Chronic Maxillary Sinusitis Recorded in Archaeological Samples: Geographical Distribution and Predisposing Factors
Автор: Zubova A.V., Moiseyev V.G., Ananyeva N.I., Stulov I.K., Andreev E.V.
Журнал: Archaeology, Ethnology & Anthropology of Eurasia @journal-aeae-en
Рубрика: Anthropology and paleogenetics
Статья в выпуске: 1 т.50, 2022 года.
Бесплатный доступ
The study explores social and climatic factors affecting the occurrence of chronic maxillary sinusitis (CMS) in ancient and historical samples of Europe, North and South America, Asia, and Africa. The main database consists of 23 cranial samples. According to the results of univariate (correlation analysis and Wilcoxon-Mann-Whitney test) and multivariate (principal component) analyses, only climatic factors reveal a statistically significant effect on the frequency of CMS. The principal factor is temperature, which shows a negative correlation with CMS at the world level: the higher the mean annual temperature and the maximal temperature of the three hottest months, the lower the occurrence. At the regional level, significant correlation was also found between CMS and the number of rainy days per year. Rather than direct dependence, however, this result suggests that the correlation between climatic variables is different in Europe and North America. None of the socio-economic factors that we analyzed (sex, urban versus rural residence, subsistence strategy) demonstrated significant correlation with the prevalence of CMS at the world level. Assessing the effect of social status evaluated by archaeological criteria was impossible because of the complex nature of stress-inducing factors.
Chronic maxillary sinusitis, climate, bioarchaeology, paleopathology, respiratory tract diseases
Короткий адрес: https://sciup.org/145146853
IDR: 145146853 | DOI: 10.17746/1563-0110.2022.50.1.147-157
Текст научной статьи Chronic Maxillary Sinusitis Recorded in Archaeological Samples: Geographical Distribution and Predisposing Factors
Chronic sinusitis or chronic maxillary sinusitis (hereinafter—CMS) is a long-term (more than 12 weeks) inflammation of the mucosa of the maxillary sinus (Khronicheskiy rinosinusit…, 2014: 11). This pathology is manifested as nasal breathing obstruction, headaches, general weakness, and at exacerbations is accompanied by nasal discharge and fever. Today, CMS is one of the most widespread chronic otolaryngologic (ENT) diseases worldwide (Slavin, Sheldon, Bernstein, 2005; Brook, 2009). The crucial factors affecting its spread at present are various anthropogenic air pollutions, iatrogeny, the presence of concomitant diseases, and climatic conditions (Mercer, 2003; Kaur, Nieuwenhuijsen, Colvile, 2005; Peled et al., 2005; Roberts, 2007). But deciphering the actual role of any of those
Archaeological skeletal samples have, despite the lesser consistency of diagnostics, some advantages for the study of CMS epidemiology as compared to clinical data. First, the influence of the iatrogenic factor is virtually absent in such samples, and the morbidity pattern is not “blurred” by the use of modern remedies. Second, the level of variability of environmental stressful factors in ancient populations is expected to be lower than in modern groups. In addition, archaeological cranial collections fit better to the criteria of a random sample than patients of a single hospital, and they provide both people affected by CMS and healthy individuals (control group) for the study. Despite these advantages, there have been only a few works on the epidemiology of CMS in ancient and historical populations. No consensus exists regarding the morbid factors of CMS. It was pointed out that the prevalence of this pathology could be related to air pollution (which differed between rural and urban areas) (Lewis, Roberts, Manchester, 1995), to the social status of individuals (Roberts, 2007), or to the frequency of dental diseases (Panhuysen, Coenen, Bruintjes, 1997; Zubova et al., 2020). It was suggested that females had a higher risk of the developments of CMS owing to sex-differences in lifestyle (Roberts, 2007).
As well as for the modern population, no statistical exploration of the influence of various factors on the prevalence of CMS has ever been performed for archaeological groups. The most geographically broad bioarchaeological survey was carried out by C. Roberts, who compared 14 samples from Europe, North America, and Africa (Ibid.). Of all the populations, only two North American samples exhibited statistically significant sexual differences (Ibid.: Tab. 5). No differences were detected between either urban vs. rural groups, or agriculturalists vs. hunters-gatherers. Despite this apparent lack of associations, the author postulated lesser morbidity in the rural and hunter-gatherers populations, as well as the importance of anthropogenic pollution for the spread of CMS (Ibid.: 804).
The aim of the present study was a statistical analysis of the environmental factors influencing the prevalence of CMS in ancient and historical populations of Europe, North and South Americas, Asia, and Africa. To these ends, we analyzed the association between the frequency of CMS and temperature and humidity conditions and the geographic locations of the studied groups. The study also sets out to test the previously formulated hypotheses regarding the influence of various social and cultural factors on the spread of the disease in different parts of the globe.
