Comparative evaluation of the effectiveness of a local hemostatic agent modified with a bio-organic composition
Автор: Budko E.V., Yampolsky L.M., Chernikova D.A., Khabarov A.A.
Журнал: Cardiometry @cardiometry
Статья в выпуске: 18, 2021 года.
Бесплатный доступ
Constant attention to the study of the experience of using hemostatic products proposed for local bleeding arrest encourages new developments in this field. Modern hemostatics are porous multilayer systems with an inclusion of active coagulants. The results of the assessment of hemostatic activity obtained with the help of clinical and laboratory methods often do not lend themselves to cross-checking and statistical processing, and do not allow us to study objects with different physical and chemical properties. Methods of chemometrics, namely planimetry, allow you to visualize the parameters of sorption and hemocoagulation activity. A comparative planimetric study of commercial local hemostatic agents like Celox powder, sponges and napkins of various companies, zeolite powder, as well as new hemostatic compositions, which were given provisionally label A52 and A58, was carried out. It is shown that the hemostatic composition labeled as A52 leads to the activation of absorbent materials, the formation of a stable volumetric primary and secondary thrombus. A comparative evaluation of the effectiveness of a new hemostatic agent in an acute experiment shows a high expression of hemostasis (the time of primary hemostasis is 15-20 seconds) and no recurrence of bleeding for the studied samples compared to the reference.
Local hemostatic agent, hemostatic sponge, hemocoagulating activity, rabbits, venous-arterial bleeding, planimetry
Короткий адрес: https://sciup.org/148321602
IDR: 148321602 | DOI: 10.18137/cardiometry.2021.18.100112
Текст научной статьи Comparative evaluation of the effectiveness of a local hemostatic agent modified with a bio-organic composition
metry.2021.18.100112; Available from: issues/no18-may-2021/local-hemostatic-agent
According to statistical data, the need for local hemostatic agents (LHA) increases exponentially every year. Modern LHA by the mechanism of activity are vasoconstrictive and proaggregational drugs, plasma clotting factors, fibrinolysis inhibitors, aggregation and adhesion stimulators, agents that promote protein denaturation [1], as well as coagulation factor concentrators and mucoadhesive (able to remain on the mucosa) agents [2]. Hemostatic activity is observed in various classes of substances: protein compounds (collagen and gelatin); polysaccharides (cellulose, chitosan, starch and their derivatives); acrylic acid derivatives; aluminosilicate minerals; heavy metal compounds [3]. To date, complex preparations are being actively developed: matrix, fibrin and / or thrombin and combined products (matrix + fibrin and / or thrombin hemostatic agents) [4]. An example is gauze impregnated with procoagulants such as zeolite and kaolin [5], hemostatic spongy products produced by MedTrade (UK), Etikon and Z-Medica (USA), “Green Oak Grove”, Belkozin (Russia), Nycomed (Norway) etc.
Modern versions of LHA are based on the development and production of new materials, for example, polymer sponges and foams with shape memory [6], alginate calcium microspheres [7], nanostructured fibrin in agarose hydrogel [4], magnetically controlled hemostatic preparations of thrombin with gamma-Fe2O3 nanoparticles [8] and other nanoscale agents [9]. These materials differ from each other in their composition, shape, and the mechanism of action. They have shown outstanding performance and versatility compared to commercially available hemostatics, but many problems have remained still unresolved.
Recent trends are aimed at a rapid and effective hemostatic action along with a beneficial contribution to the healing and restoration of tissues. At the same time, any interference with the body's functions carries a potential risk. In particular, allergic reactions are possible as a result of exposure to exogenous collagen, thrombin and prothrombin, high exothermic reactivity and poor bio-degradability of inorganic hemostatic materials can easily cause thermal damage and inflammatory reactions [10]. Some important bio-safety issues and the high cost of new hemostatics significantly limit their medical use. The reference source [11] defines the main requirements for a modern hemostatic agent. High hemostatic activity should be accompanied by such specific characteristics as no sudden changes in pH and a temperature in the wound, bio-degradability, bio-compatibility, mechanical strength; not excessive, but sufficient swelling coefficient; apyrogenicity, stability, adjustable adhesion. Accordingly, the ideal LHA should simultaneously have the capability to quickly provide hemostasis, sound bio-compatibility, properly selected degradation; it shall exclude or avoid adverse effect on the tissues and contribute to the acceleration of the healing process. In addition, important issues of quality, production cost, stability, swelling rate, safety, and regulated recoverable volume should also be considered and addressed [11]. Efficiency, safety, usability, cost, and regulatory approval are required as the five main conditions for commercial use of a hemostatic agent [12]. Therefore, all the developments of hemostatic agents are accompanied by numerous and multi-directional studies in in vivo and in vitro formats.
In vitro experiments should mainly focus on the assessment of hemostatic activity. The mechanism of hemostatic activity is determined by measuring four main parameters of blood clotting (activated partial thromboplastin time (APTT), thrombin time (TT), prothrombin time (PT), fibrinogen content (FIB), and platelet count (PLT) [9]. The few methods of laboratory research [13] are often limited to visual examination and some other subjective criteria, depending on the experience and professionalism of the experimenter. The most effective method for comparing hemostatic activity is considered to be thromboelastography [14]. Tests are performed in contact with human donor blood after recalcification [15] or freshly collected rat arterial blood [9].
An assessment of the characteristics of the hemostatic agent, in addition, should be carried out in two more areas: the description of the technical object and its impact on the biological substrate.
We will not dwell on the need to understand the chemical structure of materials and composites. All possible methods are used here, including infrared spectroscopy [15, 16], fluorescence spectroscopy, liquid and other types of chromatography [9] etc. The scope of application of these methods includes the assessment of the main toxicity profile for plant-derived hemostatics [17], antioxidant activity [15] and other tests based on the study of chemical structures and physico-chemical interactions between them.
For LHA in the form of nanoparticles, the microstructure and morphology, and the particle size distribution are studied [7, 9]. Hemostatic sponges are evaluated by porosity (number of pores per cm2 of cross-section) and density [15]. Sponges and sorbents mainly work as aggregation and adhesion promoters, and it is desirable for them to determine the surface type of the adsorbent (hydrophobic, hydrophilic) for further evaluation of the interaction with the surgical site and the external organs. Sorption activity is more often evaluated, and swelling coefficients are calculated [6]. Micrographs allow us to visualize the processes of adhesion of blood cells to the surface of the biomaterial [15].
