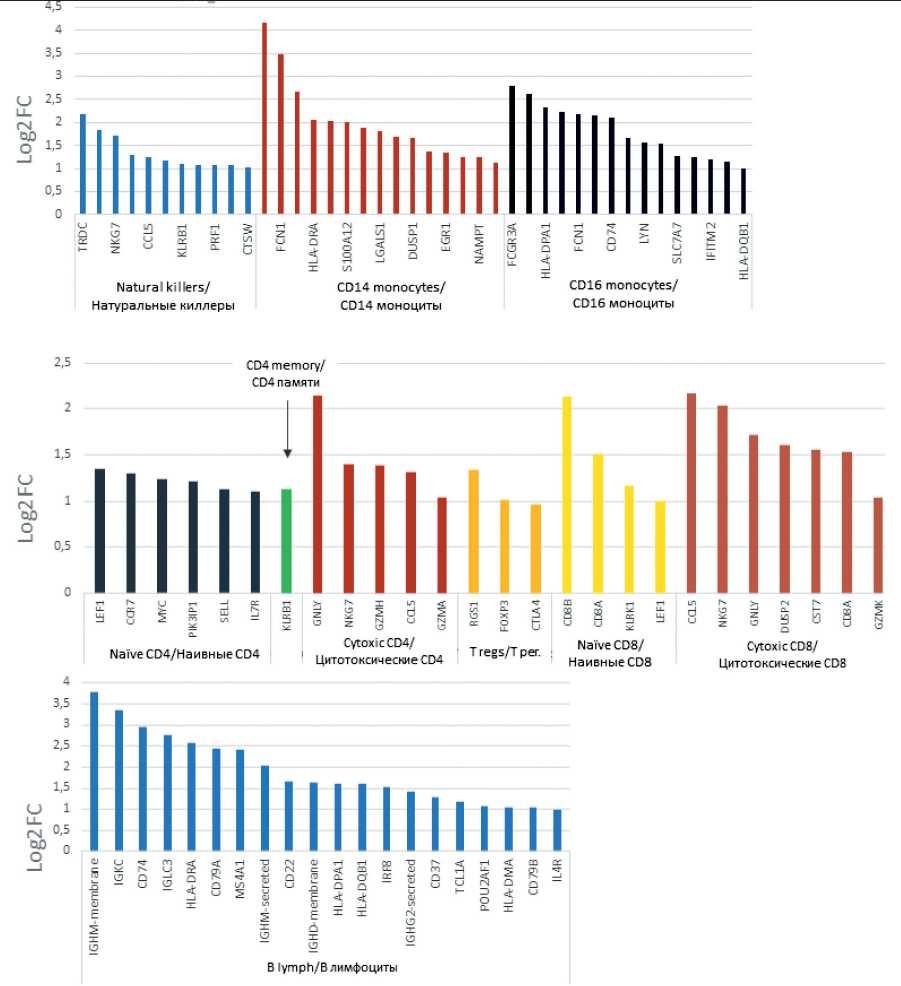
Fig. 2. Molecular phenotype characteristics of blood mononuclear populations of the patient with TNBC before treatment. Note: created by the authors Рис. 2. Характеристика молекулярного фенотипа популяций мононуклеаров крови пациентки с ТНРМЖ до начала лечения. Примечание: рисунок выполнен авторами
FCER1G and FCN1, components of the histocompatibility complex HLA-DPA1, HLA-DRA and CD74, galectins LGALS1 and LGALS9, interferon response factors IFITM3 and IFITM2 as well as transcription factor LYN, which is involved in monocyte response to major monocyte migration factor (4) (Log2FC≥1.1, FDR≤0.05) (Fig. 2). Cytotoxic T lymphocytes that contained an increased number of transcripts of gran-zymes GNLY, NKG7, GZMH, GZMA (Log2FC≥1.1, FDR≤0.05) and also had a hyperexpressed CCL5 chemokine ligand gene (Log2FC≥1.3, FDR≤0.05) (Fig. 2). Interestingly, CCL5 expression was also 8-fold elevated in the CD8 T-killer population (Log2FC≥1.3, FDR≤0.05). The cluster of regulatory T cells performing immune response suppression was characterized by the expression, as follows, of the RGS1, FOXP3, and CTLA4 genes characteristic of this cell population (Log2FC≥1.0, FDR≤0.05) (Fig. 2).
