Cerulein induced acute pancreatitis in experiment
Автор: Saparbaeva Gulshirin, Atadjanov Shukhrat
Журнал: Бюллетень науки и практики @bulletennauki
Рубрика: Медицинские науки
Статья в выпуске: 12 т.8, 2022 года.
Бесплатный доступ
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas, which ranges from mild, self-limiting disease to a severe form that is associated with multiple organ dysfunction syndrome (MODS), high morbidity, and mortality. Unpredictable nature of the disease, heterogeneity of disease presentations, and limited access to human samples, make research on human tissues impractical and often very difficult. We tried to identify crucial events in the pathophysiology of AP, in the course of several in vivo experimental models of the AP induction. In vivo experiment was carried out on rats using the analog of cholecystokinin octapeptide - Cerulein. The rats were divided into groups, in each group there was a different dosing regimen of the drug. As a result of a series of experimental studies, it was found that the interval low-dosage induction of AP causes more severe damage of pancreas tissue than a single administration of higher doses of the test substance.
Pancreatitis, pathophysiology, pancreas
Короткий адрес: https://sciup.org/14126163
IDR: 14126163 | УДК: 616.718.4-001.5-08 | DOI: 10.33619/2414-2948/85/39
Текст научной статьи Cerulein induced acute pancreatitis in experiment
Бюллетень науки и практики / Bulletin of Science and Practice
UDC 616.718.4-001.5-08
Acute pancreatitis (AP) is an inflammatory disorder of the pancreas, which ranges from mild, self-limiting disease to a severe form that is associated with multiple organ dysfunction syndrome (MODS), high morbidity, and mortality [1].
The treatment of AP is one of the most important problems of emergency abdominal surgery. Over the past 20-30 years the number of AP patients has increased from 1-2% to 10-11% in the structure of acute surgical diseases of abdominal organs, the frequency of its destructive forms increased up to 27,3-58,3% and lethality up to 20-30%, with its increase at pancreatic duct destruction and infected forms of pancreatitis up to 50-80% [2].
Ideally, studies on the etiology, pathogenesis, and treatment of AP should be carried out on the human pancreas. However, the unpredictable nature of the disease, heterogeneity of disease presentations, and limited access to human samples, make research on human tissues impractical and often very difficult. For these reasons, experimental models have been widely used to study AP for more than a century [3]. In recent years, the most commonly used AP models are carried out on rodents (rats and mice), which are relatively inexpensive to maintain, easy to handle, accessible, and allow induction of moderate to severe pancreatic injury. These experimental models not only provide an opportunity for mechanistic studies but also enable development of therapeutic strategies.
In recent years, there has been an increasing number of medical studies in the pancreatic field[4]. Animal models play an indispensable role by bridging basic science with translational research. Animal models allow detailed analysis of the crucial events in the pathophysiology of the disease and are thus important in establishing causality. However, it pays to remember that even very promising findings made on animal models, particularly those regarding novel therapeutic agents, may not always show efficacy in clinical trials [5]. The latter caveat may be improved by understanding critical thresholds for cellular events [6] and assessing potential pharmacological therapeutics in different models including in vivo and in vitro with multiple biochemical, immunological and histopathological indices, before designing appropriate clinical trials.
Experimental AP models can be divided into in vivo and in vitro models. Further in vivo experimental AP models can be generally sub-divided into non-invasive and invasive models. Although these categories describe the logistic differences in inducing each respective model, certain models may have greater utility over others depending on which mechanisms they focus on, which animal species they are induced in, and which disease outcomes are reproduced. Here we briefly review the history, development, and current use of important experimental AP models as well as explore their mechanisms, advantages, limitations, clinical relevance, and the scope for future work.
Early in 1895, Mouret [7] found that excessive cholinergic stimulation causes vacuolization and necrosis of the pancreas, which are typical features of AP. Later in 1929, Villaret et al. [8] reported the first secretagogue hyperstimulation-induced AP model by injection of acetylcholine, a cholinergic agonist, into the canine pancreas and this was later reproduced in a rat model [9]. Subsequently, cholecystokinin octapeptide (CCK-8) and its analog caerulein [10], as well as carbamylcholine [11], anticholinesterase[12], and scorpion toxin [13] have been shown to induce AP.
