Carcinogenicity of malathion and estrogen in an experimental rat mammary gland model
Автор: Calaf Gloria M.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 4 т.17, 2018 года.
Бесплатный доступ
Breast cancer is considered a major and common health problem in both developing and developed countries. The etiology of breast cancer, the most frequent malignancy diagnosed in women in the western world, has remained unidentified. Chemicals as the organophosphorous pesticide malathion have been used to control a wide range of sucking and chewing pests of field crops, and are involved in the etiology of breast cancers. The association between breast cancer initiation and prolonged exposure to estrogen suggests that this hormone may also have an etiologic role in such a process. However, the key factors behind the initiation of breast cancer remain to be elucidated. The effect of environmental substances, such as malathion and estrogen was analyzed in an experimental rat mammary gland model. Different cytoplasmic proteins are key in the transformation of a normal cell to a malignant tumor cell and among these are the Ras super family and Ras homologous A (Rho-A). both types of proteins were greater in animals treated with malathion than those with estrogens. E-Cadherins constitute a large family of cell surface proteins. results showed greater expression of E-Cadherin and vimentin than c-Ha-ras and Rho-A in rats treated by estrogens. In breast cancer, analysis using immunohistochemical markers is an essential component of routine pathological examinations, and plays an important role in the management of the disease by providing diagnostic and prognostic strategies. the aim of the present study was to identify markers that can be used as a prognostic tool for breast cancer patients.
Carcinogenesis, rat, mammary gland, malathion, estrogen, etiology, proteins
Короткий адрес: https://sciup.org/140254199
IDR: 140254199 | УДК: 618.19-006.6-02:577.175.6]-092.9 | DOI: 10.21294/1814-4861-2018-17-4-5-13
Текст научной статьи Carcinogenicity of malathion and estrogen in an experimental rat mammary gland model
Breast cancer is considered a major and common health problem in both developing and developed countries. The etiology of breast cancer, the most frequent malignancy diagnosed in women in the western world, has remained unidentified. The initiation and progression of breast cancer follows a complex multistep process that depends on various endogenous (hormonal imbalances, proliferative lesions, inherited mutations) and exogenous (diet, smoking, radiation, chemical exposures as pesticides) factors [1, 2]. Some of them are widely used with a huge potential for human exposure. Environmental factors are considered to be among the major influencing components causing increase in the incidence of breast cancer risk [3]. An important but unclarified question is the effect that environmental substances may play in the neoplastic process. Epidemiological studies have demonstrated the association between cancer in humans and agriculture pesticide exposure [4, 5].
Breast cancer models, where normal human breast epithelium cells undergo stepwise transformation into malignant cells after treatment with several agents provide the opportunity to understand the cellular and molecular mechanisms involved in breast carcinogenesis. Knowledge of factors that control cell proliferation of normal and neoplastic mammary epithelium, and the molecular basis of such action, is essential for a deeper understanding of the progression of human breast cancer. In rat mammary gland models whereby individual neoplastic transformed stages can be dissected and studied provide an excellent opportunity to address cellular and molecular mechanisms involved in environmental-induced mammary carcinogenesis [6].
Experimental studies have shown that environmental substances (e.g., DDT, polychlorinated biphenyls, 4-nonylphenol,4-octylphenol) seem to be involved in the etiology of breast cancers and can promote mammary cancer [7, 8]. Such substances have been associated with the use of organophosphorous insecticides in agriculture and in non-occupational situations as exposure to contaminated clothing, soil, ground and surface water, as well as drifts from aerial spraying of pesticides [9-11] and it also has been associated with prolonged exposure to female hormones [12].
Chemicals as the organophosphorous pesticide malathion (M) have been used to control a wide range of sucking and chewing pests of field crops, and are involved in the etiology of breast cancers [13]. The association between breast cancer initiation and prolonged exposure to estrogen (E) suggests that this hormone may also have an etiologic role in such a process. However, the key factors behind the initiation of breast cancer remain to be elucidated. Studies have found an association between human cancer and exposure to agricultural pesticides as demonstrated by IARC [4, 5].
An adequate animal model system has been developed to study the morphological changes that occur during mammary gland carcinogenesis. Mammary gland development is an attractive experimental animal model for understanding the effect of carcinogens since cell is not a random event , but is related to the topography of the mammary parenchyma and changes affected by age , hormonal variations and parity history. The structure of a normal rat mammary gland is composed of a single primary or main lactiferous duct that branches into alveolar buds (ABs) and secondary ducts that are narrow and straight and end in small clubshaped terminals, called terminal end buds (TEBs) that are equivalent to the terminal ductal lobular unit in the human breast , considered the site of origin of human breast carcinomas [14, 15]. The susceptibility of the mammary gland to M alone and in the presence of E was previously analyzed in an established rat mammary gland model already reported [2, 12].