In our opinion, the most important properties of hemostatic products are their pH of a water extraction and heat of adsorption. They are often defined as auxiliary parameters in testing of hygroscopicity (swelling). Issues on the proper selection of the medium for an extract product are topical. Possible is use of water and blood or serum; in [16] described is a technique of application of a 0,9% sodium chloride solution and cotton seed oil. Another very important property, which is found only in a few of the products, is bio-degradability, which is assessed according to data on swelling, especially in the presence of human lysozymes [15] or blood and serum.
The following biological tests are widely used in the in vitro studies: hemolysis test [16], bio-compatibility with human tissues, for example, with human dermal fibroblasts (HDFs) [15], cytotoxicity test (for instance, in testing of gel-type materials [16,7]) and nanoparticles [9]. Antibacterial properties of hemostatic products are desirable [15,6], but they are not always achievable.
To characterize the process of hemostasis of biological objects, in vivo experiments are used: a test for skin sensitization, acute systemic toxicity (pyrogenicity) [16], toxic effects and tissue reactions [7]. And most importantly is to provide an assessment of hemostatic efficiency [16], [7], [6], [9] using acute (invasive) experiments [10, 18.].
Thus, modern LHA are, to some extent, multicomponent application systems. At the same time, the use of each of them in practical surgery has certain limitations. Improvement of clot formation and, consequently, the quality of coagulation can be achieved by
Issue 18. May 2021 | Cardiometry | 101
the following mechanisms: introduction of additional amounts of thrombotic elements, concentration of endogenous coagulation elements in the wound through rapid absorption of fluid from blood, stimulation of the non-platelet coagulation pathway. To improve the efficiency of the development and visualization of results, the method of thromboelastography is not always applicable, especially if the hemostatics are dense composites. Hemostasis is not always caused by porosity: high porosity may not ensure the formation of a blood clot. The results obtained with the help of clinical and laboratory methods often do not lend themselves to cross-checking and statistical processing (due to a small number of parallel experiments). On the other hand, a wide access to specialized computer programs allows you to use them for processing results of an analysis, makes it possible to combine various methods to assess the effectiveness of hemostasis. The development of chemometrics has led to the creation of new, more accurate methods for assessing the specific biological activity of drugs and substances [19], for example, the planimetric method, which is quite clear and accurate, has great capabilities and does not require complex equipment. JMicroVision v1. 27 is an application that allows you to calculate the exact area, for example, of the wound and sorption surface, etc. The quantitative data obtained with its use are subject to interpretation and visualization (Corol Draw, Microsoft Excel, Statistica, Mathematica, Statgraphics, etc.). Therefore, the development and subsequent verification of methods for evaluating the effectiveness of existing hemostatic drugs and the resulting new hemocoagulating compositions is a promising field in modern medical research.
Materials and methods
Research objects. Powdered preparation Celox (MedTrade, Great Britain), zeolite (size about 1 mm of the Sakhaptinskoye field of the Krasnoyarsk Territory), new bio-organic hemocoagulating compositions (developed by the Department of General and Bio-organic Chemistry at the Kursk State Medical University) under the provisional label. Hemostatic planar materials (see Figure 1): bio-degradable collagen hemostatic sponge produced by LLC “Luzhsky Zavod “Belkozin” (50,0±2,0×50,0±2,0×7,0±2,0 mm), hemostatic collagen sponge with silver produced by CJSC “Green Oak Grove” (50,0±2,0×50,0±2,0×7,0±2,0 mm), hemostatic agent “EversLife-Hemo” napkins (13x18cm), atrau- matic hemostatic dressing (PAG) non-woven with aminocaproic acid sterile “APPOLO-Hem” (6x10 cm). All materials were cut into square pieces of the same size (volume 1x1x1 cm, and planar size 1x1 cm).
Determination of the sorption rate. The time of complete absorption of 30 µl of blood into the material was measured.
Determination of the level of hematocrit. Venous blood samples were taken by venopuncture of the marginal ear vein of male Chinchilla rabbits with a body weight of 4.0 – 4.6 kg. 10 ml of venous blood was collected from each animal in tubes containing a preservative (sodium citrate). Then blood was mixed and divided into reference and experimental groups, each of them had 4 test tubes. For the convenience of determining the volume of platelet clots, we used measuring tubes with a graduated scale. After mixing, the samples were centrifuged for 30 minutes at a speed of 3000 rpm, and in the transmitted light, the height of the platelet clot was noted. To calculate the hematocrit value, the obtained data were processed using the formulas given below:
HTC = (V blood total / V sediment) * 100 %, where:
HTC is hematocrit (%);
-
V blood total is the volume of blood placed in a test tube;
-
V sediment is the volume of sediment.
After calculating the hematocrit value for each series, a graphical interpretation was performed using the Microsoft Excel software.
