Comparison of totally automated and manual processing for calculation of left ventricular volumes and ejection fraction using gated myocardial perfusion spect
Автор: Al-shammari A. F., Afzal U.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 22, 2022 года.
Бесплатный доступ
ated single-photon emission computed tomography (SPECT) allows for the assessment of myocardial left ventricular ejection fraction and left ventricular volumes. There is conflicting data regarding the difference between automated and manual processing of gated myocardial SPECT images. The purpose of this retrospective “cross-sectional study is to compare the degree of variability between automated and manual processing of Quantitative Gated SPECT algorithms for assessing left ventricular volumes and ejection percent”. Study was carried out in the Nuclear Medicine department “at the Security Forces Hospital, Riyadh, Saudi Arabia, and comprised of 96 participants who “underwent” both stress and “rest gated” myocardial perfusion “imaging” (MPI) from February to May 2021. Data were analyzed “using SPSS for Windows (version 22; IBM Corp., Armonk, NY, USA). The mean of EF, ESV, and EDV on stress test using the automatic technique were 62.46 ± 14.62%, 45.00 ± 37.10 ml, and 107.01 ± 43.70 ml, respectively while using the manual technique were 57.21±14.80%, 44.35±35.93 ml, and 93.26±39.14 ml, respectively. The mean of EF, ESV, and EDV on rest test using the automatic technique were 62.85± 15.47%, 43.44± 36.35 ml, and 104.27±42.51 ml, respectively while using the manual technique were 57.74±15.33%, 43.30±36.42 ml, and 91.72±41.50 ml, respectively. For LVEF and EDV, the difference between automated and manual techniques is statistically significant (p0.05). Discrepancies were observed that exist by using the fully automated and manual technique for determining LVEF and Left ventricular volumes by gated MPI.
Gated spect, processing method, left ventricular ejection fraction, left ventricular volumes, myocardial perfusion
Короткий адрес: https://sciup.org/148324587
IDR: 148324587 | DOI: 10.18137/cardiometry.2022.22.136142
Текст научной статьи Comparison of totally automated and manual processing for calculation of left ventricular volumes and ejection fraction using gated myocardial perfusion spect
Amirah Fahad Al-Shammari, Uzma Afzal. Comparison of totally automated and manual processing for calculation of left ventricular volumes and ejection fraction using gated myocardial perfusion SPECT. Cardiometry; Issue 22; May 2022; p. 136142; DOI: 10.18137/cardiometry.2022.22.136142; Available from:
The most often utilized basic metrics to estimate global LV function for therapy, clinical follow-up, and epidemiology are left ventricular (LV) volume and ejection fraction (EF) 5 . End diastolic and end-systolic volumes (EDV/ESV) and left ventricular (LV) ejection fraction (EF) are regarded as critical parameters in the clinical management of heart disease 29,30 . Determining suitable threshold levels is critical for defining clinical relevance.
A variety of variables influence the accuracy of ventricular volume and EF estimation. Any considerable change in LVEF from baseline (a drop of 10%) is an early signal of heart failure and may precede any symptoms of cardiac illness 3-6. Technological advancements resulted in the emergence of automatic and semi-automatic processing modes and the growth of diverse processing software. However, these advancements raised the possibility of variance in estimating the LVEF 14-16 . Once the gated image is acquired, algorithms and software are used to determine the LVEF from the left ventricle at end-systole and end-diastole regions of interest 12-13 . In patients with coronary artery disease and decreased left ventricular function, LVEF and LV volume values predict survival 21–24 . Identifying people at risk of having a cardiac event is thus the cornerstone of non-invasive examination of patients with reduced LV function 25,26 . Gender substantially affects normal LVEF readings, whereas age and body weight have a lower effect 28 . Left ventricular ejection fraction and LV chamber sizes should be assessed qualitatively and objectively 27 . Since serial monitoring of LVEF fluctuations can alter patient treatment and administration, the computation of
LVEF must be accurate, reproducible, and repeatable 12 . The SPECT is considered as the best detection for cardiac failure 1 . Other modalities to detect cardiac disease are cardiac MRI or “a planar multi-gated cardiac blood pool acquisition (MUGA) 2. However, the CMR has the same significance as SPECT scan 3 .