The Ras super family and Ras homologous A (Rho-A) have been shown to promote both cell proliferation and cell invasion [16, 17]. Genetic evolutionary changes may occur in a preferred sequence in solid tumors and among the steps in this sequences include c-Ha-ras oncogene over-expression in breast cancer [18, 19]. This oncogene plays an important role in the progression of mammary cancer. An over expression of mutated c-Ha-ras oncogene has been previously reported in 10% of breast cancer patients [18]. The Ras gene has been reported to be involved in chemically induced mammary carcinomas, in breast cancer cell lines, and in primary breast cancers, in which point mutations, loss of heterozygosity are present [20].
Accumulating data indicate that Rho-A proteindependent cell signaling is important for malignant transformation [21-23]. Once activated, Rho-A triggers a complex set of signal transduction pathways. Rho-A is over-expressed during tumorigenesis [24]. Authors [25] examined several breast cancers and found that the over-expression of Rho-A is involved in human carcinogenesis. All breast tumors analyzed contained large amounts of Rho-A protein, whereas was hardly found in adjacent normal tissue. Breast cancer progression from grade I to grade III, classification of World Health Organization (WHO) was accompanied by a significant increase in Rho-A protein levels.
E-cadherins constitute a large family of cell surface proteins, including E (epithelial), N (neural), VE (vascular endothelial), P (placental), R (retinal) and K (kidney) cadherins [26]. Classical cadherins are single-pass transmembrane proteins which participate in Ca2+-dependent cell adhesion that is necessary to form solid tissues [27, 28]. E-cadherin is functionally linked to the generation of a polarized epithelial phenotype [29, 30]. The extracellular region of E-cadherin extends from the cell surface and binds to cadherins present on adjacent cells [31] whereas its intracellular region contains binding sites to interact with catenins and other regulatory proteins [32].
Different cytoplasmic proteins are important in the transformation of a normal cell to invasive tumor cell and among them is the vimentin. It is a 57-kDa intermediate filament protein which forms a part of the cytoskeleton. It is one of the cytoplasmic intermediate filament proteins, which are the major components of the cytoskeleton normally found in embryonic or mesenchymal stem cells [33, 34].
The aim of the present study was to evaluate Ras, Rho-A, E-Cadherin and Vimentin protein expression by immunohistochemistry in a transformed rat model induced by an environmental substance as malathion and an endogenous substance as estrogen to provide evidences that it can be used as good a prognostic tool for breast cancer patients.
Materials and Methods
Experimental designs: Thirty-nine-day-old virgin female Sprague-Dawley rats were obtained from the Catholic University of Chile (Santiago, Chile) and housed and bred in a barrier animal facility operated in accordance with the standards outlined in Guide for the Care and Use of Laboratory Animals [35]. All animals were allowed continuous access to a standard laboratory chow diet (Champion, Santiago, Chile). Experimental design: i) control group received saline solution, 250 μg/100 g body weight. Treated animals were injected subcutaneously (s.c.) for 5 days, twice a day with: ii) malathion (M) (Fyfanon TM, Cheminova, Denmark) that received 22 mg/100 g bw, iii) 17β-estradiol (E) (Sigma-Aldrich Chemical Co., Milwaukee, USA), 30 μg/100 g bw and iv) combination of both (M+E). The LD50 values of the substances were 1.000 mg/kg. However, the dose used in these experiments was 1/6th of the LD50 for M, which allowed a 100% survival of animals after a 5-day treatment [2]. Animals were housed three per cage and palpated weekly to detect formation of tumors and sacrificed after 400 days following a 5-day treatment. Animals to be sacrificed were anesthetized by intraperitoneal injections of sodium pentobarbital (8 mg/100 g bw) and opened by a midline incision from the pubis to the sub-maxillary area to remove the tissues.