The patient was treated with 4 cycles of NACT according to the “paclitaxel + carboplatin” scheme, followed by 2 courses according to the “AC” scheme (adriamycin + cyclophosphane). Due to the absence of the effect of NACT (an increase in the size of the tumor against the background of therapy), a radical mastectomy of the left breast was performed on 12/15/2022. Histological examination of the surgical material: Invasive carcinoma of a non-specific type, G3 (3+3+2=8/9) with necrosis of tumor tissue in the central parts. RCB-III (3.9965). No neural invasion was detected. The minimum distance to the tumor is 2 mm (blue paint). Lymph nodes of cellulose (9 l/y) in 1 of 9 metastatic lesion (4 mm). ypT3N1Mx. ICD-o code 8500/3. Taking into account the lack of effect from NACT, the patient was recommended to take capecitabine in the standard dosage in the adjuvant regimen. Computed tomography revealed metastases in the pelvic bone. The patient was transferred to palliative care.
Discussion
Analysis of the parameters of the immune system components is difficult due to its high degree of heterogeneity. Molecular markers that characterize the functional status of blood cell populations may be associated with the response to treatment. The emergence of new high-tech methods of analysis allows the study of a large number of parameters with a high level of resolution (up to single cells). In our study, we found a reduced content of cytotoxic CD4 T cells and monocyte classic (CD14+) and non-classic (CD16+) populations in the patient. The latter observation may be related to the migration of monocytes
Table/Òàблицà
Cell population distribution among blood mononuclear cells
Ðàñпðåдåлåниå êлåтîчныõ пîпóляциé ñðåди мîнîнóêлåàðныõ êлåтîê êðîви
|
Parameteres/Параметры |
Study patient/ Пациентка Ж. |
Healthy volunteers/ Здоровые лица |
Reference/Источник |
|
Naive CD4 T cells/ Наивные CD4 |
28,9 |
25,0–40,0 |
[11] |
|
Memory CD4 T cells/ CD4 памяти |
20,22 |
15,0–20,0 |
[12] |
|
Regulatory CD4 T cells (Treg)/ Регуляторные CD4 |
1,6 |
0,7–3,6 |
[13] |
|
Cytotoxic CD4 T cells/ Цитотоксические CD4 |
4,3* |
10,0–20,0 |
[14] |
|
Naive CD8 T cells/ Наивные CD8 |
5,7 |
3,0–10,5 |
[15] |
|
Cytotoxic CD8 T cells/ CD8 киллеры |
20,5 |
1,55–27,0 |
[16] |
|
B lymphocyte/ В-лимфоциты |
4,3 |
3,5–13,5 |
[16] |
|
Natural killer cell/ Натуральные киллеры |
5,79 |
3,5–9,0 |
[16] |
|
CD14 monocytes/ CD14 моноциты |
7,72* |
10,0-30,0 |
[17] |
|
CD16 monocytes/ CD16 моноциты |
0,2* |
0,5–4,5 |
[17] |
Note: *– indicator is lower than that of healthy volunteers; created by the authors.
Примечание: * – показатель ниже, чем у здоровых добровольцев; таблица составлена авторами.
from the blood into the tumor tissue, which is known to produce circulating cell recruitment factors [7]. In transcriptome marker analysis, cytotoxic CD4 T cells were characterized by hyperexpression of the CCL5 gene, responsible for maintaining tumor cell growth and chemoresistance (Fig. 2) [8]. Monocytes showed expression of S100A9 and S100A12 factors (for CD14+ population) and LYN (for CD16+ population), which related to the ability of monocytes for tissue migration and their subsequent differentiation into tissue macrophages, supporting tumor growth [9, 10]. Thus, circulating immune cells demonstrate the ability to support tumor growth, which may be associated with the progression of TNBC in the study patient.
Список литературы Blood mononuclear cell molecular landscape associated with tumor progression in triple-negative breast cancer
- García-Teijido P., Cabal M.L., Fernández I.P., Pérez Y.F. Tumor- Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin Med Insights Oncol. 2016; 10(S1): 31–9. doi: 10.4137/CMO.S34540.
- Zarotti C., Papassotiropoulos B., Elfgen C., Dedes K., Vorburger D., Pestalozzi B., Trojan A., Varga Z. Biomarker dynamics and prognosis in breast cancer after neoadjuvant chemotherapy. Scientific reports. 2022; 12(1): 91. doi: 10.1038/s41598-021-04032-x.
- Loi S., Sirtaine N., Piette F., Salgado R., Viale G., Van Eenoo F., Rouas G., Francis P., Crown J.P., Hitre E., de Azambuja E., Quinaux E., Di Leo A., Michiels S., Piccart M.J., Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02- 98. J Clin Oncol. 2013; 31(7): 860–7. doi: 10.1200/JCO.2011.41.0902.
