Cochlear models used in cochlear implant research
Автор: Mutalipova G.A., Asretov D.N., Temirova D.A., Magomedov M.A., Fetalieva S.I., Magomedsaidova S.Z., Temirov A.T.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 32, 2024 года.
Бесплатный доступ
Over the past few decades, cochlear implants have undergone significant changes due to intensive research through experimental and computational analysis. However, obtaining an accurate and reliable cochlear model remains an open issue. Invasive measurements on the human ear are hardly possible, and the only alternative is animal models, but even this is not an ideal option, as animal cochleae are anatomically significantly different from the human cochleae. In this context, an ear model based on the latest knowledge of the physiology and molecular principles of hearing will allow the study of hearing disorders, whether they are caused by some genetic or external factors. It will also enable the experts to learn more about the detailed mechanisms of various forms of hearing impairment and open up avenues for the improvement of cochlear implants. With this in mind, the aim of this article is to explore different approaches to creating models of the human cochlea used in cochlear implant research. In the process of the study an individual emphasis is made on the variability of the human cochlea, sizes of its separate elements and shapes. Also considered are herein such methods of cochlea model creation as 3D-modeling, computer graphics and finite element method, as well as computational approaches. The results obtained allow us to state the fact that there are no ideal approaches and techniques to date available. The limitations of the models are related to difficulties in reproducing the microenvironment of the human cochlear apparatus, the need for clear validation and accurate parameterization of the main parameters of the cochlea. At the same time, we can expect that with a rapid increase in the available computational resources and development of effective computational methods, the models of the cochlea and cochlear implant will become more accurate and allow analyzing the cochlear micromechanics and the temporal response of the tissue to external stimulation.
Model, reference points, cochlear implant, cochlea, ear, 3d modeling, computational method, finite element method
Короткий адрес: https://sciup.org/148329308
IDR: 148329308 | DOI: 10.18137/cardiometry.2024.32.1320
Текст научной статьи Cochlear models used in cochlear implant research
Imprint
Gulzhagan A. Mutalipova, Dzhabrail N. Asretov, Djamila A. Temirova, Magomed A. Magomedov, Svetlana I. Fetalieva, Sarat Z. Magomedsaidova, Alibulat T. Temirov. Cochlear models used in cochlear implant research. Cardiometry; Issue No. 32; August 2024; p. 13-20; DOI: 10.18137/cardiometry.2024.32.1320; Available from:
RELEVANCE OF THE PROBLEM
“Invisible disability,” as hearing loss is often called, is the most common sensory deficit, affecting approximately 5% of the world’s population. The World Health Organization estimates that more than 1.5 billion people of all ages, nationalities and economies live with some degree of hearing loss that affects their mental and cognitive health and quality of their daily life [1]. These numbers are growing every year. Treatment of hearing loss depends largely on the degree of actual hearing loss. For most people, conventional hearing aids are a satisfactory option. However, for some other human individuals, these devices may not provide enough acoustic amplification to be beneficial in everyday life. This includes people who are fully deaf and those who have minor residual hearing.
In this case, the solution to the problem is cochlear implants (CIs), which represent a more advanced treatment option for restoring hearing. The CIs overcome the limitations of conventional amplification by directly stimulating the auditory nerve fibers within the cochlea [2]. The use of implants and bio-electronic devices for neuromodulation is growing rapidly and is expected to shape a new era in medicine. By delivering local electrical stimuli to tissue, these electronic implants restore the lost neural function in the tissue or the nerves and modulate signaling patterns to achieve the desired therapeutic outcomes. To date, more than 1 million CIs have been implanted worldwide, and their prevalence is only expected to grow with an anticipated increase in the elderly population.
By bypassing faulty peripheral auditory mechanisms through direct neurostimulation, the CI electrode array is designed to restore sound perception. In a broad sense, it also attempts to reproduce the to-notopic architecture of the cochlea by delivering frequency-specific programmed stimulation to localized areas of the cochlea, which in turn stimulates individual auditory neural elements (see Figure 1 herein), where low sound frequencies are presented apically and high frequencies basally, accordingly.