Several authors have published algorithms and validation studies for automated and semi-automated chamber segmentation, regional mapping, and quantification of global myocardial function from SPECT datasets 17 . The quantification of SPECT is dependent on myocardial radioactivity count densities, which may be altered by attenuation 28 . Visualization of ventricular perfusion in any plane has been made possible by SPECT. SPECT has enabled the mapping of the function of the overall left ventricular myocardium into single images to produce ungated three– dimensional and gated four-dimensional images and calculate the volumes of the chambers 17.
Most software systems calculated LVEF by gated SPECT and reported in women as much higher than in males, maybe because women’s hearts are smaller than models used and there are more inaccuracies or physiological differences. Normal, slightly, moderately, or severely increased volume can be classified 28 .
Furthermore, leading to further recent developments, automated quantitation remains an important field of research. New software, for example, that evaluates automated LV contours for possible errors has been demonstrated to minimize the degree of human monitoring. Recent generations of nuclear medicine computer systems have significantly lowered the overall processing interval of gated SPECT scans with quicker processing speeds. Algorithms can automatically execute gated SPECT data filtering, restoration, and reorientation, decreasing or excluding the requirements for operator intervention 33 . This automation enables the batch processing of many gated experiments, reducing the time necessary to generate final images and enhancing the consistency of results. Software programs that automatically calculate endocardial and epicardial surfaces from data in each gating interval display these surfaces in cinematic mode as contours overlaid on images in two or three dimensions and derive global ejection fraction values from end-systolic and end-diastolic intervals save time (and increase accuracy).
Another exciting advancement has been the application of machine learning to integrate automated imaging parameters with clinical data for improved clinical diagnosis and individualized prediction of prognostic results 18,19. On the other hand, one research approved that the development increased the variation in determining the cardiac volume and LVEF. However, other research proves that there are no significant differences between automated and manual processing, but on the contrary, it saves time for workers 4. In addition, rapid detection of variations allows for increased surveillance to prevent subsequent problems, implement preventative measures, or identify changes in therapies or patient care 10-11.
This study aimed to investigate the substantial differences in “cardiac” volume (end-systolic and end-diastolic volume) and EF depending on whether the processing was automated or manual.
Materials and Methods
Study Population
A retrospective cross-sectional study was carried out in the Nuclear Medicine department at the Security Forces hospital located in Riyadh, Saudi Arabia. The total number of patients included in the study was 96 who underwent both “stress and rest” gated myocardial “perfusion imaging” (MPI) as per the 2-day protocol from February till May 2021. Imaging was performed on Siemens E-cam and Symbia SPECT systems. Ejection fraction (EF), end-diastolic volume (EDV), and end-systolic volume (ESV) were first” processed totally automatically, followed by manually modifying above mentioned parameters using Cedar-Sinai QGS software.
Ethical consideration
The research was carried out in accordance with the principles described in the Helsinki Declaration. All processes were carried out in accordance with the applicable norms and legislation.
Statistical Analysis
Data were analyzed using “SPSS for Windows (version 22; IBM Corp., Armonk, NY, USA). The values are given as mean SD. P-values of 0.05 are regarded as statistically significant. The coefficient of variance (CV) was determined by dividing the standard deviation of the difference between data by their mean. The t-test was used to investigate differences in mean difference.
Results
Ninety-six patients were scanned in the period from February to May 2021. Demographics and clinical data are demonstrated in ‘Table 1’. The mean age is 61.1 and the range is from 51 to 71. The cardiac disease is more in males is 64.6% and the female about 35.4%. Because the majority of the patients have risk factors for heart disease such as “diabetes mellitus, hypertension, dyslipidemia”, smoking, and a family medical history of cardiac disease, we conducted an analysis to retrieve the risk factors that impact the study’s results and observed that hypertension is the most common risk factor for cardiac disease (77.1 percent).
The mean of EF, ESV, EDV on stress test using the automatic technique were 62.46 ± 14.62%, 45.00 ± 37.10ml, and 107.01 ± 43.70 ml, respectively. The mean of EF, ESV, EDV on stress test using the manual technique were 57.21±14.80%, 44.35±35.93ml, and 93.26±39.14ml, respectively.
The mean of EF, ESV, EDV on the rest test using the automatic technique were 62.85± 15.47%, 43.44± 36.35ml, and 104.27±42.51ml, respectively. The mean of EF, ESV, EDV on the rest test using the manual technique were 57.74±15.33%, 43.30±36.42ml, and 91.72±41.50ml, respectively.
We classified the patients according to documented findings on stress test into normal and abnormal. Comparison between automatic and manual techniques for patients with documented normal findings are demonstrated in detail in ‘Table 2’, respectively.