- Lin G.N., Peng J.W., Liu D.Y., Xiao J.J., Chen Y.Q., Chen X.Q. Increased lymphocyte to monocyte ratio is associated with better prognosis in patients with newly diagnosed metastatic nasopharyngeal carcinoma receiving chemotherapy. Tumour Biol. 2014; 35(11): 10849–54. doi: 10.1007/s13277-014-2362-6.
- Peng Y., Chen R., Qu F., Ye Y., Fu Y., Tang Z., Wang Y., Zong B., Yu H., Luo F., Liu S. Low pretreatment lymphocyte/monocyte ratio is associated with the better efficacy of neoadjuvant chemotherapy in breast cancer patients. Cancer Biol Ther. 2020; 21(2): 189–96. doi: 10.1080/15384047.2019.1680057.
- Lu C., Zhou L., Ouyang J., Yang H. Prognostic value of lymphocyteto- monocyte ratio in ovarian cancer: A meta-analysis. Medicine (Baltimore). 2019; 98(24). doi: 10.1097/MD.0000000000015876.
- Geiger R., Duhen T., Lanzavecchia A., Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J Exp Med. 2009; 206(7): 1525–34. doi: 10.1084/jem.20090504.
- Nausch N., Bourke C.D., Appleby L.J., Rujeni N., Lantz O., Trottein F., Midzi N., Mduluza T., Mutapi F. Proportions of CD4+ memory T cells are altered in individuals chronically infected with Schistosoma haematobium. Sci Rep. 2012; 2: 472. doi: 10.1038/srep00472.
- Duggleby R., Danby R.D., Madrigal J.A., Saudemont A. Clinical Grade Regulatory CD4+ T Cells (Tregs): Moving Toward Cellular-Based Immunomodulatory Therapies. Front Immunol. 2018; 9: 252. doi: 10.3389/fimmu.2018.00252.
- Hashimoto K., Kouno T., Ikawa T., Hayatsu N., Miyajima Y., Yabukami H., Terooatea T., Sasaki T., Suzuki T., Valentine M., Pascarella G., Okazaki Y., Suzuki H., Shin J.W., Minoda A., Taniuchi I., Okano H., Arai Y., Hirose N., Carninci P. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc Natl Acad Sci USA. 2019; 116(48): 24242–51. doi: 10.1073/pnas.1907883116.
- Pulko V., Davies J.S., Martinez C., Lanteri M.C., Busch M.P., Diamond M.S., Knox K., Bush E.C., Sims P.A., Sinari S., Billheimer D., Haddad E.K., Murray K.O., Wertheimer A.M., Nikolich-Žugich J. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol. 2016; 17(8): 966–75. doi: 10.1038/ni.3483.
- Kleiveland C.R. Peripheral Blood Mononuclear Cells. In: Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet]. Cham (CH): Springer; 2015. Chapter 15.
- Патышева М.Р., Стахеева М.Н., Ларионова И.В., Тарабановская Н.А., Григорьева Е.С., Слонимская Е.М., Кжышковска Ю.Г., Чердынцева Н.В. Моноциты при злокачественных новообразованиях: перспективы и точки приложения для диагностики и терапии. Бюллетень сибирской медицины. 2019; 18(1): 60–75. [Patysheva M.R., Stakheeva M.N., Larionova I.V., Tarabanovskaya N.A., Grigorieva E.S., Slonimskaya E.M., Kzhyshkowska J.G., Cherdyntseva N.V. Monocytes and cancer: promising role as a diagnostic marker and application in therapy. Bulletin of Siberian Medicine. 2019; 18(1): 60–75. (in Russian)]. doi: 10.20538/1682-0363-2019-1-76-83.
- Kohli K., Pillarisetty V.G., Kim T.S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2022; 29(1): 10–21. doi: 10.1038/s41417-021-00303-x.
- Aldinucci D., Borghese C., Casagrande N. The CCL5/CCR5 Axis in Cancer Progression. Cancers (Basel). 2020; 12(7): 1765. doi: 10.3390/cancers12071765.
- Das P., Pal S., Oldfield C.M., Thillai K., Bala S., Carnevale K.A., Cathcart M.K., Bhattacharjee A. A PKCβ-LYN-PYK2 Signaling Axis Is Critical for MCP-1-Dependent Migration and Adhesion of Monocytes. J Immunol. 2021; 206(1): 181–92. doi: 10.4049/jimmunol.1900706.
- Larionova I., Kazakova E., Gerashchenko T., Kzhyshkowska J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers (Basel). 2021; 13(13): 3253. doi: 10.3390/cancers13133253.