Material and methods
This study employs the published frequencies of CMS in 21 samples from North America, Europe, Africa, and India, alongside with authors’ own data on two South American groups (Table 1) studied via computed tomography (CT) at the Bekhterev Psychoneurological Research Institute (see (Zubova et al., 2020: 146) for methodological details).
Owing to the relatively small number of analyzed samples, the non-parametric Wilcoxon-Mann-Whitney (Wilcoxon Matched Pairs) test was used for pair-wise comparisons between males and females, urban and rural groups, hunter-gatherers and agriculturalists. The association between the prevalence of CMS and the climatic and geographic variables was assessed using parametric Pearson’s (worldwide/intercontinental sample, 23 groups) or non-parametric Spearman-Kendall’s (continental sub-samples) correlation coefficients. The set of climatic variables included the mean annual temperature, mean minimal temperatures of the three coldest months, mean maximum temperatures of the three warmest months (Fahrenheit scale), annual
Table 1. Frequency of CMS in the samples employed in this study
|
ф о о со |
СО О СМ ГО ф it |
Е о ^ ф Z с го ^ 2 w ° |
о о см S ф о £ |
in со „ о ф ^ 1м о ^ (Г «" ё □ 2 |
с ф с о ф 2 о Й о 2 с 2 £ ш |
о см ч^ -С ф 1 |
о о см £ ф о £ |
о см о см го ф го о 52 |
и "с ф ф 2 □Z |
о о см £ ф о £ |
|||||||||||||||
|
го £ |
о'' |
см Cxi |
СО о |
со |
о о |
со |
in о о |
°? |
о о LO |
о см |
05 |
со |
со со см |
со см |
in ю |
in со со |
со со со |
см |
со |
°? со со |
со со см |
о |
°? 3 |
||
|
1 с |
о ю |
ю О ю |
3 £2- |
со см |
со о со LO |
со о о о |
см со |
со со со со |
со 52 |
о о см |
52 со |
с7 52 |
со |
LO £2 in |
со 52 |
со 52 со |
o' см |
со о |
со 52 со см |
o' 52 о см |
о то |
€ о |
о см см |
||
|
* со о о о ф |
и ф го Е ф |
о'' |
ю о |
со со |
со in о |
со |
со о |
см со со |
со LO |
о in |
in со |
о |
со со in |
со |
со см со |
со |
°? |
то см |
|||||||
|
1 с |
o' со со |
с7 о со |
со см |
со in 3 |
о |
LO со ^ |
с7 со |
с7 52 |
со |
со |
со 52 о |
со 52 |
со |
со |
7 |
со 52 |
|||||||||
|
и Ф го ^ |
о'' |
о о |
N |
03 LO |
03 о |
о см о |
in in |
00 |
со о со |
ш см |
со со |
со со см |
со |
in см со |
о со см со |
со со со |
N |
||||||||
|
1 |
со о со о |
с7 со 3 |
со § |
со ’EL co см |
со EL co со |
со |
о ? |
со |
со |
со |
с7 см |
то со |
то |
t |
см |
то" со |
|||||||||
|
Q < -С о го ф г о ф го _| |
|||||||||||||||||||||||||
|
ф го Q |
Q < -С ю 7 -С со |
Q < о 5 7 -С о |
Q < о о 7 -С со |
Q < о со 7 -С см |
Q < о 5 7 -С см |
Q < -С о 7 .с со |
Q < -С 5 7 -С см |
Q < -С со 7 -С о |
Q < -С со .с |
Q < .с О 7 -С 5 |
Q < .с 7 -С со |
о m -С 7 -С о |
Q со о m -С |
Q < -С о |
Q < -С 7 -С о |
о m -С ю 7 г о см |
Q < -С см 7 -С со |
Q < -С |
Q < .с то |
Q < -С то 7 -С со |
Q < -С 7 -С о |
Q < -С то 7 -С со |
|||
|
о О |
с го о 0_ |
с ф ф 5 со |
"с го СП ш |
ф го ф г ф Z |
.2 с |
< со Z) |
го го го о |
го 2 ф со < |
ф 0_ |
с го со |
|||||||||||||||
|
ф с о О |
ф о ш |
= |
= |
= |
= |
2 ■ф < |
= |
го о ф Е < -С о Z |
= |
= |
= |
= |
го о ф Е < -С о со |
= |
го о < |
||||||||||
|
Ф Е го со |
го (Г |
го 1? со |
о о _1 ф (О |
2 ф ф ф ^ ф "ф ф о о |
ф 2 Ф .ь О Ф I £ ср Р и £ га о |
? г ф р Е го ф £ с о ^ (Г |
ф 1 ф Ф .ь и г V ф с £ Ф Г ф t Si й " |
-С о Z о 0- ф Е Z ГО (Л |
го V 2 Г Ф О О о В 2 |
2 го го ф 52 ^ о "ф го го ^ |
ф с с Z со £ о "ф го го ^ |
с о го со Е го с |
го го Е о |
ф ф < |
ф со го ^ > о СО 3 р £ го X |
о ^ с .2 с |
2 о |
го о го Q -С о со |
£ 2 о Z Р ? гс £ О О |
ф ■О X Z) |
2 го о ф 'Го го о 0_ |
го Е _1 |
го 2 |
||
* n – number of pathological cases, N – number of individuals.