Comparison between automatic and manual techniques for patients with documented abnormal findings on stress and rest tests are demonstrated in ‘Table 3’, and ‘Table 4’, respectively.
There is a “statistically significant difference” between automatic and manual techniques for EF and EDV “(p<0.05) as shown in (Fig. 1) and (Fig. 2). However, there is “no statistically significant difference’’ between automatic and manual techniques for
Table 1
Sample Demographics’ and Clinical Data
|
Mean Age (Years) |
Gender (%) |
Diabetes Mellitus (%) |
Hypertension (%) |
Dyslipidemia (%) |
Smoking (%) |
Family History (%) |
Stress Test Findings (%) |
|
61.1 ±11.6 |
Male 64.6 Female 35.4 |
61.5 |
77.1 |
72.9 |
16.7 |
26.0 |
Normal 67.7 Abnormal 32.3 |
Table 2
Comparison Between Automatic and Manual Technique for Patient with Documented Normal Findings (Stress)
|
Automatic |
Manual |
|
|
EF * (%) |
66.07 |
60.52 |
|
ESV (ml) |
36.53 |
35.81 |
|
EDV* (ml) |
97.56 |
83.04 |
|
*Indicates p<0.05 statistically significant difference between automatic and manual. |
||
Table 3
Comparison Between Automatic and Manual Technique for Patient with Documented Abnormal Findings (Stress)
|
Automatic |
Manual |
|
|
EF * (%) |
54.90 |
50.29 |
|
ESV (ml) |
62.74 |
62.26 |
|
EDV* (ml) |
126.81 |
114.68 |
|
*Indicates p<0.05 statistically significant difference between automatic and manual. |
||
Table 4
Comparison Between Automatic and Manual Technique for Patient with Documented Abnormal Findings (Rest)
|
Automatic |
Manual |
|
|
EF * (%) |
56.23 |
51.77 |
|
ESV (ml) |
59.61 |
58.32 |
|
EDV* (ml) |
123.26 |
108.77 |
|
*Indicates p<0.05 statistically significant difference between automatic and manual. |
||
** Abbreviations EDV : End-diastolic volume; ESV : End-systolic volume; EF : Ejection fraction
ESV (p>0.05) as shown in (Fig. 3). We compare between automated and manual processing for EF, EDV, and ESV in stress test as shown in (Fig. 4) and rest as shown in (Fig.5).
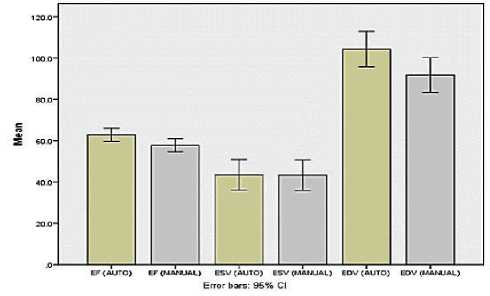
Figure 4. Stress Test
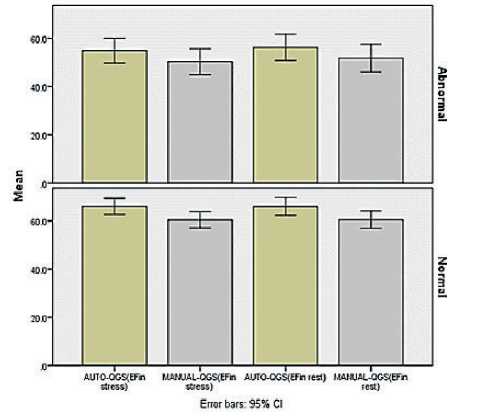
Figure 1. EF in Stress and rest Test for normal and abnormal findings.
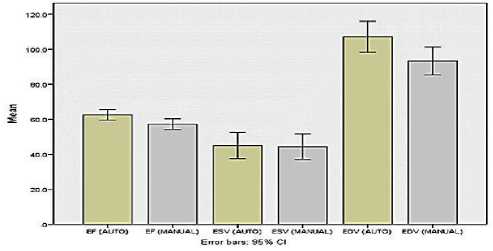
Figure 5. Rest Test
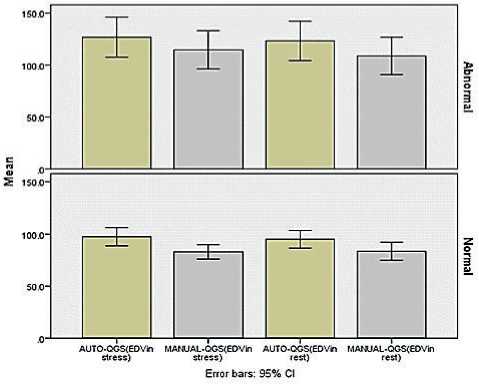
Figure 2. EDV in Stress and rest Test for normal and abnormal findings.