At the same time, the main limitation of the today’s CIs is their inaccurate control over the introduced stimulus, which is a consequence of the intrinsic conductive nature of biological tissues, and especially biological fluids in the inner ear. The appearance of the human cochlea is well described in the reference literature. However, there is still a lack of knowledge about what characteristics lead to optimal or suboptimal postoperative hearing outcomes. The success of cochlear implantation depends on various factors, such as the anatomical properties of the cochlea itself, the cochlear duct length, volume, basal diameter or malformations [3]. However, only some of these characteristics, such as the length of the cochlear duct, have been studied to a sufficient extent, while the role of others, such as volume, has not yet been fully understood.
Thus, issues related to the development of accurate models of the human cochlea to improve future treatments for CI are theoretically and practically significant that determines the choice of the topic of this article.
Three-dimensional models of voltage distribution in the cochlea have been widely developed over the past few years. Advancements in creating 3D models of the cochlea can be found in the works of such researchers as Kuzovkov V.E., Lilenko A.S., Sugarov S.B., Tanaschishina V.A., Ammosov L.I., Ilyin N.R., Julia R. Drouin, Rachel M. Theodore, Sarah C. Nyirjesy, Jessica H. Lewis, Diana Hallak BS, and Sara Conroy.
The rotationally symmetric geometry of the cochlea for calculating models of neural excitation when using various electrode configurations and stimulation schemes is treated in the papers by Tufatulin G.Sh., Levin S.V., Koroleva I.V., Pudov V.I., Zontova O.V., Tristan Putzeys, Gideon Wackers, Oliver Jamieson, Marloes Peeters, Theodor Doll, Patrick Wagner.
Methods capable of providing standardized automated extraction of pre-operative anatomical characteristics of the human cochlea such as its length, volume or basal diameter have been developing by Pashkov A.V., Klyachko D.S., Kalyapin D.D., Levin S.V., Lilenko A.S., Simone R. de Rijk, Alexander J. Boys, Iwan V. Roberts, Chen Jiang, Charlotte Garcia, Róisín M. Owens and Manohar Bance.
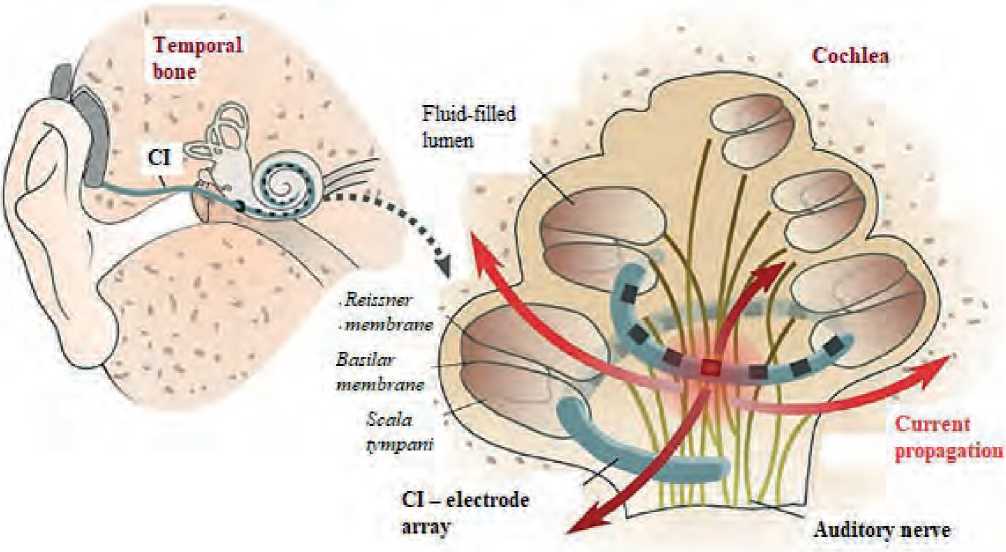
Figure 1. Scheme of the auditory system and cochlea with an implanted CI
An analysis of scientific expert publications allows us to state the fact that currently various physical and computational models of the hearing aid have been developed for testing CIs, but they are not sufficient to assess the propagation of a stimulus in the human cochlea. Animal models are well established for testing CIs in vivo, but due to the dramatic differences between the human and animal cochlear anatomy, an incomplete understanding of the human response thereto may be obtained. The in silico approaches such as finite element modeling (FEM) can overcome ethical, sample-availability and cost-related issues. However, the existing FEM modeling is limited by several factors, including incomplete and fragmented knowledge of the electrical properties of living human cochlear tissue to fit various in vivo cases, their inability to account for anatomically patient-dependent CI placement, and poorly defined boundary conditions, as well as descriptions of physical/empirical laws.