Table 2. Climatic, geographic, and socio-economic variables
|
"с О) Е ф ф со |
2 к |
с 05 = 5 |
2 |
С го 5 |
2 |
с го _ _ |
_ ф = = = = = = = = = |
с го = 5 |
2 |
|
|
о. ф о ф и ел W |
2 СП < |
СП СП с .Е ф ф ГО CD ГО СП ±^ СП с ос ^ о ^ = = = = = = С = = О С о СП о I < I |
2 о СП < |
|||||||
|
* |
о со |
со о |
со ю |
СО СО СП о г- о |
смсосою^Зсч^спю^ |
^ сч о р |
Р |
|||
|
О) о —1 |
in |
in in |
о |
7 |
о |
о о 1 1 1 |
1 17 1 1 1 1 |
Г"-' in г- со |
ел со |
|
|
* |
м ю |
сч сч LO Ш |
о |
in СП |
in со |
1П М" СП CD со |
СЧ^^’^-^ЮО^Г-СО СО^ОО^Г^СЧСЧСЧ^СЧ |
СЧ СЧ О СП |
in 0 |
|
|
го —1 |
о Ю |
о о in in |
со in |
сч ю |
со о Р LO LO LO |
осб^со^сбсбог^со^ LOLO’-’-'t’t't'tCOCO |
СЧ СЧ ^ сч |
04 |
||
|
О -i— m ГО с ф z 2 11 |
со |
со со |
со |
со |
со о |
со со со ^ сч сч т— т— |
^г-счсоРрсчюсосоР СПСПСЛСП^^Г-СОСПСЛ^ |
СЧ со со 7 |
0 |
|
|
о4 |
||||||||||
|
Е га р < 2 Е -С |
со о со |
со со о о со со |
сч CD СП |
о о о со |
in сч СП |
^ со со СП со со СП со со |
ооосчююсоооюо OOO4-NNC0OOC4O Q’t^bbcicOCDNNN Г'--Г'--СОСОГ''-Г''~ЮСОСОСОГ''- |
in со СЧ со О in со ^ |
со со со сч |
|
|
С |
с ф ф II ^ □ 2 «• Е 2 £ Е ^ * § с Е^®Е |
сч |
сч сч |
со со in со |
со со in со |
со со со |
3 ^ ^ iA о о g СО со |
coSSjPPPScoPPco СОЮО^^^СОСО^^СО |
7 р £ £ |
р со о |
|
с го |
и — ф ф Р Е ел £ ел £ Ф ^ к £ £ г Е е| ° |
со со со |
? ч со со со со |
ю со |
ю со |
сч in со |
р со со 1П СО СО со |
PPPincOCO^P^. О СО СО п п гл сч О О Q-} С^СОСО^^^ — СОСЧСЧ^ |
3 ” |
р in |
|
ГО О) ГО LU |
м сч |
сч сч сч сч |
СП СП |
СП СП |
ел г- г СП со со |
COQNWNNCOCM^^CO °. ^ ^ ^ СО СО Т- г- in in in |
со ^ о р |
1 |
||
|
ю |
LO LO |
со ^ ^ ^ in in |
bb^lDNNCObbbcO ’^-LOCOr-’^-’^-’^-LOLOLOCO |
со ^ со in |
СП |
|||||
|
Е го со |
го 2 (Л о m |
сл S сч со |
-С о Z о |
-С Z ел 1 ф г |
'ф' ел р Е го -С |
-С о i „ |
го ^ 70 2^ = -“ |
2 го |
||
|
г го го ^ |
г о .2 ф 1л 1л го го го го ^ ^ |
Е г |
И ел |
ел с го I |
^ 2 ф ^ S’ И ф Ю > -С о. И- О W |
ф | g S, 2 S | с СП Р г- ел С .Е W в Е £ со -S О .5 Р -S .^^О^хОо — "Осо^ WK^^Z)2W = ^I< |
ф '2 Е | □ 0_ |
го с 5 |
||
*Positive sign – north latitude, negative sign – south latitude.
**Positive sign – east longitude, negative sign – west longitude.
relative humidity, and the number of rainy days per year (Table 2).