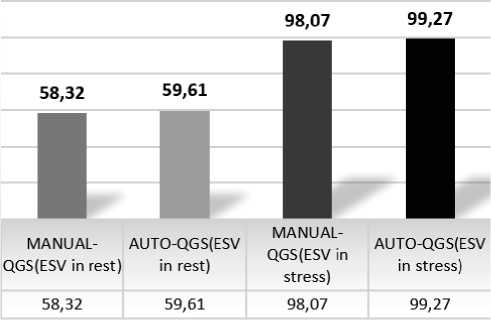
Figure 3. ESV in Stress and rest Test for normal and abnormal findings.
Discussion
Accurate and valid end-diastolic and end-systolic frame detection procedures would enable the development of entirely automated procedures for objective assessment of the LV function, such as automated computation of EF and stroke volume, strain imaging, and wall thickening 4 . However, gated myocardial perfusion single-photon emission computed tomography (SPECT) has been widely used to measure perfusion and LV functional parameters in patients with suspected heart disease. It has proven a high correlation with the results of other modalities 31 .
Automated analysis and quantification display techniques for assessing perfusion and function from myocardial perfusion SPECT have been devised and proved to work in the great majority of patients. Automated software algorithms use different feature extraction procedures when applied to radiologic images 32 . Whenever necessary, software programmers aim to integrate “sanity tests” which question if a particular contour, for example, makes rational sense of general human anatomy 1 . Simple manual manipulation techniques have also been created for the remaining cases 32 . These advancements are projected to considerably influence nuclear cardiology by reducing the time necessary for study analysis and evaluation, influencing both technical and physician expenses.
Furthermore, this method is predicted to increase the repeatability of myocardial perfusion SPECT. Many types of software can use to assess cardiac function as research said but we are use one type of software which is Cedar-Sinai QGS software {Diagnostic evaluation of three cardiac software packages using a consecutive group of patients}. In Cedar-Sinai QGS software allow using automated and manual processes. It is important to use manual processing to make sure that the placement of the organ specifically cardiac borders is accurate and can be verify the region of interest (ROI) and also to minimized the artifact from adjacent organ to the heart borders like a big breast or diaphragm {QGS+QPS Automatic Quantification Version 2013.1}. Some research says that the artifacts has a minor effect, but do not cause a significant impact on the study {Diagnostic evaluation of three cardiac software packages using a consecutive group of patients}. Despite that, we found in this research that patients who have potential artifacts related to patient or technical factors are more in need of manual processing to increase the accuracy of the cardiac volume and ejection fraction {Artifacts in Quantitative analysis of myocardial perfusion SPECT, using Cedars-Si-nai QPS Software}.
Conclusion
We found discrepancies that exist by using the fully automated and manual technique for determining LVEF and volumes by gated MPI. This study highlights the importance of a semi-automated technique using a mix of automated and manual correction of LV contours to ensure a more precise and accurate estimation of Left ventricular function. Semi-automated processing is important to improve the results, thus minimizing the potential impact on diagnosis and patient management.
Limitations
The population of the patients used in this study was small hence larger set of data is required for more in-depth analysis. Other limitation that we have only one computer of Siemens E-cam and Symbia SPECT systems using for processing. The software for the device is updated at the end of each month, and from this point of view, additional information such as manual processing which is not needed by every patient and is considered overload on the device so that why deleted.
Recommendations
The operator always should be alert to myocardial boundaries (LV epicardial and endocardial contours and apex/base endpoints) that do not look correct or mismatched to visual assessment, especially in centers lacking a newer SPECT-CT system (equipped with CT attenuation correction option).
Abbreviations
SPECT : single-photon emission computed tomography; LVEF : Left ventricular ejection fraction; EF : Ejection fraction; EV : Ejection volume; EDV : End-diastolic volume; ESV : End-systolic volume; QGS : Quantitative gated SPECT; CT : computed tomography; TID : Transient Ischemic Dilation; MP I: Myocardial perfusion imaging; MUGA : Multi-gated cardiac blood pool acquisition; MRI : Magnetic resonance imaging; CMR : Cardiac Magnetic Resonance.