Planimetric studies of hemocoagulating properties. The area occupied by one drop of blood was determined at its free fall on the following items: standard carriers made of 100% cellulose (acc. to GOST R 52354-2005 filter paper and acc. to GOST R 576412017 printing paper), on nonwovens before and after their modification with dry powders. Native blood was slowly applied to each sample of planar material by the drip method (using a standardized drip gauge), waiting for the fluid movement to stop. Before applying each drop of blood, the sample was photographed, observing the perpendicular optical axis. To calculate the area of the run-up zones, photos of the samples were uploaded to the JMicroVision v1.27 software application; the area of the blood run-up zones was determined. When studying the effect of powders on the absorbency at the preparatory stage, the same weight lots (0.3-0.5 g) of dry hemocoagulating substances and compositions were weighed. Then the suspended
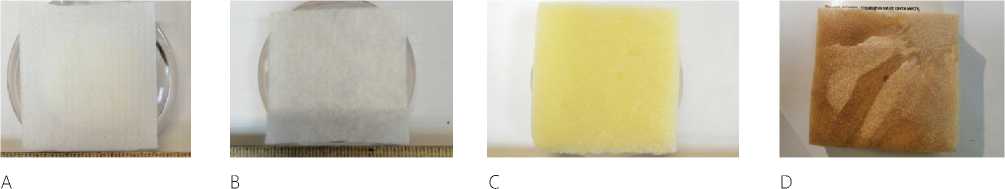
Figure 1. Planar hemostatic materials: cellulose according to GOST R 52354-2005 (A), non-woven bandage PAG “APOLLO-Gem” (B), hemostatic sponge produced by LLC “Luzhsky Plant “Belkozin” (C), hemostatic sponge produced by CJSC “Green Oak Grove” (D)
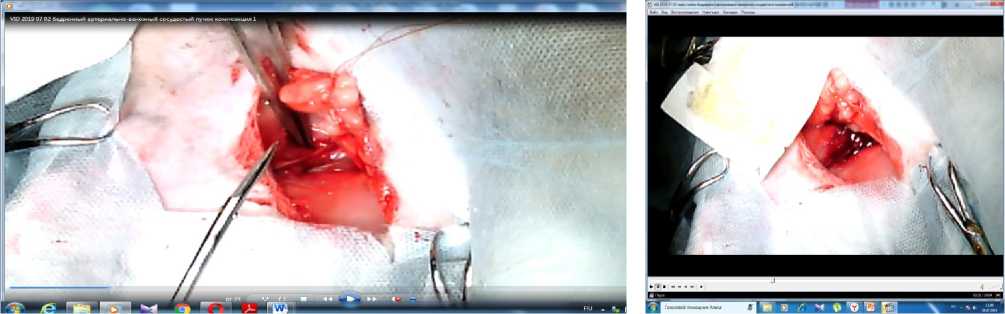
Figure 2. External view of the wound in the groin area of the rabbit before excision of the arteriovenous bundle (A) and after it with intense arteriovenous bleeding (B)
weights of substances and compositions were placed evenly over the area of the lower layer of the carrier.
An experiment on animals. To evaluate the effectiveness of a new hemostatic composition on a model of a stab wound in an acute experiment, the authors ' team based on the developments of N. Alam (2003) proposed his own method for evaluating the effectiveness of local application hemostatics with subsequent dynamic observation of surviving animals. The essence of the method is to record the dynamics of blood pressure and heart rate (HR) values in animals in the absence of the stage of wound revision for animals of both groups with their subsequent mandatory euthanasia.
For the experiment, 10 male Chinchilla rabbits with a body weight of 4.0 - 4.6 kg were selected. The experiment was performed in a veterinary operating room at the laboratory of “Experimental Surgery and Oncology” of the Research Institute of EM at the Kursk State Medical University in compliance with the requirements of the local Ethics Committee and the applicable Helsinki Declaration on Animal Experimentation. In the course of the study, in accordance with the ethical requirements of the Helsinki Declaration, only those animals whose condition was assessed as critical by the operating surgeon were removed from the experiment.
At the preparatory stage, the experimental animals were divided into two groups (the reference group and the experimental group) and deprived of food with free access to water. Each group consisted of 5 animals. As a method of general anesthesia, endotracheal anesthesia with “Isoflurane” was chosen at a dosage calculated based on the weight of each animal and the duration of the operation (on average, 4 ml per rabbit to provide anesthesia for 40 minutes) [20]. For invasive monitoring of blood pressure, a 5Fr introducer was installed in the carotid artery. Liquid media were infused through a 6Fr catheter into the external jugular vein of the animal [21]. A scalpel was applied to an oblique linear incision 5 cm long in the upper third of the right thigh below the inguinal fold in the projection of the femoral artery. After cutting the skin in a blunt and sharp way, the femoral artery and the femoral vein were isolated from the muscles for 2-3 cm (Figure 2), which was simultaneously excised by a transverse incision, resulting in intense arteriovenous bleeding. After a latent period of 30 seconds, each animal lost 1/3 of the BCC, which caused a decrease in blood pressure by 25 mmHg.
Before applying hemostatic agents to the wound surface, the wound bed was drained with sterile wipes to determine the volume of blood loss. After that, a prototype LHA or a standard reference agent (a bio-degradable hemostatic collagen sponge produced by the Luzhsky BELKOZIN Plant) was applied and tamponed into the wound. Finger tamponade was performed for 1 minute. If necessary, the tamponade was repeated up to 3-5 operations, each for no more than 1 minute. Simultaneously with the temporary stop of bleeding, the lost BCC was replenished with 0.9% sodium chloride solution. After 1 minute, tamponade was stopped, and the result was evaluated. When the bleeding resumed, the LHA was re-compressed into the wound for 1 minute. A similar scheme of actions was performed on each animal as needed, until the complete stop of arteriovenous bleeding was achieved (on average, 3-5 procedural operations) [22]. Blood pressure and heart rate readings were recorded throughout all stages of the operational procedure. After achieving hemostasis, the LHA was left in the wound bed or sewn to the edges of the wound if additional fixation was necessary. Then the skin was sutured, and each animal was transferred to a separate cage, where it was observed for 3 hours.
The general condition of the animal was evaluated, namely, the wound area was subjected to visual examination with an assessment of the flow of blood through the stitches in the wound. The evaluation of the effectiveness of LHA was carried out according to the following indicators: the number of animals that managed to achieve primary hemostasis; the volume of blood loss and survival. The entire study was recorded using video recording technique, photographs, and blood pressure and heart rate readings recorded throughout all stages of the operation, using the MR-150 BIOPACK.
Study of the heat of dissolution. The tested liquids (water, blood) were sequentially introduced into the calorimeter measuring vessel, and the system equilibrium temperature was recorded. The temperature of the media was 250C. Then the samples of the studied compositions were added within the range of mass ratios from 0.05 to 0.5 to 1 g of liquid, and the temperature of the mixture was measured at regular intervals until its change became insignificant (thermodynamic equilibrium was established). The temperature increase corresponded to the heat of dissolution of the sample in question.
Determination of the acidity of media. The pH values of the solutions were studied using standard laboratory ionometer ETAN (Tom Analyte, Russia).
104 | Cardiometry | Issue 18. May 2021
The measurement was carried out with the magnetic stirrer switched on until the pH values were stabilized. The test liquids (water or blood) were successively poured into a measuring cup, the initial pH value was fixed, then a sample of the composition was added and mixed until they were dissolved and parameters stabilized, and the pH value was measured again. The temperature of the media was 250C.