Unfortunately, it was not possible to obtain detailed climatic data for the time of existence of the studied archaeological sites; thus, modern values of the variables were compiled from the open source . The geographic coordinates of the locations of the sites were employed in the analyses as well. The coordinates of an area rather than a single geographic point were used for the sites lacking the data on the exact location, as well as for composite samples.
In order to obtain cumulative descriptors of climate, a principal component analysis (PCA) was carried out for all the climatic variables, and the PCA values were then employed in the correlation analyses. The frequencies of CMS were calculated for sex-combined samples except for the analysis of sexual dimorphism.
Results
As a first step, the differences between various demographic and socio-economic cohorts hypothesized in previous studies were tested.
Sex difference in the prevalence of CMS. Seventeen samples were analyzed: one African, seven European, seven North American, and two South American (see Table 1). At the intercontinental level, the differences between males and females were not significant: Wilcoxon-Mann-Whitney Z = 1.55, p = 0.12. The same is true for the European samples: Z = 0.676, p = 0.499. But in the New World, females exhibit a high incidence of the disease. The difference is statistically significant: male mean 38.75 %, female mean 53.79 %; Z = 1.96, p = 0.05. Notably, this trend is highly dependent on one “influential” North American sample from Harding Village, displaying a difference higher than 50 % (see Table 1). No similar pattern is observed in other groups; moreover, in the South American female skulls, only one case of CMS was detected. Thus, except for the Harding Village sample, no significant sexdifference in the prevalence of CMS was observed. Consequently, the unequal occupational stress experienced by males and females does not seem to influence the frequency of the disease at either a global or a continental scale.
Prevalence of CMS in different socio-economic groups. Urban vs. rural and hunter-gatherer vs. agriculturalist groups were compared (see Table 2). No statistically significant differences between the urban and rural samples were detected either at the global scale ( Z = 1.613, p = 0.1) or in Europe or North America ( Z = 0.8, p = 0.42). Separate analyses for Asia, Africa, and South America were not carried out owing to their low sample-sizes. No statistically significant differences between the agriculturalist and hunter-gatherer groups were detected at the intercontinental scale ( Z = 1.29, p = 0.19), likely as a result of the low number of the latter (only four samples). The only continent where such comparison was possible was North America, showing a marginally significant level of association ( Z = 1.96, p = 0.0495). However, the North American agriculturalist groups inhabited colder areas than the hunter-gatherers from the same continent; thus, this apparent difference might be explained by climatic rather than social factors. On the basis of the results of the present analysis, an influence of social or economic factors on the epidemiology of CMS, in general, cannot be completely ruled out; but, according to these results, there is no relation between this and the studied socio-economic cohorts.
Associations of the prevalence of CMS with climate. As a first step, the correlation of the climatic variables with the geographic differentiation of the compared samples was explored via a PCA. The analysis has shown that only PC1 is statistically significant, and explains 67.8 % of the total variance. All the climatic variables display high loading on PC1, though these are positive for the features of humidity and negative for the temperature markers (Table 3). Negative values
Table 3. Loading on PC1 and PC2 of the analysis of climatic variables
|
Variable |
PC1 |
PC2 |
|
Number of rainy days per year |
0.749 |
–0.267 |
|
Minimal temperature |
–0.760 |
–0.618 |
|
Mean temperature |
–0.959 |
–0.258 |
|
Maximal temperature |
–0.893 |
0.130 |
|
Humidity |
0.730 |
–0.55 |
|
Eigenvalue |
3.39 |
0.84 |
|
Proportion of variance described |
0.678 |
0.168 |
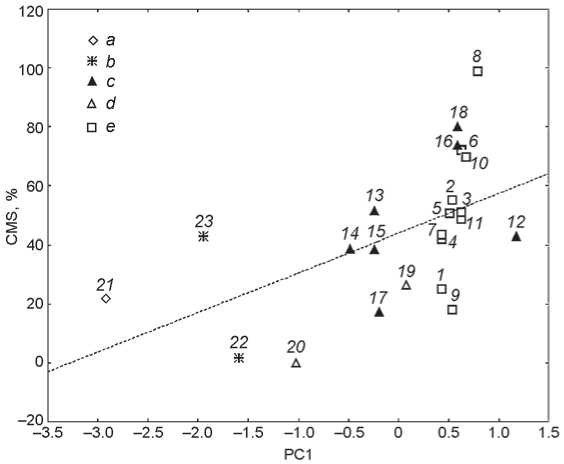
The frequencies of CMS and PC1 coordinates in the 23 cranial samples employed in this study.