Immunohistochemistry
Mammary glands and palpable tumors were fixed in 10% neutral buffered, embedded in paraffin, then serially sectioned at a thickness of 5 μ and stained with hematoxylin-eosin (HE). Rat mammary gland tissues were excised to analyze protein expression by immunohistochemistry. Tissues were analyzed using a binocular microscope (Olympus CX31) with lens of 10 x in which a 1-mm2 grid was installed in one of the oculars. The localization of the antibody was visualized using 3, 3’-diaminobenzidine tetrahydrochloride (DAB) and counterstaining with Mayer’s hematoxylin (Sigma-Aldrich Chemical Co., Milwaukee, USA). All samples investigated were tested for anti-mouse monoclonal or polyclonal antibodies: H-Ras (mouse, sc-29), Rho-A (sc-418), E-Cadherin (mouse, sc-8426) and Vimentin (sc-6260) (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Immuno peroxidase staining of protein expression in the slides after treatment with pesticide and estrogen were examined in 10 fields of microscope. Five slides were counted per animal to determine per mm2 the number of stained proliferative ducts and lobules filled with hyaline casts in the mammary gland from control and treated-animals. Fifty ducts in stage of proliferation (dsp/mm2) and fifty lobules filled with secretion were counted. Such results were scored according to scale from 0 to 30 points. The ratings were: none (0 points), weak (10 points), slight (15 points), moderate (20 points) and intense (30 points) protein expression. Structures were graded as 0 when morphology of normal structure was present and there was not proliferative ducts and lobules with hyaline casts in the center of the structures. Immunochemical data were expressed as the average ± standard error (SE) of the mean. Statistical comparison between groups and controls were made by ANOVA and Dunnet´s test with P < 0.05 between groups was considered to be significant.
Results