The aim of the study is to investigate different approaches to creating models of the human cochlea used in CI research.
MATERIALS AND METHODS
Systematic analysis of the reference literature, which was carried out using PubMed Medline data, grouping, synthesis, synthesis, and visualization.
RESULTS
The cochlea, embedded in the temporal bone, is a fluid-filled structure that is part of the bony labyrinth, also called the otic capsule. It consists of the semicircular canals, which are responsible for the perception of head rotation, which is necessary to maintain balance, the vestibule, which contains linear acceleration sensors (otolith organs), and the cochlea itself [4].
Variability of the cochlea is reflected in the range of length of the cochlear canal from 30.8 to 43.2 mm, and also, to a lesser extent, in the variable number of turns [5]. Thus, an individual cochlea is not only an enlarged version of the same basic shape, but also represents real morphological variations. Table 1 given herein summarizes some of the key anatomical features of the cochlea.
Table 1
Brief description of the sizes and shapes of various elements of the human cochlea [6, 7]
|
Cochlear component |
Measurement |
N1 |
|
|
Average ± CO2 (range) (mm) |
|||
|
Cochlear canal length |
37.9 ± 2.0 (30.8–43.2) |
436 |
|
|
35.8 ± 2.0 (30.7–42.2) |
310 |
||
|
40.9 ± 2.0 мм |
108 |
||
|
Angular length 966.7 ± 45.1 (outer wall) |
|||
|
Число оборотов 2.69 ± 0.13 |
|||
|
Number of turns |
Number of convolutions |
Percentage (%) |
|
|
2.5 |
1 |
68 |
|
|
2.5 |
13 |
||
|
2.5–2.75 |
74 |
||
|
2.75–3 |
12 |
||
|
Round window membrane |
Height (mm) |
Width (mm) |
|
|
1.91 ± 0.78 |
1.37 ± 0.43 |
20 |
|
|
0.69 ± 0.25 |
1.16 ± 0.47 |
34 |
|
|
1.62 ± 0.77 |
1.15 ± 0.39 |
50 |
|
|
Transverse diameter (mm) |
|||
|
1.65 ± 0.21 |
558 |
||
|
Thickness (mcm) |
|||
|
69.4 ± 4.3 |
37 |
||
1 N – количество образцов
2 СО – стандартное отклонение
|
Cochlear component |
Measurement |
N |
||
|
Window shape |
Oval (60%), round (25%) and triangular (15%) |
20 |
||
|
Oval (50%), round (20%), triangular (12%), comma-shaped (10%), quad (6%) and pear-shaped (2%) |
50 |
|||
|
Round window slot |
Width (mm) |
Depth (mm) |
||
|
1.66 ± 0.34 |
1.34 ± 0.26 |
541, 460 |
||
|
Face opening |
Width (mm) |
|||
|
4.01 ± 0.56 |
356 |
|||
|
Basilar membrane (except organ of Corti) |
Base |
Upper limit |
||
|
Width (mm) |
||||
|
~ 80 |
498 |
25 |
||
|
138 |
573 |
Up to 15 |
||
|
126 |
418 |
1 |
||
|
201.9 |
475.2 |
Up to 14 |
||
|
Thickness (mcm) |
||||
|
1.26–1.92 |
0.53–0.89 |
Up to 13 |
||
|
1.46–1.51 |
0.2–0.96 |
1 |
||
|
Organ of Corti |
Base |
Upper limit |
Up to 15 |
|
|
Height (mcm) |
||||
|
66.02 |
62.69 |
|||
|
Reissner membrane |
Width (from spiral branch to spiral limbus) (mcm) |
18 |
||
|
770 ± 180 |
||||
|
Thickness (mcm) |
||||
|
6.4 ± 2.6 |
||||
|
Cochlear septal bridge |
Base |
Upper limit |
||
|
Width (mm) |
||||
|
130 |
519 |
Up to 15 |
||
|
228.6 |
499.2 |
Up to 13 |
||
|
Bone spiral plate |
Base |
Upper limit |
||
|
Width (mm) |
||||
|
1143 |
~ 400 |
Up to 15 |
||
|
726.6 |
335.9 |
Up to 13 |
||
|
Thickness (mcm) |
||||
|
1100 |
~ 400 |
Up to 15 |
||
|
Helicotrema |
Length (along the side wall) (mm) |
14 |
||
|
1.6 ± 0.9 |
||||
|
Scala tympani |
Width (mm)) |
Height (mcm) |
||
|
Base (0°) |
2.5 ± 0.3 |
0.9 ± 0.25 |
9 |
|
|
Maximum (900°) |
1.2 ± 0.3 |
0.4 ± 0.1 |
||
|
Scala vestibuli |
Width (mm) |
Height (mcm) |
||
|
Base (0°) |
2.5 ± 0.2 |
1.3 ± 0.1 |
9 |
|
|
Maximum (900°) |
1.3 ± 0.3 |
0.5 ± 0.15 |
||
Currently, 3D modeling has become widely adopted to create a model of the human cochlea.