-
1 – Maastricht-1; 2 – Chichester; 3 – Fishergate House; 4 – Maastricht-3; 5 – Raunds; 6 – Rurka; 7 – Maastricht 2;
-
8 – Sigtuna; 9 – Spitalfields; 10 – St. Helen-on-the-Walls;
-
11 – Wharram Percy; 12 – Aleuts; 13 – Harding Village; 14 – Illinois; 15 – Indian Knoll; 16 – Moatfield; 17 – South Dakota; 18 – Uxbridge; 19 – Pucará de Tilcara; 20 – Lima;
21 – Kulubnarti; 22 – Inamgaon; 23 – Kodumanal.
a – Africa; b – Asia; c – North America; d – South America; e – Europe.
of PC1 are found for the populations from the warmest and driest climates (Sudan and India), while positive values are typical for the North and Western European groups, Canada and the Aleutian Islands. The rest of the American samples display intermediate values (see Figure ).
The proportion of the total variance described by PC2 is substantially less than that of PC1. The eigenvalue of PC2 is also less than 1, which means that this vector explains less variation than any of the raw variables. Thus, the results for PC2 are not considered further.
The correlation analysis between PC1 coordinates, raw climatic variables, and CMS frequencies carried out across three continents has shown significant (though moderate in factor values) associations: negative between temperatures and frequency, and positive between
Table 4. Pearson’s correlation coefficients between the frequency of CMS, raw climatic variables, and the integral climatic factors (PCs) (23 samples)
|
Variable |
ф го —1 |
ф СП с о _I |
о ^ И -О |
2 ■= Р £ го .£ Е 2 го |
2 го с го го & 2 го |
2 га го
Е га Е 2 го |
X |
о 0_ |
о Ф О' сл и. о |
|
Latitude |
1.000 |
||||||||
|
Longitude |
0.064 |
1.000 |
|||||||
|
Number of rainy days per year |
0.258 |
0.013 |
1.000 |
||||||
|
Minimal temperature |
–0.578 |
0.623 |
–0.405 |
1.000 |
|||||
|
Mean temperature |
–0.525 |
0.481 |
–0.605 |
0.878 |
1.000 |
||||
|
Maximal temperature |
–0.343 |
0.159 |
–0.579 |
0.528 |
0.844 |
1.000 |
|||
|
Humidity |
0.442 |
–0.009 |
0.525 |
–0.272 |
–0.570 |
–0.670 |
1.000 |
||
|
PC1 |
0.521 |
–0.317 |
0.749 |
–0.760 |
–0.959 |
–0.893 |
0.730 |
1.000 |
|
|
Frequency of CMS |
0.527 |
–0.001 |
0.367 |
–0.516 |
–0.504 |
–0.471 |
0.372 |
0.544 |
1.000 |
Note. Italicized are significant correlations ( p < 0.05).
PC1 scores and frequency (Table 4). The latter correlation is higher than those for the raw climatic variables, i.e. their combination summarized with PCA is associated with climate more than single variables.
It is of note also that PC1 is positively correlated with latitude, while the latter is associated with the prevalence of CMS as well (Table 4). This observation confirms the dependence of climatic conditions on the geographic location of the populations, which suggests to an extent that the use of modern climatic data has not affected the outcome of the analyses. As the geographic locations of the groups have not been changing with time, the simultaneous correlation of latitude with climate and morbidity confirms the significance of the association between these.
In order to obtain a more detailed picture of the association between various climatic variables and the prevalence of CMS at the continental level, three more analyses were performed: for Europe, for the New World in a broad sense, and for North America separately. In Europe, the strength and sign of the correlations between the frequency of CMS on one hand, and latitude, annual and maximal temperature, on the other hand, remain the same. In addition, a negative correlation between morbidity and the number of rainy days per year was detected (Table 5).
In the New World (including the continental part of the Americas and the Aleutian Islands), the pattern is different. A significant correlation with morbidity was only detected for latitude, but not for any other variable. The coefficients for the annual average number of rainy days and mean temperature were higher than at the intercontinental level (Table 6). But unlike Europe, precipitation in the New World was positively associated with morbidity. If the South American samples are excluded, the loading on the annual number of rainy days becomes significant (Table 7). Also, the correlation between morbidity and longitude increases strongly, but still does not reach significance owing to the small number of analyzed samples.
The results for the North American groups should be in general treated cautiously because of the low sample size. Nevertheless, the results of all the analyses lead to the preliminary conclusion of the importance of climatic conditions for the epidemiology of CMS. But the moderate values of the correlation coefficients demonstrate that the association between climate and morbidity is far from being absolute, and can be affected by the influence of non-climatic factors.