Analysis of stage for treatment of breast cancer by using markers of immunohistochemistry remains an essential component of routine pathological examinations, and plays an important role in the management of the disease by providing diagnostic and prognostic strategies. The evaluation of Ras, Rho-A, E-Cadherin and Vimentin protein expression by immunohistochemistry in a rat mammary gland cancer model of transformed cells by pesticides in the presence of hormones as estrogens gave us evidences that they can be used as a good prognostic tool for breast cancer patients.
We have previously reported a synergism in the type of structures by the effect of M and E in the structures present in rat mammary gland development by using morphological measurements [13]. In the present study such effect was confirmed by immunohistochemistry by determining Ras, Rho-A, E-Cadherin and Vimentin protein expression. Results indicated that M induced significantly (p > 0.05) higher number of ducts in stage of proliferation per mm2 in mammary glands and had significantly greater (P<0.05) c-Ha-ras (Figure 1A), Rho-A (Figure 1B), E-Cadherin (Figure 1C) and Vimentin (Figure 1D) protein expression at 400 days after a 5-day treatment in comparison to control, E and M+E treated rats, as seen in graphs. Figure 1E shows relative protein expression determined by peroxidase staining and correspond to M+E-treated animals on similar proteins where there was significantly (P<0.05) greater c-Ha-ras and Rho-A than Cadherin and Vimentin expression.
Representative images of cross section of mammary gland immunostained with c-Ha-ras (Figure 2 B a-e), Rho-A (Figure 2 C a-e), E-Cadherin (Figure 2D a-e) and Vimentin (Figure 2E a-e) protein expression are seen. It can be observed that the control rats had normal mammary gland duct formation. A normal duct is shown in Figure 2 A a). Cross section of ducts in stage of proliferation can be seen in M-treated animals. Figure 2A b-d) represents ducts in stage of proliferation of M-treated rat stained with HE. Figure 2A e) corresponds to a cross section of a tumor formed in animal treated with M.