As a rough approach to reproducing the electro-anatomical features of the cochlea in CI studies, some scientists represent the cochlea as a single spiral cavity with an ever-narrowing diameter, which does not take into account the internal soft tissue membrane structures within the cochlea, such as the basilar membrane and the Reissner’s membrane. The researchers explain this by the fact that, firstly, when performing a pre-op-erative CT scan of a typical patient as a part of a routine clinical assessment, the scan resolution allows only the shape of the cochlear lumen to be determined, but not the fine micro-anatomical structures of the soft tissues. Secondly, FEM shows that the influence made by the basilar membrane and the Reissner membrane within the cochlea on the electric field image profile after stimulation is likely to be negligible, since boundary resistances dominate in the surrounding bone tissue [8]. Therefore, the biomimetic cochleae are constructed by embedding a 3D mock-up of the conical and spiral lumen cavity of the cochlea inside an electromimetic bone matrix. The spiral cavity is filled with a physiological saline solution to simulate the ionic conduction environment in the cochlea (perilymph) and the conductivity properties at the electrode-electrolyte interface.
To create a 3D model, Garcia, Charlotte et al. chose four geometric descriptors to parametrically describe anatomical variation in the human cochleas: the basal lumen diameter, the conicity ratio, the cochlear width, and its height. For electro-anatomical modeling of cochleae with CI, a fifth descriptor was also included: the array specific resistivity, which is controlled by the proportion of voids in the electromimetic bone matrix [8]. It was resulted in a creation a library of physical models that made it possible to artificially reconstruct a wide range of electro-anatomical features of the human cochlea with a uniform distribution of characteristics.
Figure 2 given herein exhibits some bio-mimetic 3D-printed cochleae types, which replicate a wide anatomical spectrum of the human cochleae, provide geometrically controlled CI positioning, and produce patient-specific electric field imaging profiles. The validity of the obtained models has been tested using a neural network analysis, and it has been found that they have high clinical predictive value.
Also in practice, attempts are being made to create some personalized models of the cochlea using computer graphics. In this context, the results of Swedish scientists who created a computer model of a drawing of a cochlea using the Inventor software deserve
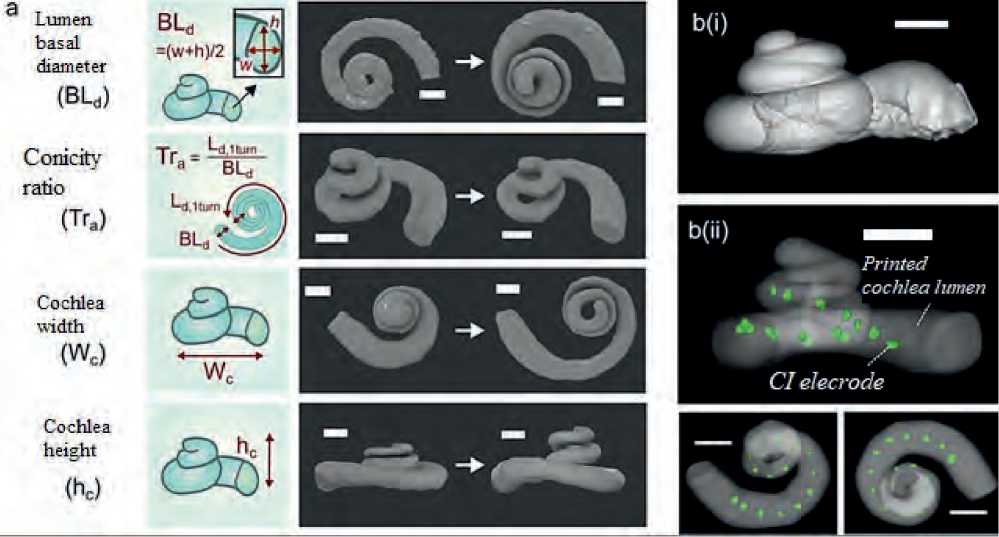
Figure 2. Some 3D printed biomimetic cochlea [8]
а) reconstructed images of the spiral lumen of a biomimetic cochlea with different geometric characteristics. Scale bar = 2 mm; b) reconstructed images of (i) a deceased human cochlea and (ii) the lumen of an exemplary 3D printed biomimetic cochlea with an implanted CI electrode array (marked green). Scale bar = 2 mm.