To measure the mass, we used laboratory electronic scales VK-150.1 (manufactured by JSC “MASSA-K”, Russia), accuracy class II (acc. to GOST R 53228-2008), the absolute error of weighing was (± 5 - ± 10 mg).
In the course of the study, purified water was used (water distillation system AE-5 “LivaM”, Russia).
A mechanical pipetator was used as a standardized drop gauge.
Every measuring procedure was performed in at least 3 times.
Results and discussion
We have selected various porous materials and powders as the objects of our research. The area of a single drop of blood, as well as the rate of absorption of blood depends on the adhesive properties of the surface and its absorbency. The minimum suction time was shown by loose materials: paper, a dry Ever-sLife-Gemo napkin, and the maximum suction time was reported for the Green Oak Grove sponge.
When applying a drop of blood to a cellulose carrier acc. to GOST R 52354-2005 (filter paper), it is absorbed in the area occupied by a free-falling drop and then the blood penetrates into the adjacent and underlying layers of the carrier (see Figure 3). Replacing the cellulose carrier with a non-woven material or a porous sponge logically leads to a decrease in the area and an increase in the absorption time.
When applying powders between the layers of the cellulose carrier, the capability to move blood along the plane of the carrier is sharply reduced. We can only partially to talk about the sorption of the liquid part of blood due to the properties of granules/crystals. An introduction of sorbent powders (Celox, zeolite) leads to a reduction in the area, but the new hemocoagulating compositions of a powdery form under the temporary labels A52 and A58 do not have the sorption capacity, create an almost “non-absorbent” surface (see Figure 3B). In this case, the introduction of powders of acetylsalicylic acid and hexamethylenetetramine (blood-thinning substances) leads to blood spreading
А
C
E

B

D

F
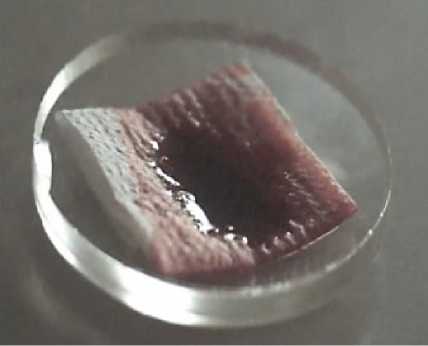
Figure 3. The increase in the area occupied by the first (A, B) and seventh (C, D) drops of native blood (reference A, B on the cellulose carrier acc. to GOST R 52354-2005 (filter paper) and after applying hemostatic powder A52 – B, D between the layers of the cellulose carrier); E, F – the reference and the test sample on a glass substrate
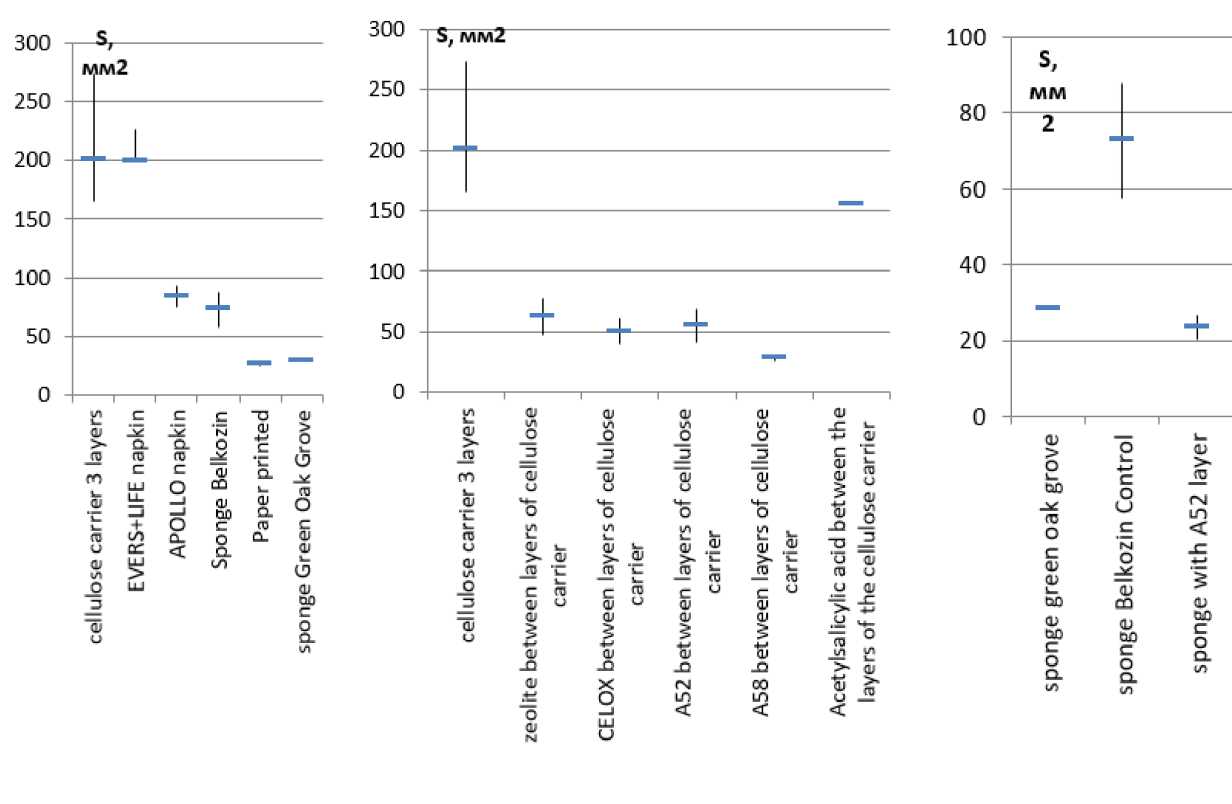
Figure 4. The average area (indicating scattering of values) occupied by 1 drop (30 µl) of blood on surfaces with different absorbency and hemocoagulation capacity. The lines show the range and the average of the obtained values.