The relative c-Ha-ras, Rho-A, E-Cadherin and Vimentin protein expression in lobules can be seen in Figure 3A-D. Graphs correspond to the quantification of mammary glands of rats treated with E on relative average number of lobules with casts in stage of proliferation/mm2 in immune-stained cells. It was studied the effect of control, malathion (M), estrogen (E) and combination of both on relative protein
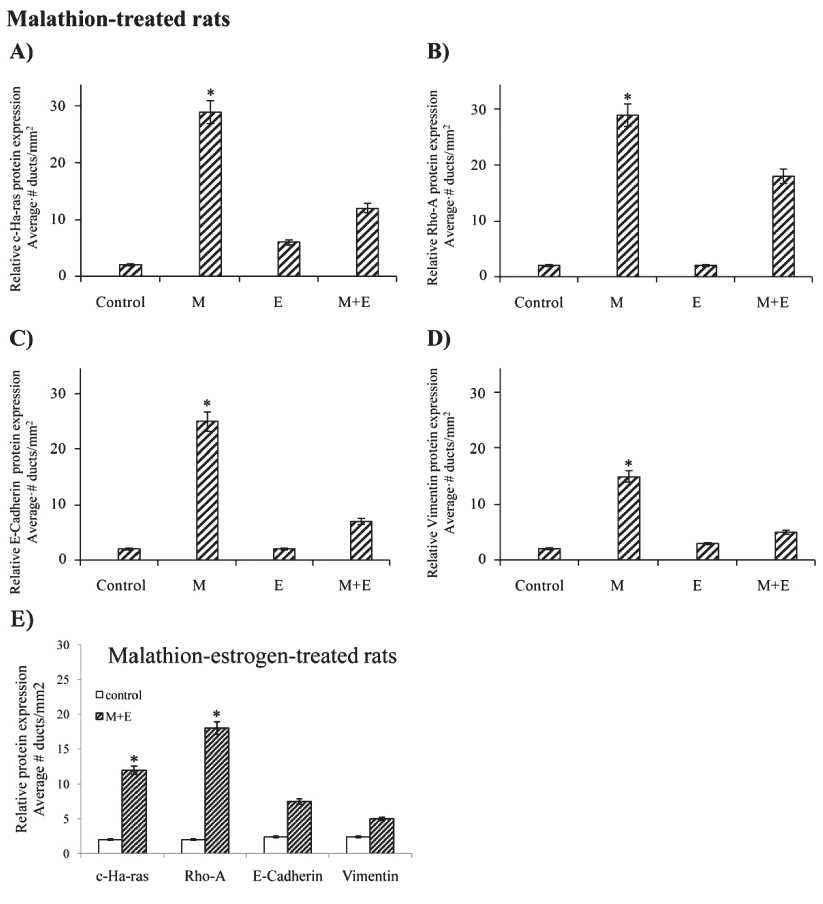
Figure 1. Effect of control, malathion (M), estrogen (E) and combination of both on relative protein expression determined by peroxidase immune-staining in cross sections of rat mammary gland tissues and tumors derived from such animals: Graphs correspond to the quantification of relative average number of ducts in stage of proliferation (dsp/ mm2) in sections immune-stained with: i ) Malathion on: A) c-Ha-ras, B) Rho-A, C) E-Cadherin and D) Vimentin, and ii) Malathion+Estrogen on similar proteins. E) Graph corresponds to Malathion+Estrogen-treated animals on similar proteins where c-Ha-ras and Rho-A expression was greater than Cadherin and Vimentin (P < 0.05)
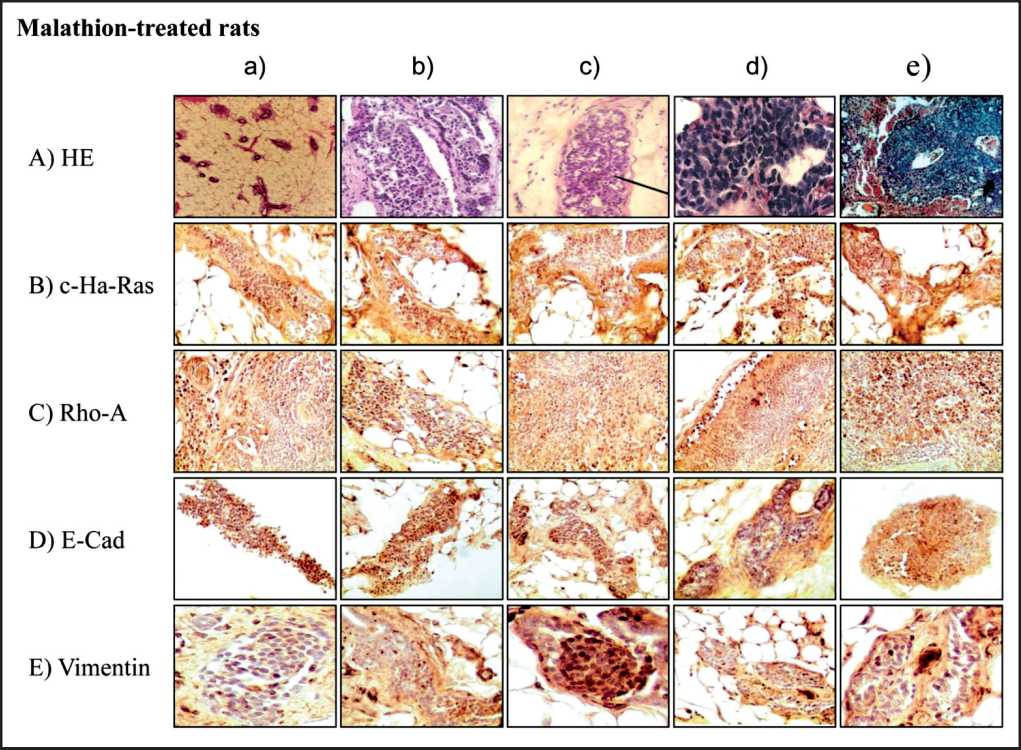
Figure 2. Representative images of cross section of rat mammary gland tissues and tumors stained with hematoxin and eosin) (2A a-e) and immune-peroxidase stained. Effect of M on: c-Ha-Ras
(2B a-e), Rho-A (2C a-e), E-Cadherin (2D a-e) and Vimentin (2E a-e) protein expression
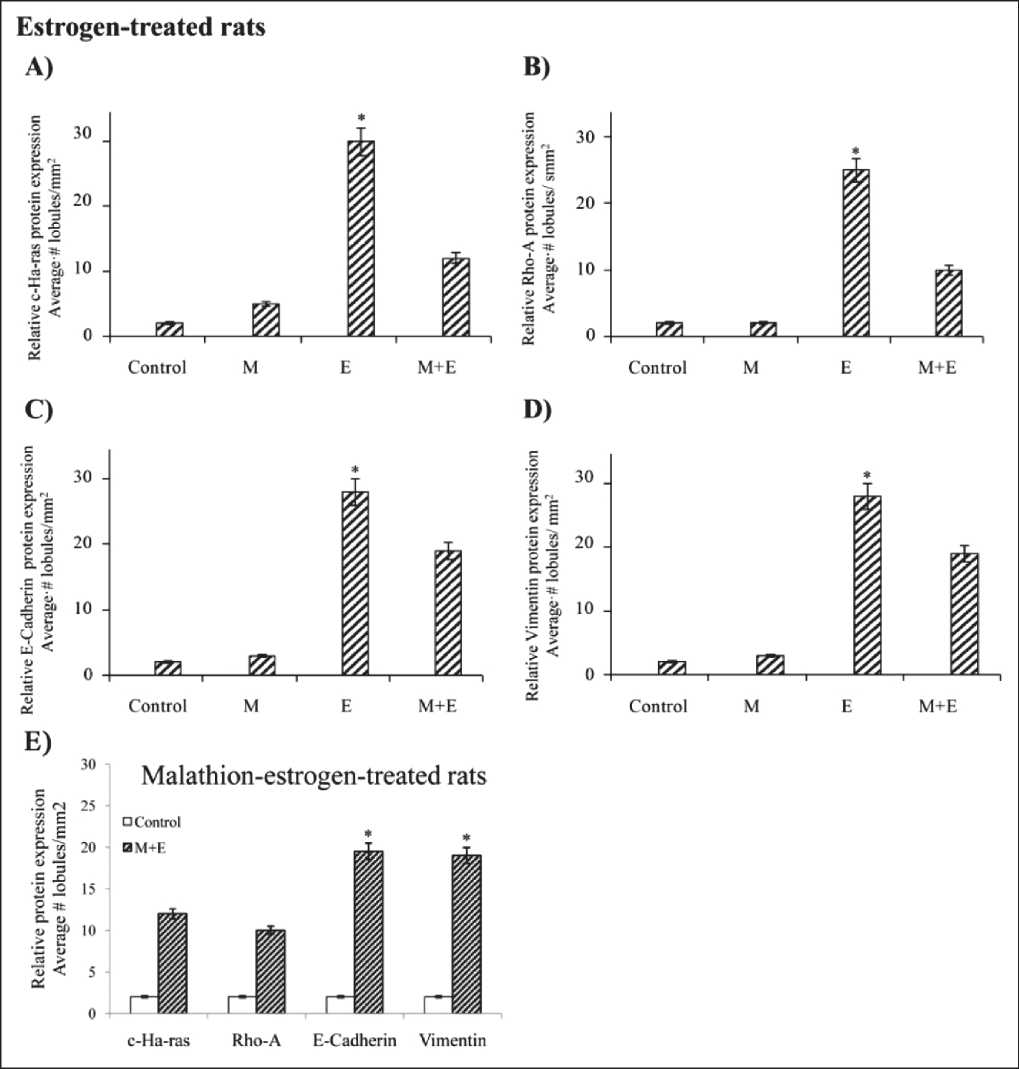
Figure 3. Effect of control, malathion (M), estrogen (E) and combination of both on relative protein expression determined by peroxidase immune-staining in cross sections of rat mammary gland tissues and tumors derived from such animals:
Graphs correspond to the to the quantification of mammary glands of rats treated on relative average number of lobules with casts in stage of proliferation/mm2 immune-stained with i) A) c-Ha-ras, B) Rho-A, C) E-Cadherin and D) Vimentin protein expression in control, M, E and combination of both-treated- group. ii) E) Graph corresponds to Malathion+Estrogen-treated animals on similar proteins where Cadherin and Vimentin expression was greater (P<0.05) than c-Ha-ras and Rho-A expression