CCK was named after its main function related to promoting contraction of gallbladder smooth muscle and bile discharge. Later, it was found that it can act on the pancreas to stimulate the secretion of pancreatic digestive enzymes [14] and insulin [15]. The fundamental mechanism of pancreatic pathology induced by CCK and its analogs is based on the action of these chemicals on CCK receptors, which in turn leads to activation of second messenger pathways related to secretion of pancreatic enzymes (e.g., amylase) in pancreatic acinar cells (PACs) like phospholipase C- inositol trisphosphate-calcium (Ca2+). There are also protein-protein interaction pathways that mainly regulate non-secretory processes, including biosynthesis and growth, such as three major mitogen-activated protein kinase pathways (ERK, JNK, and p38 MAPK) and several other pathways that are still unknown. While CCK-8 is most well studied, CCK-58 is the main circulating form in humans and dogs, and the only endocrine form of CCK-58 in rats (Reeve et al., 2004). CCK-8 and CCK-58 have the same effect on Ca2+ signaling, zymogen activation, and cell death in PACs at high and low agonist concentrations in vitro [16]. A recent review has summarized the regulation of the CCK pathway in PACs in detail [17].
Caerulein , a CCK analog, was first isolated from skin extracts of the Australian green tree frog (Litoria caerulea) and was immediately acknowledged for its physiological activity mimicking natural hormones [18]. CCK and caerulein have a very similar amino acid sequence, but compared to the CCK, caerulein is a decapeptide that has methionine substituted to threonine and two additional N-terminal residues. Both peptides show almost the same potency in vitro, but caerulein is more potent to induce AP in vivo. The increased biological activity is related to the additional N-terminal residues, and a result of the substitution of methionine for threonine [19]. To date, caerulein remains the most widely used compound to induce AP (CER-AP) in rodents.
There is a clear dose-response relationship between the structural and biochemical changes of the pancreas in response to caerulein administration [20]. Continuous infusion of maximal physiological doses of caerulein (0.25 μg/kg/h) causes rapid degranulation of the exocrine pancreas in rats [21]. Administration with a supramaximal dose leads to vacuolization within the acinar cells, followed by regeneration of the pancreas [22]. At an even higher dose, caerulein causes pancreatic interstitial edema and inflammatory cell infiltration together with a significant increase of the pancreatic enzyme levels in the blood [23]. Based on the above findings, in 1977 Lampel and Kern[24] described a non-lethal CER-AP model in rats, after which CER-AP was successfully reproduced in mice [25].
The caerulein/CCK model exhibits the closest parallel with clinical AP induced by scorpion venom or organophosphate insecticides. It is non-invasive, easy to conduct, highly reproducible, and reflects a vast number of in vitro studies, making it a favorable model for AP. It is also compatible with other models, sharing histopathological changes consistent with early phases of human AP[26]. All these factors explain why CER-AP is so widely accepted and commonly used by pancreatic investigators [27,28].
Materials and Methods
In vivo experiments were carried out in the RRCEM (Republican Research Center of Emergency Medicine, Tashkent, Uzbekistan) experimental laboratory. The permission for experimental work on animals of the ethical committee was received in May 20, 2021, protocol № 3/2-1518. Animals were kept in standard conditions of laboratory vivarium, stipulated by "Protection and use of fauna", Chapter 5, Article 31: "Fauna objects use for medical, sanitary, epidemiologic and veterinary purposes", approved by the Law of Republic of Uzbekistan from December 26, 1997 № 545-I. Manipulations with experimental animals were carried out in accordance to regulations on humane treatment of animals, methodological recommendations on withdrawal from experiment and euthanasia.
The animals were kept at a temperature of 18-20.5°C, free access to water and food was provided. Experiments were performed on mature male white rats. Mature animals were 6-8 months old and weighed 250±50 g. Experimental studies are connected with the necessity of fulfilling the set task - evaluation of morphofunctional changes of the pancreas in case of its damage. In order to realize this task we needed to model pancreatic pathology, determine laboratory blood and morphometric indices, which required invasive interventions.
Methodology of anesthesia . The animals were weaned from food 2 hours before the operation, but had free access to water. Ten minutes before anesthesia, the animals were transported to the operating room in their cages to adapt to the operating room conditions.
Anesthesia and oxygen were administered during the operation using a Fabius anesthesia machine with a Vapor 2000 vaporizer (Dräger, Germany). For anesthesia, the animal was first placed in an induction chamber, which was a 32x32x20 cm plastic box, and 5% isoflurane (" Isoflurane USP "Piramal Enterprises Limited, India ) was started in an oxygen flow of 2 L/min until loss of motor activity.