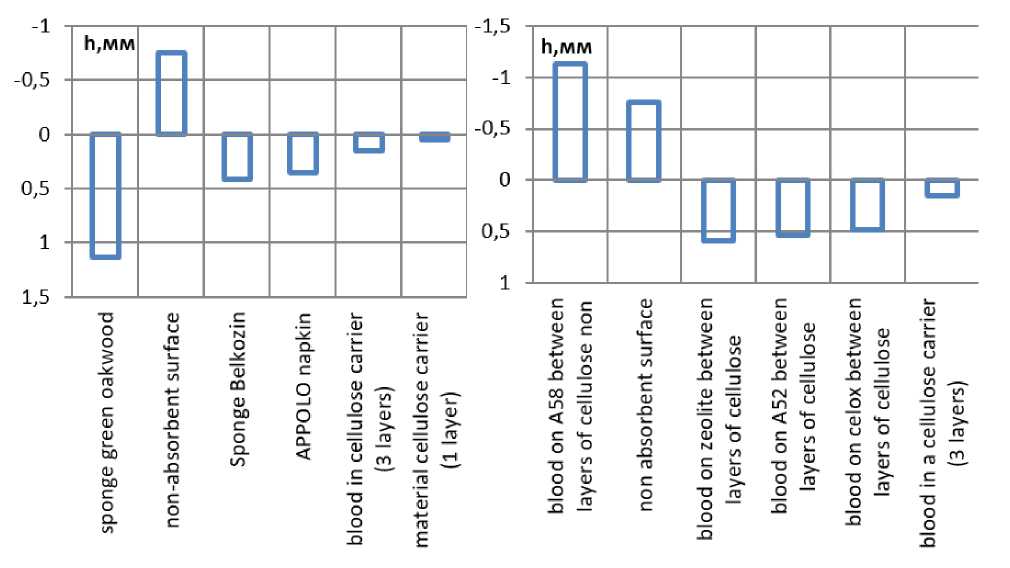
Figure 5. The height of the virtual cylinder of the 1st drop of blood in materials with different absorbency (“+”- absorbent, non-absorbent material)
“-” -
over the surface. Volumetric materials (sponges) help to reduce the area occupied by the first drop of blood, but when introducing powders between their layers, the drop area decreases (see Figure 4) comparable to the experience with thin layers.
If the volume occupied by a drop of blood is represented in the form of a virtual cylinder proportional to the area occupied by this drop, then a comparison of the heights of the cylinders in different porous systems allows us to estimate the “depth” of blood penetration into the material (see Figure 5). Based on Figure 3, the first drop on a thin cellulose carrier occupies the maximum area, being distributed in a single absorbent volume. The heights of the cylinders calculated by experimental and theoretical values (Figure 5 cellulose carrier in 1 and 3 layers) are comparable that confirms the reasoning. Napkins made of non-woven material are soaked to the entire thickness of the material, which we take for the height of the cylinders. The sponge does not get wet to the full depth from a single drop of blood, and its surface is heterogeneous, the formation of the height of the virtual cylinder in the sponge is influenced by multidirectional factors, but the obtained values for the porous system of the Belkozin sponge significantly differ from both a well-absorbing surface and a non-absorbing one. Due to the component composition of the sponge “Green Oak”, the blood penetrates deeply into its body, with- 106 | Cardiometry | Issue 18. May 2021
out spreading horizontally. At the same time, the sponge quickly gets wet in the place of contact with the blood, remaining dry in the rest of the area. The values of the heights of the virtual blood cylinders in the cellulose carrier after its modification are specifically located. The apparent height of the cylinder is much greater than the thickness of the pulp layer and even the apparent height of the cylinder in the sponge. In terms of the range of height values, the cellulose modified by hemostatics differs little. The virtual cylinder of the composition A58 is located in the negative region, since the blood formed a stable drop that is practically not absorbed by the cellulose carrier.
The kinetics of the increase in area with an increase in the number of blood drops was studied planimetri-cally. The increase in the area on the cellulose carrier acc. to GOST R 52354-2005 (filter paper), the Belkoz-in sponge and the non-woven napkin was expected to be observed in an almost linear relationship. When placing hemocoalating powders between the carrier layers, the area of the zone soaked in blood is stabilized by adding a certain number of drops (see Figure 3 herein). The number of blood drops forming a “stable zone” depends on the activity of the powder: the greater the sorption-coagulating ability it has, the less the area of the blood drop on the outer layer of the carrier changes. The type of kinetic curve depends on the mechanism of powder activity: an almost lin-
Table 1.
Results of planimetric estimation of the area of the blood zone on the film carrier when introducing powders with hemocoagulating activity Celox and composition A52 between its layers
|
Blood drop number |
Average area of the blood run-up zone, mm2 |
The Mann-Whitney test criterion* |
Blood drop number |
Average area of the blood run-up zone, mm2 |
The Mann-Whitney test criterion* |
|
Сelox |
Composition А52 |
||||
|
1 |
58,9 |
1 |
119,2 |
||
|
2 |
104,75 |
0 p≤ 0.01** |
2 |
204,8 |
0 p≤ 0.01** |
|
3 |
142,4 |
4 p≤ 0.01** |
3 |
191,3 |
0 p≤ 0.01** |
|
4 |
138,4 |
0.05≤33p≤ 0.01*** |
4 |
197,8 |
65 p≥ 0.05 |
|
5 |
138.1 |
69 p ≥ 0.05 |
5 |
193,5 |
0.05≤34p≤ 0.01*** |
|
6 |
135,7 |
2,5 p≤ 0.01** |
6 |
193,2 |
72 p≥ 0.05 |
|
7 |
271,9 |
0 p≤ 0.01** |
7 |
193,7 |
72 p≥ 0.05 |
* Compared to the previous drop, ** The data is in the area of significance, *** The data is in an area of uncertainty,
**** The data is in the area of insignificance
ear increase in area was observed when zeolite powder was introduced into the carrier, but if Celox was placed between the layers of the absorbent carrier, the curve became stepwise. An almost linear increase in the area was observed when the A58 composition was introduced into the carrier, while the A52 composition led to the formation of a step graph. The planimetric method made it possible to conduct a comparative analysis of the hemocoagulating and sorption properties of 6 objects in 4-12 series of experiments without resorting to in vivo methods. The reliability of the quantitative data obtained in the course of the study was confirmed by statistical processing using the nonparametric Man-Whitney test (Table 1).