determined by peroxidase immune-staining in cross sections of rat mammary gland tissues and tumors derived from such animals. Results showed that E significantly (P<0.05) increased the number of lobules filled with casts parallel to greater of A) c-Ha-ras, B) Rho-A, C) E-Cadherin and D) Vimentin protein expression than control, M, E and combination of both-treated- group at 400 days after a 5-day treatment in comparison to controls, M and M+E combined. In Figure 4A a can be seen that the control rat mammary tissue presented a single main lactiferous duct that branches into smaller ducts from which TEBs and ABs are formed. Figure 4A b) shows a normal mammary gland lobule. Cross section of lobules of mammary glands of E-treated rat stained with HE can be seen in Figure 4A c-d. Figure 4A e corresponds to a cross section of a tumor formed in animal treated with E. Figure 1E shows relative protein expression determined by peroxidase staining and correspond to M+E-treated animals on similar proteins where
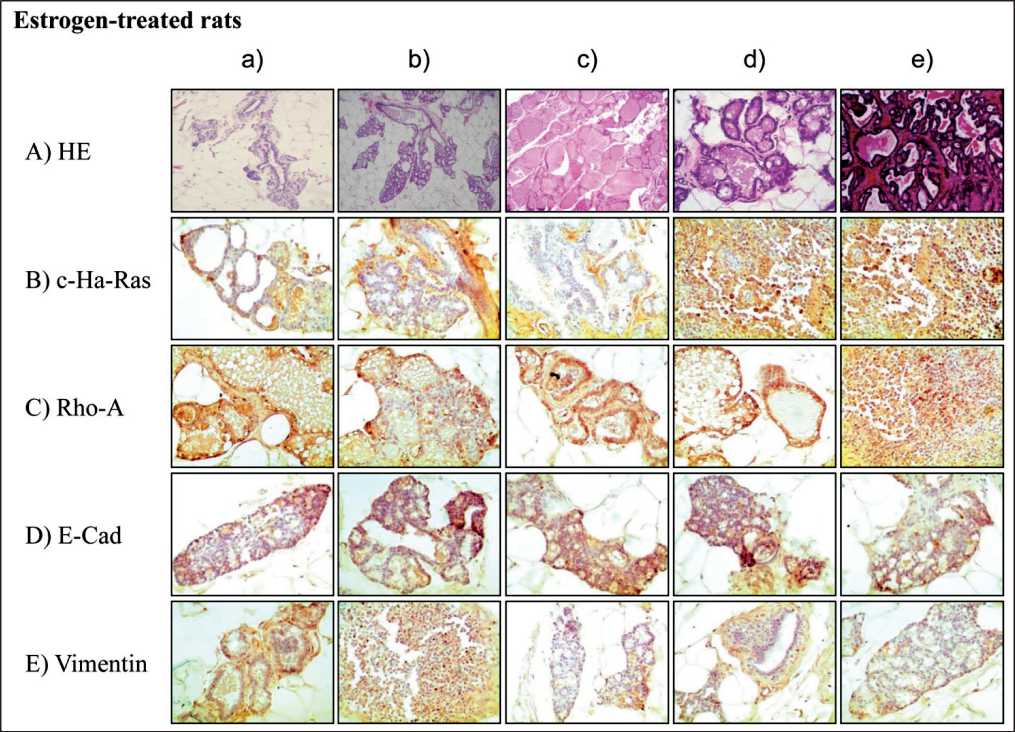
Figure 4. Representative images of cross section of rat mammary gland tissues and tumors stained with hematoxin and eosin) (4a-e ) and immune-peroxidase stained . Effect of E on: c-Ha-Ras (4B a-e), Rho-A (4C a-e), E-Cadherin (4D a-e) and Vimentin (4E a-e) protein expression
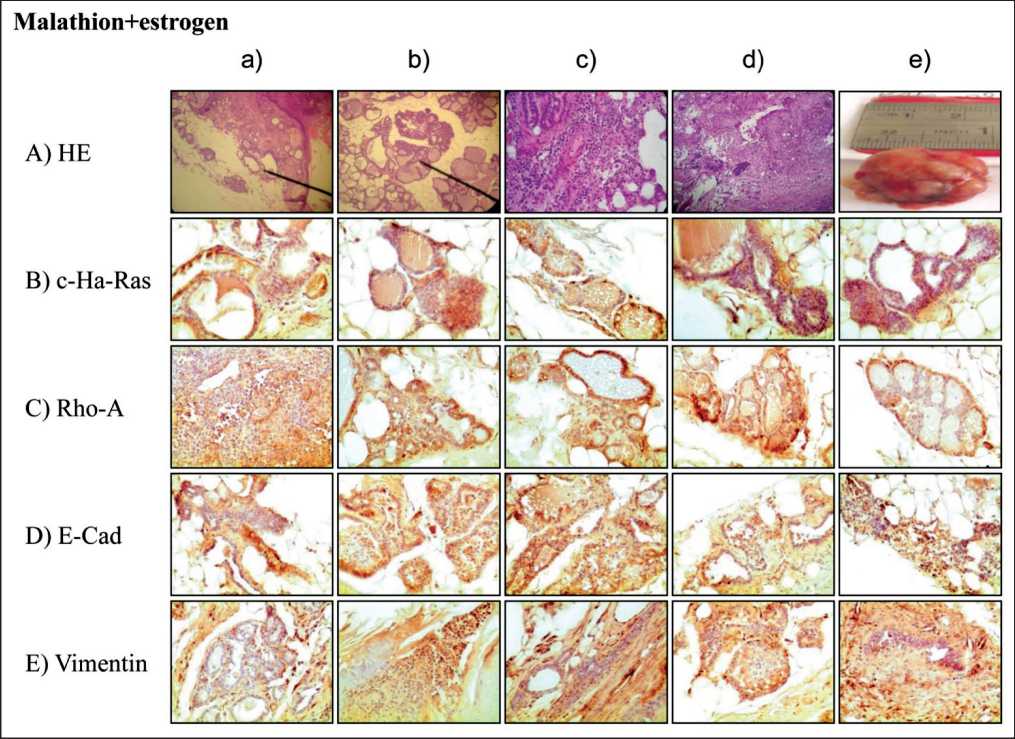
Figure 5. Representative images of cross section of rat mammary gland tissues and tumors stained with hematoxin and eosin) (4a-e ) and immune-peroxidase stained . Effect of M and E on: c-Ha-Ras (5B a-e), Rho-A (5C a-e), E-Cadherin (5D a-e) and Vimentin (5E a-e) protein expression
E-Cadherin and Vimentin was significantly (P < 0.05) greater than c-Ha-ras and Rho-A expression.
Cross section of mammary glands of M+E-treated rat and stained with HE can be seen in Figure 5A a-d). Figure 5A e) corresponds to a tumor formed in animal treated with M+E. Results indicated that mammary glands of such animals showed numerous lobules with secretion or casts in stage of proliferation as well as ducts in stage of proliferation