our attention. Next, a tetrahedral mesh was generated using a general physics algorithm. To investigate the mesh convergence, three different meshes were generated with different levels of refinement. Later on, the scientists developed a non-specific model with an electrode array located in a standard position, based on the general cochlea shown in Figure 3 herein. The minimum dimensions of the elements are 5 × 10-4, 2 × 10-4 and 7.5 × 10-5 m, and the total number of elements is 16,229, 309,670 and 387,451 for each refinement level [9].
The FEM for this geometry was solved using the iterative conjugate gradient solver COMSOL Multiph- ysics v5.0. Steady-state simulations were carried out based on an assumption that permeability effects are negligible. For this reason, the electrical permittivity for all materials was accepted to be 1. The result was a cochlear model produced for a standard cochlear geometry that was not tailored specifically for the CI user (see Figure 4 herein).
Current density flow lines were used to estimate the direction of current flow from the surface of the stimulating electrode to the return electrode. The estimated proportion of current passing through the basal end, the modiolus, and the cochlear wall was 20, 24, and 56%, respectively. At the same time, the scientists
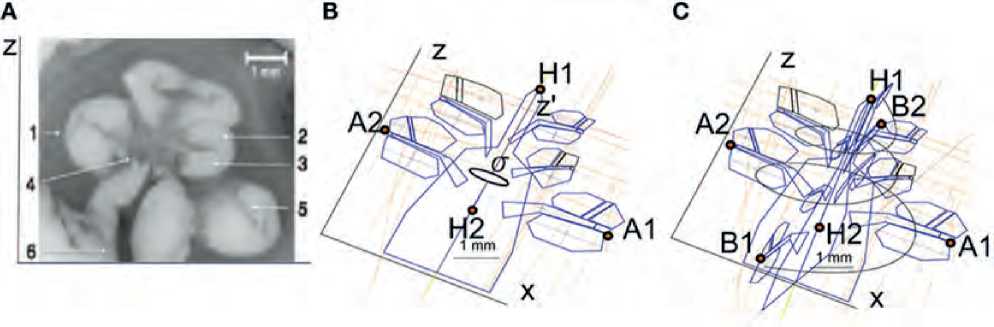
Figure 3. Results of parametric cochlear modeling [9] (A) Microphotograph of a mid-modiolar cross-section of the histological structure of the human cochlea. 1 – spiral ligament; 2 – cochlear duct; 3 – tympanic duct; 4 – modiolus; 5 – vestibular process; 6 – auditory nerve. (B) Sections of the cochlea in the XZ plane. The cochlear structures repeat every 90° around the z′ axis with angle σ. (C) Splines used to interpolate cochlear compartments around the z′ axis.