Next, the rat was moved to the operating table and anesthesia was maintained with 1-1.5% isoflurane in an oxygen flow of 1 L/min using a special mask. To prevent sclerae drying, the animal's eyes were lubricated with ophthalmic gel ( Viscotears, Alcon Pharmaceuticals, Germany ). Monitoring of vital functions during anesthesia and surgery was performed by visual control of the animal's respiratory movements and periodic auscultation of the heart with a pediatric stethoscope.
For intraoperative anesthesia, fentanyl ( Kharkiv Pharmaceutical Enterprise «Zdorovie Narodu», Ukraine ) was administered intraperitoneally (IP) at 0.2 mg/kg after induction and repeatedly at 0.1 mg/kg for duration of surgery over 30 min. Also, for postoperative analgesia, 1-2 ml of 1% lidocaine solution was infiltrated into the anterior abdominal wall before the surgical incision. To replenish the water balance during surgery, isotonic sodium chloride solution was injected at the rate of 1 ml/100g subcutaneously every 30 minutes. For antibiotic prophylaxis, ceftriaxone 10mg/kg subcutaneously was also administered. All animals were injected with 5 ml of 10% glucose subcutaneously after the experiment to prevent hypoglycemic coma. Intraperitoneal and subcutaneous injections were performed using a 1-mL syringe with a needle size of 27G.
Cerulein model
Cumulative effect of the studied substance was determined on 10 male white rats weighing 200-240 g. To assess cumulative properties, we took into account low toxicity of the drug established in the acute experiment, as well as the expected duration of the treatment course (single intraperitoneal injection), so we chose a period of seven days in accordance with the "Methodological guidelines for the study of general toxic effects of pharmacological substances" ("Guidelines for experimental (preclinical) study of new pharmacological substances", Remedium, 2005).
The substance was administered intraperitoneally to three groups of experimental white rats. The fourth group served as a control group. The test substance was administered in the following doses: Group 1 - once in a dose of 50 mg/kg; Group 2 - once in a dose of 200 mg/kg, Group 3-150 mg/kg (three times 50 mg/kg with an hour interval).
After the experiment, some animals from experimental and control groups were slaughtered by decapitation under mild ether anesthesia. Then macroscopic and microscopic studies of internal organs were performed. The objects of study were lungs, liver and pancreas. Blood was taken to study the levels of amylase, AST/ALT ratio.
Results and discussion
3.1.The general condition was assessed from day 1 to 7 on the basis of daily monitoring of body weight and clinical signs, based on a semi-quantitative three-step grading system: active (+++), weak (++) and dying (+). Hair loss, shine, jaundice of the skin and general weakness, and refusal to eat were recorded. Clinical characteristics of the animals are presented in Table 1.
Table 1
CLINICAL CHARACTERISTICS OF THE RATS
Indicators Observation time (days)
|
1 |
3 |
5 |
7 |
10 |
|
|
Behavior |
+++ |
+++ |
++ |
++ |
++ |
|
Coat and skin changes |
Shiny, light gray |
Shiny, light gray |
Pale, light gray |
Pale, light gray |
Pale, light gray |
|
Vomiting times/day |
Absent |
Absent |
2-3 |
4-5 |
4-5 |
|
Urine color |
Bright, yellow |
Bright, yellow |
Bright, yellow |
Bright, yellow |
Dark yellow |
|
Survival |
5 |
5 |
5 |
5 |
4 |
The toxicity indicators were: animal behavior, survival rate, time of death, appearance of intoxication symptoms, local skin changes, weight dynamics, and respiration rate. During the experiment, the general condition of the experimental animals was not disturbed; symptoms of intoxication and death of animals were not observed. No local changes were found on the skin, no focal alopecia or ulcers were noted. The animals were neat, active, their coats were smooth and shiny, and they ate food willingly, and reacted adequately to external stimuli. The dynamics of the weight of the white rats under repeated intraperitoneal exposure of Cerulein are presented in Table 2.