parallel to c-Ha-ras (Figure 5B), Rho-A (Figure 5C), E-cadherin (Figure 5D) and Vimentin (Figure 5E) protein expression at 400 days after a 5-day treatment in comparison to controls, E and M. The relative c-Ha-ras, Rho-A, E-Cadherin and Vimentin protein expression in ducts in stage of proliferation and lobules can be seen in graphs of Figures 5A-D. Figure 5E shows relative protein expression determined by peroxidase staining and correspond to M+E-treated animals on similar proteins where E-Cadherin and Vimentin was significantly (P < 0.05) greater than c-Ha-ras and Rho-A expression. Cross section of ducts in stage of proliferation and lobules filled with secretion can be seen in M+E-treated animals. Figure 5A a-d) stained with HE. Figure 5A e) corresponds to a tumor formed in animal treated with M+E. Representative images of cross section of mammary gland of these animals immunostained with c-Ha-ras (Figure 5B a-e), Rho-A (Figure 5C a-e), E-Cadherin (Figure 5D a-e) and Vimentin (Figure 5E a-e) protein expression are seen.
Discussion
The evaluation of Ras, Rho-A, E-Cadherin and Vimentin protein expression by immunohistochemistry in this transformed model induced by pesticides as malathion and an endogenous substances as estrogen provided evidence that it can be used as good a prognostic tool for breast cancer patients. The ducts markedly increased in size and number of cells per mm2. Furthermore, such structures increased until tumors started to appear with similar type of cells and after 400 days of pesticide of 5 day treatment those structures were referred to as proliferative ducts. M alone induced changes exclusively at the level of ducts that increased in size and number of cells per mm2, these were defined as ductal in stage of proliferation (dsp/mm2) and the interesting finding was that as time progressed those structures were transformed in mammary gland tumors that revealed a similarity to ductal carcinomas as described by WHO [15] in comparison to controls. Sections of the ducts were filled with increased number of cells with dark nucleus.