To test the capability of the powders to hold the blood flow, their identical masses were placed in measuring cylinders, compacted, and the same volumes of blood were placed on top. Figure 6 shows that A52, A58 and zeolite absorb blood. Celox is not saturated with blood, but when the closing valve is removed in the measuring cylinders, Celox continues to create a plug, which is well comparable with the experience of using Celox series hemostatics [2]. The zeolite has passed through blood and has been washed away by the stream that also corresponds to the recommendations for the use of zeolites in the composition of dressings and other carriers. In contrast to Celox, the composition A 52 retains a column of blood despite getting wet, and the composition A 58 in the form of a gel clot has been washed out of the cylinder. This experiment visualizes the behavior of powders at excess and average blood pressure, the result of the experiment is well comparable with conclusions of many authors. The observed processes are associated with various mechanisms of coagulation processes of the studied powdered hemostatics.
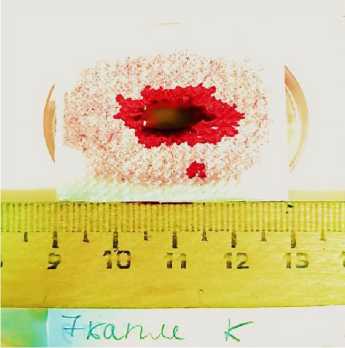
Hematocrit was studied in blood of animals. Its value for the reference samples was 37.5-39.0 %. The powders were injected into the blood samples and mixed, sponges and napkins were placed in test tubes before blood was injected, and the samples were centrifuged. When zeolite was injected into the blood sample, the hematocrit value did not change, while blood was separated into three fractions (platelet clot, suprathrom-bic fluid, and powder layer). When Celox powder was injected into blood, the entire volume of blood formed a primary blood clot, which was not stable with time (Figure 4 A, B). The increase in hematocrit to 100% in all samples was revealed due to the capability of the drug to swell as a result of the absorption of the liquid part of blood. The developed compositions A52 and A58 initiated stable blood coagulation (Figure 4 C, D), and the hematocrit level reached 64.7%. The difference between them was in the amount of suprathrombic serum released and the degree of its transparency. The resulting clots thickened with time. The suprathrom-bic serum released during coagulation was examined for the protein content. For this purpose, qualitative reactions were performed according to known methods. Negative test results showed the almost complete absence of peptides over the clot.
One of the most significant characteristics of LHA is the heat of absorption and the change in the pH value of the wound. Acidification of the medium is a negative factor when using oxidized cellulose, heavy metal salts, etc. Commercial LHA products eliminate this disadvantage by introducing neutralizing components. To evaluate the new hemostatic compositions, the pH values of blood and the aqueous medium were studied depending on the mass ratios of the composition and the liquid. As can be seen from the graph

A
B
C
Figure 6. Measuring cylinders with hemostatic powders (0.3 g) before (A) and after (B) filling with blood (3 ml). In the measuring cylinders, the closing valve is removed (В).

A

B CD
Figure 7. Appearance of platelet clots (immediately after centrifugation and after a day) obtained by adding Celox powders (A,B) and composition A52 (C,D) to native blood
(Figure 8), the medium is acidified, but the experimental data in water and blood do not coincide with each other and with the calculated pH values. Lower pH values were not detected due to a deep change in the structure and properties of blood (coagulation).
The heating of the medium accompanies the absorption of liquid by the solid body, and it was the main problem of the first LHA product lines like Qui-kClot, Celox, and other brands using zeolite and chitosan. Modern commercial LHA products are almost free of this drawback. For the new hemostatic composition A52, changes in the enthalpy gain in water and in blood were studied. The transition of the enthalpy gain (dH) through 0 with a change in the mass ratios indicates the absence of an exothermic effect in the optimal state of the system for the hemostatic effect (see Figure 8 herein).
To test the effectiveness of the new hemostatic composition A52 in an acute experiment, a method for evaluating the effectiveness of local application hemostatics with subsequent dynamic observation of surviving animals is proposed. A bio-degradable hemostatic collagen sponge produced by the Luga BELKOZIN Plant was used as a reference sample. The composition A52 was placed between the layers of the same sponge, taking into account the planar and volumetric dimensions.
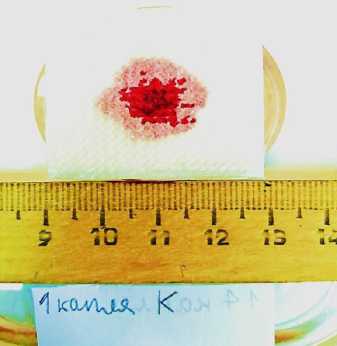
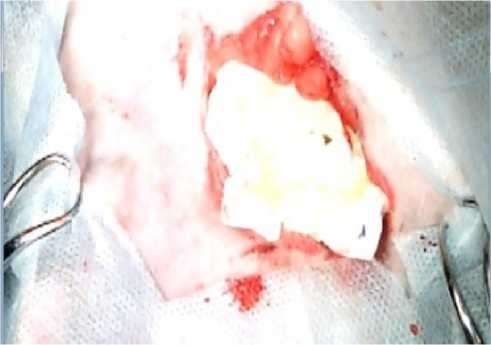
The visual and parametric examination of the onset of primary hemostasis was performed by our operating surgeon according to the following signs: tight fit of LHA to the excised vascular bundle; no need for an additional fixation of LHA in the wound bed (suturing, etc.); no leakage of blood under LHA into the wound bed with its subsequent filling; achievement of primary hemostasis by 1 LHA unit; time of onset of primary hemostasis less than 1-1.5 minutes; restoration of the initial indications of blood pressure and heart rate after installing the LHA in the wound bed; no relapses of postoperative bleeding through sutures applied to the wound. When using the A52 composition to stop bleeding, primary hemostasis was achieved within 15-20 seconds in all animals of the experimental group (Figure 9, 10). All test parameters fully corresponded to the onset of primary hemostasis. All the animals from the experimental group were able to stand up 30 to 40 minutes after the operation, and no blood leaking through the sutures was observed in any of the animals in the group. Within 3 hours of the observation, all the animals of the experimental group remained alive.