Acknowledgments
I would like to thank the head of the nuclear medicine department and the nuclear medicine staff for their assistance in facilitating data collection and analysis.
Funding
No external funding was acquired for this research.
Availability of data and materials
All data analyzed during this research are included in this article.
Declarations
Informed consent
Ethical Approval
This study has been reviewed and approved by the Research Ethics committee (REC) at the university of Hail (Research # H-2022-114).
Competing interests
The authors declare that they have no competing interests.
Список литературы Comparison of totally automated and manual processing for calculation of left ventricular volumes and ejection fraction using gated myocardial perfusion spect
- Bresser P, De Beer J, De Wet Y. A study investigating variability of left ventricular ejection fraction using manual and automatic processing modes in a single setting. Radiography. 2015 Feb 1;21(1): e41-4.
- Motwani M, Berman DS, Germano G, Slomka P. Automated quantitative nuclear cardiology methods. Cardiology clinics. 2016 Feb 1;34(1):47-57.
- Lin GS, Hines HH, Grant G, Taylor K, Ryals C. Automated quantification of myocardial ischemia and wall motion defects by use of cardiac SPECT polar mapping and 4-dimensional surface rendering. Journal of nuclear medicine technology. 2006 Mar 1; 34(1):3-17.
- Zolgharni M, Negoita M, Dhutia NM, Mielewczik M, Manoharan K, Sohaib SA, Finegold JA, Sacchi S, Cole GD, Francis DP. Automatic detection of end‐diastolic and end‐systolic frames in 2D echocardiography. Echocardiography. 2017 Jul;34(7):956-67.
- Shibayama K, Watanabe H, Iguchi N, Sasaki S, Mahara K, Umemura J, Sumiyoshi T. Evaluation of automated measurement of left ventricular volume by novel real-time 3-dimensional echocardiographic system: validation with cardiac magnetic resonance imaging and 2-dimensional echocardiography. Journal of cardiology. 2013 Apr 1;61(4):281-8.
- Bailey EA, Bailey DL. Results from an Australian and New Zealand audit of left ventricular ejection fraction from gated heart pool scan analysis. Nuclear Medicine Communications. 2012 Jan 1;33(1):102-11.
- Davis BA, O’Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004 Jul 1;104(1):263-9.
- Christian PE, Waterstram-Rich KM, editors. Nuclear medicine and PET/CT: technology and techniques. Mosby/Elsevier; 2007.
- De Geus-Oei LF, Mavinkurve-Groothuis AM, Bellersen L, Gotthardt M, Oyen WJ, Kapusta L, van Laarhoven HW. Scintigraphic techniques for early detection of cancer treatment–induced cardiotoxicity. Journal of Nuclear Medicine Technology. 2013 Sep 1;41(3):170-81.
- Altena R, Perik PJ, Van Veldhuisen DJ, De Vries EG, Gietema JA. Cardiovascular toxicity caused by cancer treatment: strategies for early detection. The lancet oncology. 2009 Apr 1;10(4):391-9.
- Slart RH, Poot L, Piers DA, Van Veldhuisen DJ, Nichols K, Jager PL. Gated blood-pool SPECT automated versus manual left ventricular function calculations. Nuclear medicine communications. 2004 Jan 1;25(1):75-80.
- Abe M, Kazatani Y, Fukuda H, Tatsuno H, Habara H, Shinbata H. Left ventricular volumes, ejection fraction, and regional wall motion calculated with gated technetium-99m tetrofosmin SPECT in reperfused acute myocardial infarction at super-acute phase: comparison with left ventriculography. Journal of Nuclear Cardiology. 2000 Nov 1;7(6):569-74.
- Bresser P, De Beer J, De Wet Y. A study investigating variability of left ventricular ejection fraction using manual and automatic processing modes in a single setting. Radiography. 2015 Feb 1;21(1):e41-4.
- Davis BA, O’Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004 Jul 1;104(1):263-9.
- Christian PE, Waterstram-Rich KM, editors. Nuclear medicine and PET/CT: technology and techniques. Mosby/Elsevier; 2007.
- Lum DP, Coel MN. Comparison of automatic quantification software for the measurement of ventricular volume and ejection fraction in gated myocardial perfusion SPECT. Nuclear medicine communications. 2003 Mar 1;24(3):259-66.
- Lin GS, Hines HH, Grant G, Taylor K, Ryals C. Automated quantification of myocardial ischemia and wall motion defects by use of cardiac SPECT polar mapping and 4-dimensional surface rendering. Journal of nuclear medicine technology. 2006 Mar 1;34(1):3-17.