E induced significant progressive alterations in lobules in comparison to control in the rat mammary gland after 400 days of the 5-day treatment. Congested tubules were filled with pink eosinophylic deposits. Furthermore, such structures increased in number per mm2 and also in size until tumors started to appear.
E-treated rats showed that the density of the number of terminal end buds per mm2 decreased as time progressed and lobules became markedly abnormal, while large and dilated congested structures increased in size and number per mm2. Results indicated that E alone increased the average number of lobules per mm2 of rat mammary glands in comparison to control and M treatment alone at 400 days after a 5-day treatment. Such structures were referred to as secretory lobules since they were congested tubules filled with pink deposits until lobular carcinomas started to appear and revealed that the tumors originated were pathologically similar to lobular carcinomas according to WHO [15]. Mammary gland of E-treated animal had altered lobules full of hyaline casts; the control rats had normal lobule formation. The increase in the number of secretory lobules was higher in the E-treated animals after 400 days of the 5-day injections than in the other treated group. Lobules increased in size with the time and mammary gland tumors were induced by the effect of E alone.
Environmental endocrine disrupting chemicals, including pesticides and industrial chemicals, are also considered among the environmental agents that are released into the environment producing deleterious effects on wildlife and humans. Lacassagne in 1932 [36] was the first to demonstrate that the administration of estrogens to experimental animals increased the incidence of mammary cancer, indicating at the same time that sex hormones were involved in the development of neoplasias and their progression in hormone-target organs such as the prostate and the breast. This knowledge was soon applied to cancer treatment [13].
Combination of M+E induced greater cellular changes in the rat mammary glands than E or M alone in comparison to control animals after 400 days of the 5-day treatment. Increased amount of proliferative ducts and secretory lobules were induced by these two substances showing rat mammary gland tumor formation. Mammary gland tumor formed by the effect of these substances was characterized by the presence of both types of structures as ducts in stage of proliferation and secretory lobules [13].
Our studies showed that M induced greater c-Ha-ras protein expression in ducts in proliferative stage at 400 days after a 5-day treatment in comparison to control, E and M+E treated rats. High c-Ha-Ras expression in intraductal proliferative lesions is very relevant since these epithelial lesions have a risk of progression to invasive breast cancer and related issues, particularly atypical ductal hyperplasia [37]. However, the use of c-Ha-Ras expression as a diagnostic and prognostic marker should be selective and should preferably be used in conjunction with other markers. According to published data, the major value of c-Ha-Ras expression is in its clinical correlation with an improved prognosis of relapse [38-40]. Since Ras is often mutated in human cancers, much effort has been devoted to devising means of controlling the activity of Ras [41]. Breast tumors have been shown to have an elevated expression of the Harvey Ras oncogene when compared to their respective normal tissue sections [42]. The Harvey Ras oncogene has also been shown to have a significant correlation with the clinicopathological characteristics of breast cancer [43]. Immunohistochemical analyses of Ras oncogene expression in human breast lesions have been carried out [44], as well as of the p21 Ras oncogene [45-47], with a high expression in breast cancer patients indicating clinical significance.
Our studies showed that E induced greater c-Ha-ras, Rho-A, Vimentin and E-Cadherin protein expression in lobules full of casts and secretion at 400 days after a 5-day treatment in comparison to control, E
Список литературы Carcinogenicity of malathion and estrogen in an experimental rat mammary gland model
- Perera F.P. Environment and cancer: who are susceptible? Science. 1997; 278(5340): 1068-1073.
- Cabello G., Valenzuela M., Vilaxa A., Duran V., Rudolph I., Hrepic N., Calaf G. A rat mammary tumor model induced by the organophosphorous pesticides parathion and malathion, possibly through acetylcholinesterase inhibition. Environ Health Perspect. 2001 May; 109(5): 471 9.
- Knower K.C., To S.Q., Leung Y.K., Ho S.M., Clyne C.D. Endocrine disruption of the epigenome: a breast cancer link. Endocr Relat Cancer. 2014 Mar 12; 21(2): T33 55. DOI: 10.1530/ERC-13-0513
- IARC. IARC working group on the evaluation of carcinogenic risks to humans. IARC Monogr Eval Carcinog Risks Hum. 1994; 61: 45 119.
- Guyton K.Z., Loomis D., Grosse Y., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Scoccianti C., Mattock H., Straif K.; International Agency for Research on Cancer Monograph Working Group, IARC, Lyon, France. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015 May; 16(5): 490 1. DOI: 10.1016/S1470-2045(15)70134-8