Table 2
WEIGHT (g) OF WHITE RATS AFTER INTRAPERITONEAL INJECTION
OF THE TEST SUBSTANCE
|
Indicator |
Animal groups |
|||
|
М±m |
1st (50 μg/kg) |
2nd (200μg/kg) |
3rd 3x50 μg/kg with one hour interval (total = 150 μg/kg) |
4th (control group) |
|
227±11,7 |
221±10,1 |
208±15,6 |
211±13,3 |
|
|
р |
>0,05 |
>0,05 |
>0,05 |
- |
The results showed that the weight gain of the experimental animal groups did not differ from the control. Biochemical blood parameters were investigated using reagents. For biochemical studies of the disease development, blood sampling was carried out on the 7th day. The results are given in Table 3.
Table 3
DYNAMICS OF BIOCHEMICAL PARAMETERS IN RATS
|
Indicators |
Normal ranges |
Control groups |
Groups of rats with an induced pancreonecrosis |
||
|
1st |
2nd |
3rd |
|||
|
Bilirubin: |
8,55-20,5 µmol/L |
11,87±0,55 |
18,0 µmol/L |
17,0 µmol/L |
12,0 µmol/L |
|
Total Direct |
25% of total |
µmol/L abs |
2,9 µmol/L |
2,4 µmol/L |
abs |
|
Amylase |
0-100 U/L |
55,0±5,0 U/L |
803 U/L |
790 U/L |
752 U/L |
|
AST |
0-37 U/L |
39,51±5,67 U/L |
74,0 U/L |
88,0 U/L |
240,0 U/L |
|
ALT |
0-42 U/L |
25,20±3,19 U/L |
53.0 U/L |
51,0 U/L |
170,0 U/L |
In the analysis of biochemical parameters in the 3rd group of rats, there were excessive levels of blood amylase and blood transaminases (ALT, AST).
On the 7th day after the beginning of the experiment we performed relaparotomy and revision of the abdominal cavity organs. The pancreatic tissue was visualized by traction of duodenum and spleen into the operative wound.
These animals were slaughtered and material was taken for morphological study (liver, pancreas, lungs). The obtained material was fixed in 12% formalin, followed by a standard alcoholic ascending concentration and embedded in paraffin. Organ’s slices 6-7 µm thick were stained with hematoxylin and eosin. They were examined under an optical microscope at a 70x and 90x magnification. The state of internal organs, inflammatory and granulation reaction, reparation and regenerative fibrosis were evaluated by light microscopy.
The results of general examination of the animal bodies receiving the test substance (Cerulein) showed the presence of macroscopic recognizable abnormalities compared to the control group. All animals had pale light gray hair coats, no alopecia or ulcers were found. Visible mucous membranes were moist, pink, shiny and smooth in appearance. Mammary glands of females without tumor-like masses and indurations, equably soft. The external genitalia of males had no visible deformities or deviations from the control group.
In the thorax, the visceral and parietal pleural sheets and thoracic organs were without visible changes. The lungs are pale pink, airy, without thickening or destructive changes. Lung tissue of experimental rats remained its cytoarchitectonics. No signs of pathological changes of inflammatory or destructive nature were found. The wall inside the pulmonary bronchi consisted of the corresponding tissue components inherent in the large, medium and small bronchi. Respiratory bronchioles and alveolar passages are without pathological changes. Types Ι and ΙΙ alveolar epitheliocytes have typical structure and tissue properties. Interalveolar connective tissue without pathological changes. There were revealed single macrophages with characteristic dense inclusions in cytoplasm in the lumen of alveoli. Microscopic structure of all lung slices did not differ from the control group significantly.
The liver had an usual shape, did not enlarge in size, had soft consistency and smooth surface. Glisson's capsule is thin and transparent. The liver cytoarchitectonics was unchanged, and parenchyma was moderately full-blooded. No pronounced pathohistological changes were found in the liver tissue of the experimental animals. The liver capsule was not thickened, it contained longitudinally oriented bundles of collagen fibers. Parenchyma of the liver is formed by classical hepatic lobules consisting of radially oriented to the central vein hepatic plates or beams. Interlobular connective tissue was poorly developed, no signs of inflammatory infiltration and liver fibrosis were found. Hepatocytes are polygonal in shape, with a centrally located nucleus, a nucleus is often detected. Quite often there were binuclear hepatocytes. Sinusoidal capillaries of normal size. Single erythrocytes and leukocytes are detected in the lumen. Kupffer cells with intact structure were detected in the sinusoidal capillaries wall. In some cases there is a moderate dilation and blood filling of sinusoidal capillaries, central and sublobular veins. Endothelial lining without destructive changes, swollen endothelial cells with hyperchromatic nuclei were noted in some places. The structure of cholangioles and interlobular bile ducts without pathological changes. All this points to the fact that the studied substance had no negative effect on microscopic structures of the liver.