- Arsanjani R, Xu Y, Dey D, Vahistha V, Shalev A, Nakanishi R, Hayes S, Fish M, Berman D, Germano G, Slomka PJ. Improved accuracy of myocardial perfusion SPECT for detection of coronary artery disease by machine learning in a large population. Journal of Nuclear Cardiology. 2013 Aug;20(4):553-62.
- Arsanjani R, Dey D, Khachatryan T, Shalev A, Hayes SW, Fish M, Nakanishi R, Germano G, Berman DS, Slomka P. Prediction of revascularization after myocardial perfusion SPECT by machine learning in a large population. Journal of Nuclear Cardiology. 2015 Oct;22(5):877-84.
- White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987 Jul;76(1):44-51.
- White HD, Cross DB, Elliott JM, Norris RM, Yee TW. Long-term prognostic importance of patency of the infarct-related coronary artery after thrombolytic therapy for acute myocardial infarction. Circulation. 1994 Jan;89(1):61-7.
- Norris RM, Barnaby PF, Brandt PW, Geary GG, Whitlock RM, Wild CJ, Barratt-Boyes BG. Prognosis after recovery from first acute myocardial infarction: determinants of reinfarction and sudden death. The American Journal of Cardiology. 1984 Feb 1;53(4):408-13.
- Borow KM, Green LH, Mann T, Sloss LJ, Braunwald E, Collins Jr JJ, Cohn L, Grossman W. End-systolic volume as a predictor of postoperative left ventricular performance in volume overload from valvular regurgitation. The American journal of medicine. 1980 May 1;68(5):655-63.
- De Winter O, De Sutter J, Dierckx RA. Clinical relevance of left ventricular volume assessment by gated myocardial SPET in patients with coronary artery disease. European journal of nuclear medicine and molecular imaging. 2002 Jul;29(7):957-66.
- Sharir T, Germano G, Kavanagh PB, Lai S, Cohen I, Lewin HC, Friedman JD, Zellweger MJ, Berman DS. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999 Sep 7;100(10):1035-42.
- Sharir T, Kang X, Shaw LJ, Gransar H, Cohen I, Germano G, Hayes SW, Friedman JD, Berman DS, Bax JJ. Prognostic value of poststress left ventricular volume and ejection fraction by gated myocardial perfusion SPECT in women and men: gender-related differences in normal limits and outcomes. Journal of nuclear cardiology. 2006 Jul;13(4):495-506.
- Wang SY, Cheng MF, Hwang JJ, Hung CS, Wu YW. Sex-specific normal limits of left ventricular ejection fraction and volumes estimated by gated myocardial perfusion imaging in adult patients in Taiwan: a comparison between two quantitative methods. Nuclear Medicine Communications. 2011 Feb 1;32(2):113-20.
- Committee Members, Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O’Gara PT, Carabello BA, Russell RO, Cerqueira MD. ACC/AHA/ ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/ AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Journal of the American College of Cardiology. 2003 Oct 1;42(7):1318-33.
- Committee Members, Gibbons RJ, Balady GJ, Timothy Bricker J, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Journal of the American College of Cardiology. 2002 Oct 16;40(8):1531-40.
- Achtert AD, King MA, Dahlberg ST, Hendrik Pretorius P, LaCroix KJ, Tsui BM. An investigation of the estimation of ejection fractions and cardiac volumes by a quantitative gated SPECT software package in simulated gated SPECT images. Journal of Nuclear Cardiology. 1998 Mar;5(2):144-52.
- Germano G, Kavanagh PB, Berman DS. An automatic approach to the analysis, quantitation and review of perfusion and function from myocardial perfusion SPECT images. The International Journal of Cardiac Imaging. 1997 Aug;13(4):337-46.
- Germano G, Kavanagh PB, Su HT, Mazzanti M, Kiat H, Hachamovitch R, Van Train KF, Areeda JS, Berman DS. Automatic reorientation of three-dimensional, transaxial myocardial perfusion SPECT images. Journal of Nuclear Medicine. 1995 Jun 1;36(6):1107-14.
- Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, Van Train KF, Berman DS. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. Journal of Nuclear Medicine. 1995 Nov 1;36(11):2138-47.
- Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, Van Train KF, Berman DS. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. Journal of Nuclear Medicine. 1995 Nov 1;36(11):2138-47.