In contrast to the experimental group, the reference animals required additional suturing of several (on average 3 – 4) units of bio-degradable hemostatic collagen sponge to achieve primary hemostasis. After
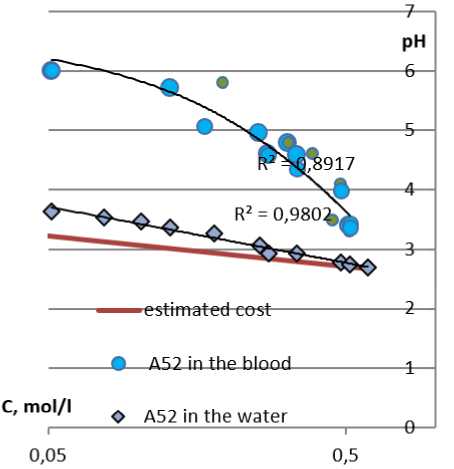
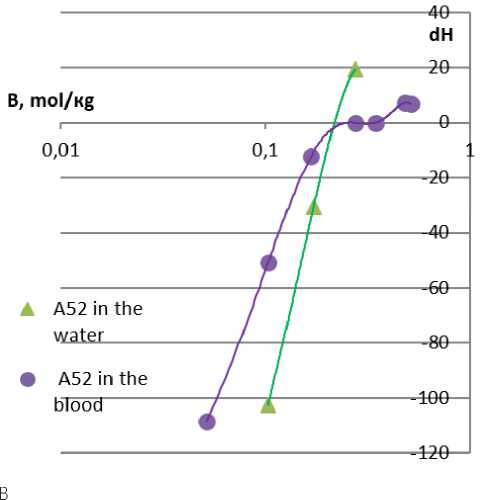
Figure 8. Results of correlation changes in acidity and heat effect when the composition A 52 is introduced into water and blood
A
Figure 9. Appearance of the wound after applying the sponge with the new hemostatic composition A52: immediately after application (A) and 20 seconds after application (B)
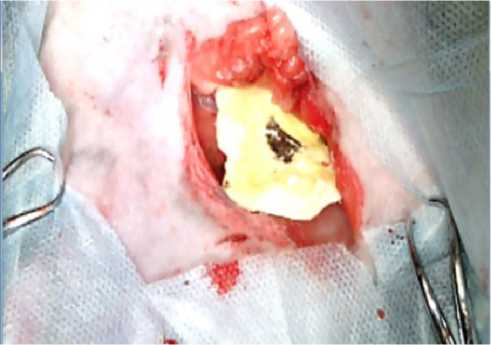
B
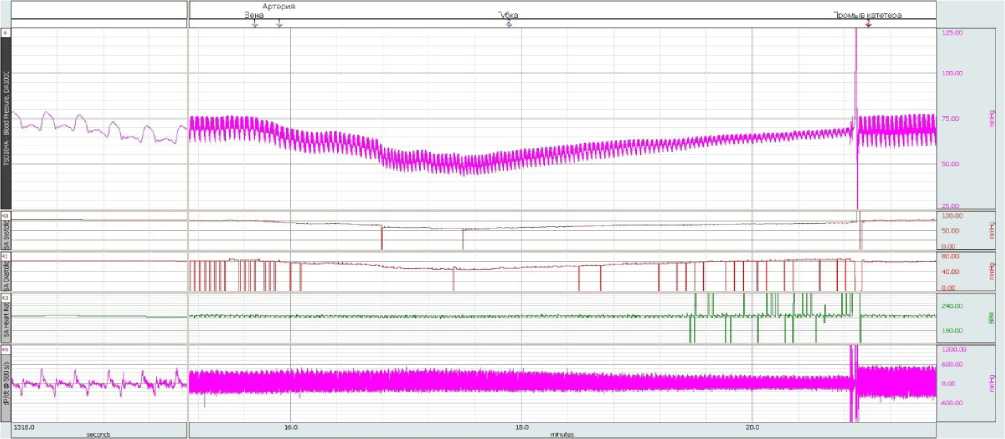
Figure 10. Dynamics of blood pressure readings and heart rate at all stages of the experimental study of the new hemostatic com-
position A52
Table 2.
Evaluation of the effectiveness of achieving hemostasis in the studied animals
Conclusion
Our analysis of scientific publications on the problem under consideration demonstrates the constant attention to the study of the experience of using hemostatic products proposed for local bleeding arrest, as well as to new developments in this field. Most LHA products are combined, and their hemostatic activity is synergistic, but it is always based on the capability to form mesh matrix structures and the possibility of forming ionic complexes, which leads to an effective adhesion of third-party macromolecules. Dehumidification of the impact site leads to an increase in the viscosity of the adjacent liquid and the concentration of coagulation factors. These characteristics bring together napkins and sponges, sorbents, aluminosilicates and polymers of protein nature, cellulose and chitosan materials. For the comparative in vitro analysis of powders and planar systems with hemostatic activity, we used the method of planimetric analysis employing cellulose carriers, which allowed us to visualize the parameters of the sorption and hemocoagulating activity of powders, sponges and napkins. Comparative planimetric analysis shows that Celox powder retains quite large volumes of blood, due to swelling and creating a plug. Zeolites absorb the liquid with the entire mass and when the mass ratio is exceeded in favor of blood, they do not cope with hemostasis. Sponges and wipes are blotted according to the absorbed volume, and the introduction of a he- mostatic agent into the volume of the porous material reduces the effectiveness of the material. However, the introduction of the new hemostatic compositions under the provisional label A52 and A58 leads to the activation of absorbent materials, the formation of a stable volumetric primary and secondary blood clot. A comparative evaluation of the effectiveness of a new hemostatic agent in an acute experiment shows a high expression of hemostasis (the time of primary hemostasis is 15-20 seconds), and there is no recurrence of bleeding for the studied samples compared to the reference samples.