Characteristics of morphological slices of pancreas (microscopy) in rats are given in Table 4.
Conclusions
Trends in experimental science development are caused by the presence of unresolved problems in modern surgery. One of these problems is the absence of acute pancreatitis lethality rate decreasing, observed during the last decades. The main number of lethal outcomes is associated with multiple organ failure in the initial phase of the disease or complications of infected AP.
Table 4
HISTOLOGICAL SLICES CHARACTERISTICS (MICROSCOPY)
1st group 2nd group 3rd group
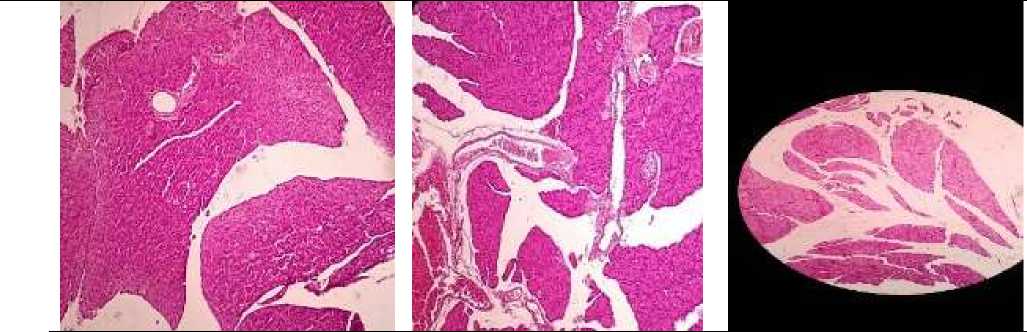
Figure 3. Pancreatic tissue with the preservation of the structure, pronounced interstitial edema, vascular plethora
Figure 1. The structure of the pancreas is preserved, there is a pronounced interstitial edema, dilated full-blooded vessels
Figure 5. Pancreatic tissue with the preservation of the structure, pronounced interstitial edema, vascular plethora
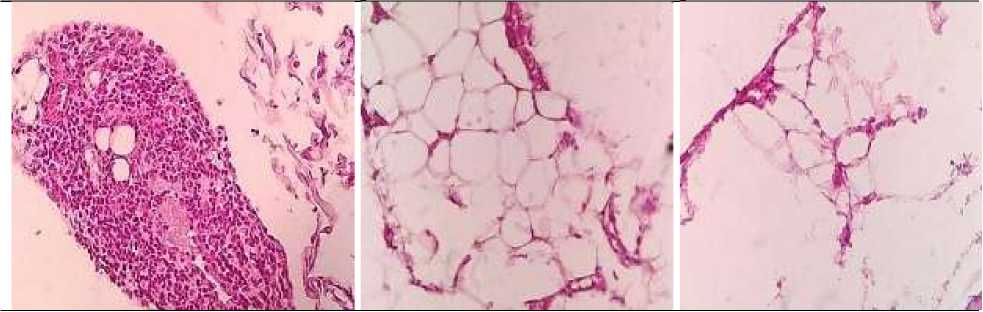
Figure 2. Foci of inflammatory Figure 4. In the adjacent Figure 6. In the adjacent infiltration represented by adipose tissue - vascular adipose tissue - pronounced lymphoid cells and full-blooded plethora edema vessels are determined in the peripancreatic tissue
For experimental study of the first stage of AP with the syndrome of systemic inflammatory reaction and multiple organ failure, it is necessary to create a model with extensive destruction of the pancreas tissue and the presence of systemic manifestations. Literature analysis shows that models of biliopancreatic duct perfusion in rats lead to corresponding morphological changes of pancreas and lethality rates, comparable with those in the human population.
To study late infectious complications of AP, models with low lethality rate and preserved conditions for bacterial translocation are optimal. The model with duodenal lumen occlusion is the most effective. When analyzing the results, it should be taken into account that in these models there is a reflux of microorganisms through the biliopancreatic duct.