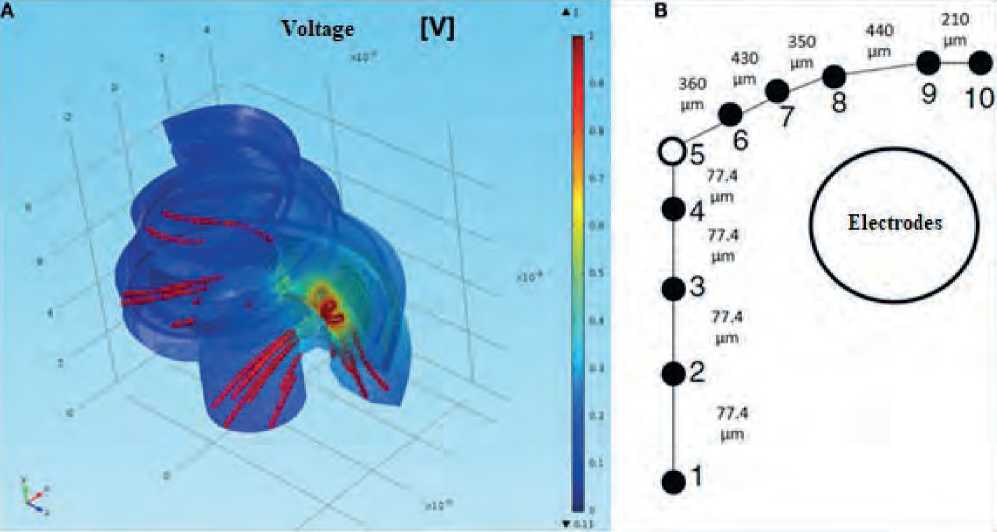
Figure 4. Cochlear modeling using FEM [9] (A) modeling of normalized intracochlear voltage for the nonspecific model; (B) Single auditory nerve fiber with k = 10 sections
note that, taking into account the individual geometry of the cochlea and the position of the electrodes, it is possible to optimize the model by changing the parameters so that the difference between the simulated and measured intracochlear voltage values is minimal. At the same time, this model has some limitations due to the fact that it does not take into account some details of the cochlear canals and the position of the reference electrode.
A separate category of models consists of computational models of the cochlea, which are based on an iterative process of searching for optimal parameters to develop a model of the cochlea. These studies are carried out by the German Hearing Center in Hannover. In addition to the number of reference points, the input parameters of the cochlea model are also adjusted to achieve a better representation and smaller errors.
To build a computational model, a cochlea of an average size and shape was employed. Then the corresponding reference points were specified, which were selected on the basis of human computed tomography data and placed almost equidistantly (every 90º) on the model. During the recording process, the model gradually converged to the size and shape of a segmented cochlea with each iteration step that should reflect the individual characteristics of the patient. Figure 5 given herein shows the iteration steps and the corresponding results.
As can be seen from Figure 5 herein, after iteration 1, the model advanced quite clearly towards the segmented cochlea, and after iteration 5, a noticeable tendency to approach has been already visible. Iteration 11 is the final result from the reference point recording that achieved a minimum mean absolute error (MAE) of 0.2383 mm for this example.
DISCUSSION
The results of this study bear witness to the fact that today scientists are developing various approaches and methods for modeling the human cochlea to
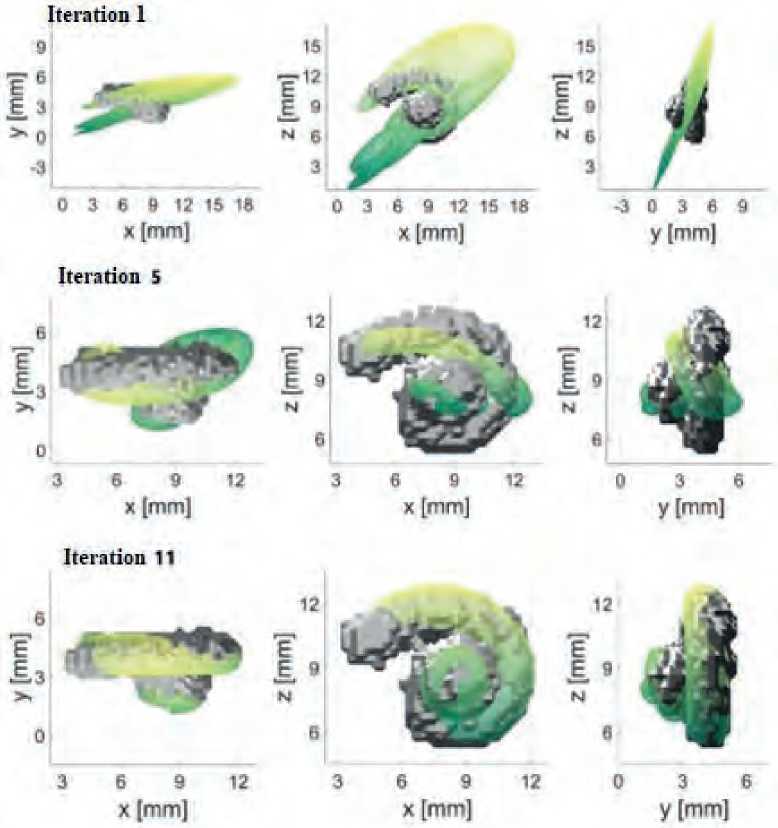
Figure 5. Iterative steps to build a cochlea model based on reference points [10]
investigate the mechanical aspects of CI implantation and the electrical properties of the electrode-nerve interface. These models are created using various software products and computing systems that allow modifying the results obtained to study the influence made by some specific parameters on the behavior of the CI.