Acknowledgements
The authors thank the veterinarian Mikhailov K. A. for his assistance in developing the research methodology and performing operations on animals, and the staff of the Research Institute of EM KSMU for providing the recording of the dynamics of blood pressure and heart rate readings, among them Senior Researcher Gladchenko M. P. and Naimzada M. D. Z for providing anesthesia of animals.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Comparative evaluation of the effectiveness of a local hemostatic agent modified with a bio-organic composition
- Belozerskaya GG, Makarov VA, Zhidkov EA, Malykhina LS, Sergeeva OA, Ter-Arutyunyants AA, Makarova LV. Hemostatic means of local action (review). Chemico-pharmaceutical journal. 2006(40;7):9-15. [in Russian]
- Samokhvalov IM, Golovko KP, Reva VA, Denisov AV, Sokhranov MV, Zhabin AV, Kaznacheev MV. Application of local hemostatic agent “Celox” in an experimental model of massive mixed external bleeding. Bulletin of the Russian Military Medical Academy. 2013(4;44):187-191. [in Russian]
- Budko EV, Chernikova DA, Yampolsky LM, Yatsyuk VY. Local hemostatic means and ways of their improvement. Russian Medico-biological Bulletin named after Academician Pavlov at the Ryazan State Medical University. 2019(27;2):274-285. [in Russian]
- Campos-Cuerva R, Fernandez-Munoz B, Farfan Lopez F, Pereira Arenas S, Santos-Gonzalez M, Lopez-Navas L, Alaminos M, Campos A, Muntane J, Cepeda-Franco S, Gomez-Bravo MA. Nanostructured fibrin-agarose hydrogel as a new hemostatic agent. Journal of Tissue Engineering and Regenerative Medicine. 2019(13;4):664-673. DOI: 10.1002/term.2831
- Yu L, Zhang X, Chen H, Xiao L, Zhu Y, Fan J. Tightly bound and flexible mesoporous zeolite-cotton hybrid hemostat. Nature Communications. 2019(10;1):1932. DOI: 10.1038/s41467-019-09849-9
- Landsman TL, Touchet T, Hasan SM, Smith C, Russell B, Rivera J, Maitland DJ, Cosgriff-Hernandez E. A shape memory foam composite with increased fluid absorption and bactericidal properties as a hemostatic agent. Acta biomaterialiau. 2016(47):91-99. DOI: 10.1016/j.actbio.2016.10.008
- Fengqi X, Jingjing R, Ming L, Xuwen Z, Jingyang S, Lijun Z, Yang L, Dan L, Fei L, Xiaozeng W, Yaling H. Evaluation of the biocompatibility and effectiveness of a new hemostatic embolizing agent: a thrombin-loaded alginate calcium microsphere. BioMed Research International. 2017(2017): 1875258. DOI: 10.1155/2017/1875258
- Shabanova EM, Drozdov AS, Fahardo AF, Dudanov IP, Kovalchuk MS, Vinogradov VV. Nanoparticles of thrombin 2Fe3O4 for use as a hemostatic agent in internal bleeding. Scientific reports. 2018(8;1):233. DOI: 10.1038/s41598-017-18665-4
- Zhao Y, Zhang Y, Kong H, Zhang M, Cheng J, Luo J, Zhao Y, Qu H. Hemostatic nanoparticle - Derived bioactivity from Selaginella tamariscina Carbonisata. Molecules (Basel, Switzerland). 2020(25;3). DOI: 10.3390/molecules25030446
- Snyder D, Tsou A, Scholes K. The effectiveness of prehospital use of tourniquets and hemostatic dressings for the control of traumatic external hemorrhage. Washington, DC: National Highway Traffic Safety Administration, 2014.
- Li D, Chen J, Wang X, Zhang M, Li C, Zhou J. Recent advances in synthetic and polysaccharide adhesives for biological hemostatic applications. Frontiers of Bioengineering and Biotechnology. 2020(8):26. DOI: 10.3389/fbioe.2020.00926
- Spotnitz WD, Burks S. State-of-the-art review: hemostats, sealants, and adhesives II: update as well as how and when to use the components of the surgical toolbox. Clinical and Applied Thrombosis/Hemostasis. 2010(16):497-514. DOI: 10.1177/1076029610363589
- Yuldashev ZM, Bibicheva YuG, Anisimov AA, Glazova AYu. Noninvasive assessment of hematocrit level by spectrophotometric method. Medical equipment. 2014(2;284):12-15.
- Xi C, Zhu L, Zhuang Y, Wang S, Sun G, Liu Y, Wang D. Experimental evaluation of porous starch loaded with Tranexamic acid as a hemostatic powder. Clinical and Applied Thrombosis/Hemostasis. 2018(24;2):279-286. DOI: 10.1177/1076029617716770
- Praglovskaya R, Pietkovsky M, Deineka V, Janus L, Kornienko V, Gusak E, Golubnicha V, Lyubchak I, Zhurba V, Serakovskaya A, Pogorelov M, Bogdal D. Biologically active hemostatic agents based on chitosan with antibacterial properties-Synthesis and characterization. Molecules (Basel, Switzerland). 2019(24;14). DOI: 10.3390/molecules24142629
- Wu Y, Wang F, Huang Y. Comparative evaluation of biological characteristics, biosecurity, and availability of cellulose-based absorbable hemostats. Clinical and Applied thrombosis/Hemostasis. 2018(24;4):566-574. DOI: 10.1177/1076029617751177
- Koluman A, Nejat A, Umit Y Malkan, Ibrahim S. Khaznedaroglu. Qualitative / chemical analysis of Ancaferd hemostat and its antioxidant content in synthetic gastric fluids. BioMed Research International. 2016(2016):8957820. DOI: 10.1155/2016/8957820
- Samokhvalov IM, Reva VA, Denisov AV, Telitsky SYu, Adamenko VN, Churkin AA, Yudin AB, Yablokov IP.Comparative assessment of the effectiveness and safety of local hemostatic agents in the experiment. Military Medical Journal. 2017(338;2):18-24. [in Russian]
- Gabrielsson J, Lindberg N-O, Lundstedt T., Hemm J. Multidimensional methods in pharmaceutical applications. Journal of Chemometrics. 2002(16):141-160.
- Yunlong L, Hui L, Liping X, Lin Z, Jianzhong S, Xumin Z, Jie F. Hemostatic efficacy and wound-healing properties of natural zeolite granules on a lethal rabbit model of complex groin injury. Materials. 2012(5;12):2586-2596. DOI: https://doi.org/10.3390/ma5122586
- Tatarevich EYu, Lebedev AO, Kulazhin OA. Comparative effectiveness of Russian and foreign drugs for stopping external bleeding on the basis of mineral sorbents. Emergency Doctor. 2016(2): 1-10. [in Russian]
- Hao C, Xiaoqiang S, Lisha Y, Liping X, Jie F. Safety assessment of a low-heat zeolite granular hemostatic dressing in a rabbit femoral artery hemorrhage model. Journal of Biomaterial Applications. 2020(34;7):988-997. DOI: 10.1177/0885328219888626 [in Russian]