Table 5. Spearman-Kendall’s rank correlation coefficients between the frequency of CMS and climatic and geographic indicators (Europe)
|
Variable |
ф го _1 |
ф СП о _I |
о ^ И -О |
2 ■= Р £ го .£ Е 2 го |
2 го с го 2 го |
2 га го
Е га Е 2 го |
Е |
о ф О' сл и. о |
|
Latitude |
1 |
|||||||
|
Longitude |
–0.042 |
1 |
||||||
|
Number of rainy days per year |
–0.779 |
–0.268 |
1 |
|||||
|
Minimal temperature |
–0.272 |
–0.756 |
0.545 |
1 |
||||
|
Mean temperature |
–0.911 |
0.07 |
0.848 |
0.393 |
1 |
|||
|
Maximal temperature |
–0.826 |
0.014 |
0.791 |
0.223 |
0.716 |
1 |
||
|
Humidity |
0.614 |
–0.721 |
–0.239 |
0.437 |
–0.615 |
–0.38 |
1 |
|
|
Frequency of CMS |
0.552 |
0.055 |
–0.701 |
–0.311 |
–0.608 |
–0.803 |
0.271 |
1 |
* See note to Table 4.
Table 6. Spearman-Kendall’s rank correlation coefficients between the frequency of CMS and climatic and geographic indicators (New World) *
|
Variable |
ф го —1 |
ф СП о _I |
ГО го го о * а е £ 5 го И -О |
2 — ? £ го ^ го |
2 2 Г- ГО ГО с ^ S |
2 го го Е го го Е ^ го |
Е X |
о о ф о- ОТ и. О |
|
Latitude |
1 |
|||||||
|
Longitude |
–0.567 |
1 |
||||||
|
Number of rainy days per year |
0.185 |
0.353 |
1 |
|||||
|
Minimal temperature |
–0.824 |
0.655 |
–0.119 |
1 |
||||
|
Mean temperature |
–0.773 |
0.269 |
–0.627 |
0.797 |
1 |
|||
|
Maximal temperature |
–0.303 |
–0.235 |
–0.627 |
0.237 |
0.712 |
1 |
||
|
Humidity |
0.377 |
0.042 |
–0.135 |
–0.034 |
–0.135 |
–0.304 |
1 |
|
|
Frequency of CMS |
0.65 |
–0.05 |
0.588 |
–0.387 |
–0.555 |
–0.151 |
0.259 |
1 |
*See note to Table 4.
Table 7. Spearman-Kendall’s rank correlation coefficients between the frequency of CMS and climatic and geographic indicators (North America) *
|
Variable |
ф го —1 |
ф о —1 |
Го го ГО о ^ Е 3 го И -О |
2 Е го .Е Е ^ го |
2 2 С го ^ го |
го го Е S го Е 2 го |
Е т |
о ф О’ ОТ и. О |
|
Latitude |
1 |
|||||||
|
Longitude |
–0.071 |
1 |
||||||
|
Number of rainy days per year |
0.546 |
0.618 |
1 |
|||||
|
Minimal temperature |
–0.655 |
–0.291 |
–0.111 |
1 |
||||
|
Mean temperature |
–0.873 |
–0.000 |
–0.666 |
0.778 |
1 |
|||
|
Maximal temperature |
–0.873 |
–0.000 |
–0.666 |
0.778 |
1 |
1 |
||
|
Humidity |
0.667 |
0.234 |
0.881 |
–0.220 |
–0.771 |
–0.771 |
1 |
|
|
Frequency of CMS |
0.464 |
0.750 |
0.873 |
0.073 |
–0.436 |
–0.436 |
0.739 |
1 |
*See note to Table 4.
Discussion
Our analyses have shown that the interpopulation differences in the prevalence of CMS can depend on climate. While the employment of modern values of climatic variables evokes a conservative interpretation of the obtained results, the main trends seem fairly robust. At the global scale, the prevalence of CMS in warm regions is significantly lower than in colder areas. The apparent association between the number of rainy days per year and morbidity at the regional level is likely a statistical effect related to the correlation between various climatic indicators, most importantly between temperature and precipitation. The contradictory nature of the correlation between the number of rainy days and temperature in Europe and the Americas suggests this.
On the basis of the value of the correlation coefficient between the frequency of CMS and PC1 scores, climate explains only slightly more than a half of the morbidity variation at the global level. This observation means that the etiology of CMS is multifactorial, and there are other non-climatic factors influencing its prevalence. But as the social and economic factors employed in this study were not found to be associated with the frequency of CMS, a detailed bioarchaeological analysis of each of the samples is warranted for detecting the influence of those non-climatic factors. None of our analyses has revealed a significant difference in morbidity between the rural and urban groups. One possible explanation of this is the archaeological application of the term “city”, whereas not only industrial centers in the modern sense but also large ancient settlements with a high population density, an architectural layout, and a system of defensive fortifications are considered cities. Such settlements clearly did not suffer from the air pollution typical for industrialized cities.
Increased prevalence of CMS in ancient “cities” might be hypothetically expected as a result of a poor epidemiological situation due to the increase in population density. But the absence of significant CMS differences between “rural” and “urban” groups argues against such a hypothesis as well.