At the same time, each of the methods used today has certain limitations and disadvantages. Since the sophisticated complex structure of the human auditory system is a natural miracle that cannot be compared with modern engineering. However, understanding of the structure of the cochlea and how it interacts with this delicate system is critical to solving huge problems in otology and audiology [11]. Improved models and the proper understanding of the cochlea performance are essential for the development of the next generation of CIs and future treatments for the inner ear.
CONCLUSIONS
The given article discusses various approaches to creating models of the human cochlea. These models are useful tools for solving scientific questions related to the use of CIs that are quite problematic to solve employing an experimental approach thereto. The application of these models in practice is intended to assist in simulating the neural activity by CIs and improve the understanding of the shortcomings of the implant to provide natural hearing.
Moreover, a clearer understanding of the variability of the cochlear anatomy and its impact on the implantation insertion parameters and the CI performance may open up ways for individualized approaches in each specific case to achieve optimal results. Addressing these issues will expand the use of CIs and improve the life quality of the growing number of people suffering from hearing loss.
Список литературы Cochlear models used in cochlear implant research
- Implants for Surgery. Active Implantable Medical Devices. Part 7, Particular Requirements for Cochlear and Auditory Brainstem Implant Systems. London: British Standards Institution, 2022.
- Theodore R. McRackan. Development and Implementation of the Cochlear Implant Quality of Life (CIQOL) Functional Staging System. Cochlear Implant Quality of Life Development Consortiu. 2022; 132:98-105.
- Pashkova AE. Peculiarities of setting parameters of processor adjustment in patients with deafness with different types of electrode array of cochlear implant. Medicinskij sovet (Medical Council). 2023;12:192-9. [in Russian]
- Tufatulin GSh. Study of input signal attenuation when using protective and fixation devices for hearing aids and cochlear implants. Innovative technologies in diagnostics of hearing impairment and rehabilitation of patients with hearing loss and deafness. Proceedings of the scientific-practical conference. 2022;64-5. [in Russian]
- Nicole T. Jiam Preserved Cochlear Implant Function After Multiple Electroconvulsive Therapy Treatments. The Laryngoscope. 2020;131:129-37.
- Perrin CK, Levin JM. NIH Toolbox-Cognition performance of older persons with normal hearing, cochlear implant candidates, and cochlear implant users. Alzheimer’s and Dementia. 2023; 19:67-72.
- Levin SV. Tonotopic adjustment of the auditory processor of cochlear implant at normal cochlear anatomy. XII forum of otorhinolaryngologists of Russia. Proceedings of the scientific conference. 2023:98. [in Russian]
- Garcia, Charlotte et al. The Panoramic ECAP Method: Estimating Patient-Specific Patterns of Current Spread and Neural Health in Cochlear-Implant Users. University of Cambridge. 2022.
- Levin SV, Lilenko AS. Tonotopic adjustment of the auditory processor of cochlear implant at normal cochlear anatomy. Medicinskij sovet (Medical Council). 2023;7:124-31. [in Russian]
- Nicholas J. Thompson Variables Affecting Cochlear Implant Performance After Loss of Residual Hearing. The Laryngoscope. 2023; 134: 4-13.
- Philip D. Littlefield Near-infrared stimulation of the auditory nerve: A decade of progress toward an optical cochlear implant. Laryngoscope Investigative Otolaryngology. 2021; 6: 67-73.