As for the study of new therapeutic strategies, in the experiment the treatment begins immediately after induction of AP. For this reason, it is rather difficult to interpret these results. On the other hand, the course of AP in rodents is more rapid than in humans, and therefore the
"therapeutic window" (possibility of effective therapy before the appearance of complications) is much narrower, which can lead to false-negative results of the study.
Many models of AP are currently irrelevant. Modern techniques are based on basic principles from the known basis of pathogenesis of this disease. In spite of this, the ideal model of AP, with the help of which it would be possible to estimate pathophysiology of complications, possibility of treatment and prevention, has not been found yet. Each of the above-mentioned models has its disadvantages, but is not devoid of advantages. This should be taken into account before designing the study and when interpreting the results obtained.
As a result of a series of experimental studies, it was found that threefold intraperitoneal injection of Cerulein at a dose of 50 mcg/kg with an hour interval, in rats caused moderate edematous pancreatitis. Pathomorphological analysis of the pancreas of these animals revealed different degrees of interstitial edema, infiltration of lobules with macrophages and partial necrosis of a part of parenchyma, which was characterized (depending on the degree of damage) by loss of zymogen granules, degradation of some acini, formation of rounded cavities in place of destroyed acini.
Conflicts of Interest: The authors declare no conflict of interest.
Список литературы Cerulein induced acute pancreatitis in experiment
- Hines, O. J., & Pandol, S. J. (2019). Management of severe acute pancreatitis. Bmj, 367. https://doi.org/10.1136/bmj.l6227
- Alidzhanov, F.V.; Allaiarov, U.D.; Rizaev, K.S.; Khoshimov, M.A.; Osobennosti diagnostiki i lecheniia ostrogo pankreatita pri ushchemlenii kamnia v bolshom duodenalnom sosochke. Vestnik ekstrennoi meditsiny 2008; 1:18-20.
- Gorelick, F. S., & Lerch, M. M. (2017). Do animal models of acute pancreatitis reproduce human disease?. Cellular and molecular gastroenterology and hepatology, 4(2), 251-262. https://doi.org/10.1016/j.jcmgh.2017.05.007
- Mukherjee, R., Nunes, Q., Huang, W., & Sutton, R. (2019). Precision medicine for acute pancreatitis: current status and future opportunities. Precision Clinical Medicine, 2(2), 81-86. https://doi.org/10.1093/pcmedi/pbz010
- Rompianesi, G., Hann, A., Komolafe, O., Pereira, S. P., Davidson, B. R., & Gurusamy, K. S. (2017). Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis. Cochrane Database of Systematic Reviews, (4). https://doi.org/10.1002/14651858.CD012010.pub2
- Barreto, S. G., Habtezion, A., Gukovskaya, A., Lugea, A., Jeon, C., Yadav, D., ... & Pandol, S. J. (2021). Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut, 70(1), 194-203. http://dx.doi.org/10.1136/gutjnl-2020-322163
- Gabe, M. (1956). Contribution à l'histogénese des glandes salivaires chez la souris albinos. Zeitschrift für Zellforschung und Mikroskopische Anatomie, 45(1), 74-95. https://doi.org/10.1007/BF00320737
- Vilaret, M. (1929). Effects de l'acetyl-choline sur la secretion pancreatique. CR Soc Biol (Paris), 101, 7-8.
- Leblond, C. P., & Sergeyeva, M. A. (1944). Vacuolation of the acinar cells in the pancreas of the rat after treatment with thyroxine or acetylcholine. The Anatomical Record, 90(3), 235-242. https://doi.org/10.1002/ar.1090900308
- Lampel, M., & Kern, H. F. (1977). Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Archiv A, 373(2), 97-117. https://doi.org/10.1007/BF00432156
- Adler, G., Gerhards, G., Schick, J., Rohr, G., & Kern, H. F. (1983). Effects of in vivo cholinergic stimulation of rat exocrine pancreas. American Journal of Physiology-Gastrointestinal and Liver Physiology, 244(6), G623-G629. https://doi.org/10.1152/ajpgi.1983.244.6.G623
- Dressel, T. D., Goodale Jr, R. L., Zweber, B. A., & Borner, J. W. (1982). The effect of atropine and duct decompression on the evolution of Diazinon-induced acute canine pancreatitis. Annals of surgery, 195(4), 424. https://doi.org/10.1097%2F00000658-198204000-00008
- Gallagher, S., Sankaran, H., & Williams, J. A. (1981). Mechanism of scorpion toxininduced enzyme secretion in rat pancreas. Gastroenterology, 80(5), 970-973. https://doi.org/10.1016/0016-5085(81)90067-6
- Harper, A. A., & Raper, H. S. (1943). Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. The Journal of physiology, 102(1), 115. https://doi.org/10.1113%2Fjphysiol.1943.sp004021
- Kuntz, E., Pinget, M., & Damgé, P. (2004). Cholecystokinin octapeptide: a potential growth factor for pancreatic beta cells in diabetic rats. Jop, 5(6), 464-475.