Historical sources suggest that the level of household anthropogenic pollution arising from the use of fossil fuels, insufficient ventilation of dwellings, etc. was not substantially different between urban and rural settlements before the 17th–19th centuries AD, and thus could not seriously affect the prevalence of CMS. When the ciliated epithelium of the sinus functions normally, the airborne pollutants are removed to the nasal cavity with liquor and do not accumulate in the sinus. The accumulation of the polluting particles begins in case of the already existing outflow violation, which may be related to infectious diseases or allergic reactions, or in case of very strong pollution. The latter triggers the development of CMS and pneumoconiosis in miners and workers in the metallurgical industry (Artemova et al.,
2016: 37). But such conditions are extreme, and the morbidity of these professional groups does not depend on their urban or rural habitation.
The social status of the deceased is traditionally thought to be an important factor of the epidemiology of CMS (Roberts, 2007), though it seems impossible to determine its actual influence at the global or even at a regional scale. The status is typically determined by archaeologists based on the differences in grave goods, which only rarely reflect the level of biological stress experienced by the population. Thus, directly opposite patterns of morbidity are often observed in archaeological populations of a similar social status. For instance, the prevalence of CMS in several groups from North Yorkshire fits well to the theoretical expectations based on their levels of welfare. Three archaeological samples were studied there: Fishergate House, Wharram Percy, and St. Helen-on-the-Walls. In the latter sample, the frequency of CMS is the highest, which is logically explained by the poverty of the part of the city’s population buried in this cemetery (Lewis, Roberts, Manchester, 1995: 501). However, for medieval Maastricht, an opposite situation is observed. In the earliest sample from that city (Maastricht-1; 7th–10th centuries AD), which, according to archaeological data, belonged to a low-status rural population (Panhuysen, Coenen, Bruintjes, 1997: 611), fewer cases of CMS were detected than in the later samples representing urban citizens of middle to high social status. In this case, as well as in many others, it is problematic to determine what the main cause of the increased morbidity in the higher-status group was. The list of possible factors might include fluctuations of climatic conditions, local pandemics, warfare, and other occasional events, which cannot be accounted for in statistical analysis. Ideally, instead of dealing with a general social status, it would be more productive to consider single stressors and their various combinations.
In order to assess the influence of social factors on the prevalence of CMS, not only the total number of affected individuals should be estimated, but the proportion of various forms of the disease needs to be taken into account: rhinogenous, associated with respiratory disorders; odontogenic, caused by the penetration of dentoalveolar infections into the maxillary sinuses; and hematogenous, caused by specific diseases such as measles, scarlet fever, or influenza, the complications of which may be CMS (Fedorova, 2011). Each of these forms can be related to a separate group of stress factors. Ambient temperature and humidity are important causes of rhinogenous sinusitis, while diet and the associated status of dental health are influential factors of the odontogenic form.
At present, a thorough wide-scale analysis of the aspect mentioned above is not possible owing to the lack of precise diagnostics of various forms of CMS. Hematogenous and rhinogenous sinusitis cannot be differentiated using only cranial data, and a reliable diagnosis of the odontogenic form requires a CT study of all the employed samples. Such a study so far has only been carried out for the Peruvian (present publication) and Argentinean (Zubova et al., 2020) samples, while the other populations were only the subject of a conventional macroscopic examination. Thus, it is not possible to determine the main sources of infections, nor the pathogenic factors involved.
Conclusions
The main outcome of the preliminary statistical analysis carried out in the present study is that of all the possible pathogenic factors considered, only the climatic variables are significantly correlated with the prevalence of CMS at the global scale. The strongest association was detected with temperature.
No correlation was detected between the frequency of CMS and the anthropogenic factors— air pollution, social status, and others, which have previously been suggested as possible causes of the disease (Mercer, 2003; Kaur, Nieuwenhuijsen, Colvile, 2005; Peled et al., 2005; Roberts, 2007). Moreover, the influence of these factors cannot productively be discussed at present, owing to the lack of relevant information in the anthropological literature. Such an influence can only be considered at the level of single populations, since the combination of the stress factors is unique to each of them. Thus, in each case, a comprehensive bioarchaeological investigation of the skeletal sample should be carried out as a first step, and precise methods of instrumental diagnostics must be employed for the differentiation of various forms of CMS. We are unaware of any studies of this type published to date, and this remains a very promising direction for future research in the fields of paleopathology and bioarchaeology of ancient and historical populations of the world.