- Reeve Jr, J. R., Wu, S. V., Keire, D. A., Faull, K., Chew, P., Solomon, T. E., ... & Coskun, T. (2004). Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. American Journal of Physiology-Gastrointestinal and Liver Physiology, 286(3), G395-G402. https://doi.org/10.1152/ajpgi.00020.2003
- Criddle, D. N., Booth, D. M., Mukherjee, R., McLaughlin, E., Green, G. M., Sutton, R., ... & Reeve Jr, J. R. (2009). Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells. American Journal of Physiology-Gastrointestinal and Liver Physiology, 297(6), G1085-G1092. https://doi.org/10.1152/ajpgi.00119.2009
- Williams, J. A. (2011). Cholecystokinin (CCK) regulation of pancreatic acinar cells: physiological actions and signal transduction mechanisms. Comprehensive Physiology, 9(2), 535- 564. https://doi.org/10.1002/cphy.c180014
- Anastasi, A., Erspamer, V., & Exdean, R. (1968). Isolation and amino acid sequence of caerulein, the active decapeptide of the skin of Hyla caerulea. Archives of biochemistry and biophysics, 125(1), 57-68. https://doi.org/10.1016/0003-9861(68)90638-3
- Shorrock, K., Austen, B. M., & Hermon-Taylor, J. (1991). Hyperstimulation pancreatitis in mice induced by cholecystokinin octapeptide, caerulein, and novel analogues: effect of molecular structure on potency. Pancreas, 6(4), 404-406.
- Bieger, W., Seybold, J., & Kern, H. F. (1976). Studies on intracellular transport of secretory proteins in the rat exocrine pancreas. Cell and Tissue Research, 170(2), 203-219. https://doi.org/10.1007/BF00224299
- Bieger, W., Seybold, J., & Kern, H. F. (1976). Studies on intracellular transport of secretory proteins in the rat exocrine pancreas. V. Kinetic studies on accelerated transport following caerulein infusion in vivo. Cell and Tissue Research, 170(2), 203-219. https://doi.org/10.1007/bf00224299
- Tardini, A., Anversa, P., Bordi, C., Bertaccini, G., & Impicciatore, M. (1971). Ultrastructural and biochemical changes after marked caerulein stimulation of the exocrine pancreas in the dog. The American journal of pathology, 62(1), 35. https://pubmed.ncbi.nlm.nih.gov/5538718
- Willemer, S., Elsässer, H. P., & Adler, G. (1992). Hormone-induced pancreatitis. European surgical research, 24(Suppl. 1), 29-39. https://doi.org/10.1159/000129237
- Niederau, C., Ferrell, L. D., & Grendell, J. H. (1985). Caerulein-induced acute necrotizing pancreatitis in mice; protective effects of Proglumide Benzotript, and Secretin. Gastroenterology, 88(5), 1192-1204. https://doi.org/10.1016/S0016-5085(85)80079-2
- Rifai, Y., Elder, A. S., Carati, C. J., Hussey, D. J., Li, X., Woods, C. M., ... & Saccone, G. T. (2008). The tripeptide analog feG ameliorates severity of acute pancreatitis in a caerulein mouse model. American Journal of Physiology-Gastrointestinal and Liver Physiology, 294(4), G1094- G1099. https://doi.org/10.1152/ajpgi.00534.2007
- Saluja, A. K., Lerch, M. M., Phillips, P. A., & Dudeja, V. (2007). Why does pancreatic overstimulation cause pancreatitis?. Annu. Rev. Physiol., 69, 249-269. https://doi.org/10.1146/annurev.physiol.69.031905.161253
- Lerch, M. M., & Gorelick, F. S. (2013). Models of acute and chronic pancreatitis. Gastroenterology, 144(6), 1180-1193. https://doi.org/10.1053/j.gastro.2012.12.043