Список литературы Chronic Maxillary Sinusitis Recorded in Archaeological Samples: Geographical Distribution and Predisposing Factors
- Artemova L.V., Baskova N.V., Burmistrova T.B., Buryakina E.A., Bukhtiyarov I.V., Bushmanov A.Y., Vasilieva O.S., Vlasov V.G., Gorblyansky Y.Y., Zhabina S.A., Zakharinskaya O.N., Izmerov N.F., Kovalevsky E.V., Kuznetsova G.V., Kuzmina L.P., Kunyayeva T.A., Logvinenko I.I., Lutsenko L.A., Mazitova N.N., Obukhova T.Y., Odintseva O.V., Orlova G.P., Panacheva L.A., Piktushanskaya I.N., Plyukhin A.E., Poteryaeva E.L., Pravilo S.V., Razumov V.V., Roslaya N.A., Rosly O.F., Rushkevich O.P., Semenikhin V.A., Serebryakov P.V., Smirnova E.L., Sorkina N.S., Tsidilkovskaya E.S., Chasovskikh E.V., Shpagina L.A. 2016 Federalniye klinicheskiye rekomendatsii po diagnostike, lecheniyu i profi laktike pnevmokoniozov. Meditsina truda i promyshlennaya ekologiya, No. 1: 36-49.
- Brook I. 2009 Sinusitis. Periodontology 2000, vol. 49 (1): 126-139.
- Fedorova M.E. 2011 Odontogenniye gaimority. Vestnik sovremennoy klinicheskoy meditsiny, vol. 4, app. 1: 57-58.
- Kaur S., Nieuwenhuijsen M.J., Colvile R.N. 2005 Pedestrian exposure to air pollution along a major road in Central London, UK. Atmospheric Environment, vol. 39: 7307-7320.
- Khronicheskiy rinosinusit: Patogenez, diagnostika i printsipy lecheniya (klinicheskiye rekomendatsii). 2014 N.A. Arefyeva, V.V. Vishnyakov, O.A. Ivanchenko, S.A. Karpishchenko, A.B. Kiselev, V.S. Kozlov, R.S. Kozlov, S.Y. Kosyakov, P.A. Kochetkov, A.S. Lopatin, Y.A. Nakatis, I.V. Otvagin, G.Z. Piskunov, D.P. Polyakov, A.B. Turovsky. Moscow: Prakticheskaya meditsina.
- Koshel I.V. 2017 Odontogenniye verkhnechelyustniye sinusity i ikh patogeneticheskoye lecheniye (eksperimentalno-klinicheskoye issledovaniye): D. Sc. (Medicine) Dissertation. Stavropol.
- Lewis M.E., Roberts C.A., Manchester K. 1995 Comparative study of the prevalence of maxillary sinusitis in Later Medieval urban and rural populations in Northern England. American Journal of Physical Anthropology, vol. 98: 497-506.
- Mercer J.B. 2003 Cold - an underrated risk factor for health. Environmental Researches, vol. 92: 8-13.
- Mushrif-Tripathy V. 2014 Maxillary sinusitis from India: A bio-cultural approach. Korean Journal of Physical Anthropology, vol. 27 (1): 11-28.
- Panhuysen R., Coenen V., Bruintjes T. 1997 Chronic maxillary sinusitis in Medieval Maastricht, the Netherlands. International Journal of Osteoarchaeology, vol. 7: 610-614.
- Patel N.A., Ferguson B.J. 2012 Odontogenic sinusitis: An ancient but under-appreciated cause of maxillary sinusitis. Current Opinion in Otolaryngology & Head and Neck Surgery, vol. 20 (1): 24-28.
- Peled R., Friger M., Bolotin A., Bibi H., Epstein L., Pipel D., Scharf S. 2005 Fine particles and meteorological conditions are associated with lung function in children with asthma living near two power plants. Public Health, vol. 119: 419-425.
- Roberts C.A. 2007 A bioarcheological study of maxillary sinusitis. American Journal of Physical Anthropology, vol. 133 (2): 792-807.
- Slavin R.G., Sheldon L., Bernstein I.L. 2005 The diagnosis and management of sinusitis: A practice parameter update. Journal of Allergy and Clinical Immunology, vol. 116 (6): S13-S47.
- Sundman E.A., Kjellström A. 2013 Chronic maxillary sinusitis in Medieval Sigtuna, Sweden: A study of sinus health and effects on bone preservation. International Journal of Osteoarchaeology, vol. 23 (4): 447-458.
- Teul I., Lorkowski J., Lorkiewicz W., Nowakowski D. 2013 Sinusitis in people living in the Medieval Ages. In Neurobiology of Respiration: Advances in Experimental Medicine and Biology, M. Pokorski (ed.). Dordrecht: Springer, pp. 133-138.
- Zubova A.V., Ananyeva N.I., Moiseyev V.G., Stulov I.K., Dmitrenko L.M., Obodovskiy A.V., Potrakhov N.N., Kulkov A.M., Andreev E.V. 2020 The use of computed tomography for the study of chronic maxillary sinusitis: Based on crania from the Pucará De Tilcara Fortress, Argentina. Archaeology, Ethnology and Anthropology of Eurasia, vol. 48 (3): 143-153.


