Compatibilization of polymer mixtures during processing of waste products from thermoplastics
Автор: N.N. Fomina, V.G. Khozin
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Manufacturing technology for building materials and products
Статья в выпуске: 4 Vol.13, 2021 года.
Бесплатный доступ
Introduction. The recycling of thermoplastic waste into building materials with a long-life cycle is a promising and technologically feasible disposal direction. It is advisable to develop the processing of mixed polymer waste. Thermoplastics are thermodynamically incompatible, and technological compatibility can be controlled at the melt stage by the introduction of compatibilizers and nanofillers, the formation of free radicals in the melt, and reaction compatibilization. Materials and methods. The work aimed to study the effect of a compatibilizer – an ethylene-vinyl acetate copolymer with grafted acrylate and epoxy groups on the properties of composites obtained from mixtures of polyethylene terephthalate and polypropylene waste. The polymer mixture was melted, filled with limestone flour (50–85 wt.%), the samples were pressed, and their physical and mechanical properties were investigated. Results and discussion, conclusions. Differential thermal analysis established the melt preparation temperature of 240оC, which is below the onset of thermal oxidative destruction of the compatibilizer. It was established that the optimal ratio of polyethylene terephthalate: polypropylene: compatibilizer as 70:25:5 wt.% provided the greatest improvement in the bending strength without compromising the compressive strength of filled composites; the optimal degree of filling was found to be 60–70%. The volume fractions of polymers in the mixture are comparable. Therefore, one can expect the formation of both the matrix structure of the composite and the interpenetrating networks type structures. In both cases, the compatibilizer is distributed along the interphase boundaries. Its effect on properties can be attributed to its plastic deformation in the interfacial layer and morphological changes in the structure of the polymer mixture. There is evidence of increased structure-sensitive properties, tensile and tensile strength, of polymer mixtures containing this compatibilizer. This fact indicates an increase in adhesion between polymer phases, which is difficult to explain only for physical reasons. Possible reactions between the functional groups of the compatibilizer and polyethylene terephthalate in the melt with epoxy ring opening were analyzed. An increased effect of compatibility was revealed; however, the actual effectiveness of the compatibilizer is difficult to assess. Additional mechanical tests are required as well as optimization of the melt temperature. It is also necessary to find a balance between activating the reaction compatibilization and minimizing destructive processes in polymers.
Polymer waste, recycling, nanofillers, polyethylene terephthalate, polypropylene, compatibilization
Короткий адрес: https://sciup.org/142227313
IDR: 142227313 | DOI: 10.15828/2075-8545-2021-13-4-229-236
Текст научной статьи Compatibilization of polymer mixtures during processing of waste products from thermoplastics
In recent decades, the disposal of polymer waste has become a serious environmental problem throughout the world [1–4]. Following the basic principles of the circular economy [5], recycling has become the most desirable strategy for the disposal of polymer waste. Recycling of thermoplastic waste into building materials through remelting, filling (optional), and molding (by extrusion or pressing) of products with a long-life cycle is a promising and technologically feasible direction, given the variability of the composition of polymer waste, their pollution, and as a result the labor intensity of more high-tech processing [6–10].
At present, the secondary recycling of polymer waste is underdeveloped both in Russia [4, 9–11] and abroad [1, 5–8, 12]. The main constraints are the relatively low cost of virgin polymers, contamination of waste and the need for their careful sorting, partial structural degradation of waste and the presence of low-molecular fraction. More-
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS over, even sorted polymer waste most often is a mixture of polymers. In fact, the main polymer product of waste sorting factories is plastic beverage bottles consisting of polyethylene terephthalate (PET), but the label on them, as a rule, is made of polypropylene (PP), the cap is made of high-density polyethylene (HDPE). Therefore, with the development of recycling technologies, it is advisable to focus on the processing of mixed polymer waste.
The theory of compatibility of polymer mixtures was developed by Flory [13] and Huggins [14] in the first half of the 20th century. In Russia, the science dealing with polymer mixtures was developed in the fundamental works of Lipatov, Kuzeznev, Tager, and Berlin. The results of foreign researchers are summarized in monographs by Paul [15] and Bucknall [16]. It is known from these works and many others, including modern studies [12, 17–24], that due to the low entropy of mixing and weak adhesion at the interphase boundaries, the compatibility and miscibility of thermoplastic polymers are rather an exception and not the rule. A mixture of thermoplastics can be characterized as a composite material with a developed phase boundary. In addition, if thermodynamically thermoplastics are incompatible, it is possible to control the technological compatibility of their mixtures in the melt. Currently, there are several directions for the development of research.
The traditional direction is the introduction into the mixture of an additional combining agent – compatibilizer, mainly of a polymer type (as a rule, these are block or graft copolymers, less often statistical). Compatibilizers reduce the surface energy of incompatible polymers, reduce the size of phase-separated structures, stabilize their morphology [25], and thus improve the physicomechani-cal properties of composites.
Another direction is the formation of free radicals in the mixture during the melting process, which is achieved, for example, by introducing peroxides into the mixture [17, 26–28] or by physical treatment of the mixture (gamma radiation, etc.). The formation of free radicals leads to chain breaks, the formation of peroxide, hydroxyl, carbonyl groups, and crosslinking with the formation of spatial structures, improving the compatibility of mixtures. Mechanochemical processes can also contribute to the formation of free radicals. The combination of shear, stretching, friction results in the rupture of linear macromolecules, making possible interchain reactions.
The most advanced approach suggests introducing nanofillers, acting as compatibilizers, into a mixture of thermoplastics. However, the main difficulty is to concentrate nanoparticles at the interphase boundaries because only in this case is the goal of improving compatibility achieved [18,19].
Another direction is reaction compatibilization, i.e. improvement of compatibility via physicochemical reactions occurring directly in the melt. In this case, an ad- ditional component is introduced into the thermoplastic melt, usually a polymer with functional groups (epoxy, acrylate, carboxyl, isocyanate, etc.) that are active with respect to the functional groups of the mixture components. This leads to the formation of complex block copolymers or graft copolymers at the interface, resulting in the formation of spatial structures. It should be noted [12] that reactive compatibilization with the participation of epoxy groups is expedient for mixtures of polymer wastes, in which terminal hydroxyl and carboxyl groups are formed as a result of destructive processes.
The combination of several methods for controlling the compatibility of polymers in a melt seems to be the most promising. Such experiments were described, for example, in [29], where the compatibility of secondary PET and primary low-density polyethylene (LDPE) was affected in two ways: introduction of a compatibilizer (copolymer of ethylene and vinyl acetate) and gamma radiation after-treatment. As a result, the mechanical and thermal properties of the mixture were improved.
The polymer composites industry is actively developing, and the market offers a range of compatible additives, including compatibilizers with grafted functional groups. Thus, modified ethylene and vinyl acetate copolymers (for example, Etatilene from New Polymer Technologies , Sevilen from Khimplex (https://himplex. ru), etc.) are positioned as additives in the processing of polymer waste. Such copolymers can act both as conventional and reactive compatibilizers. The main purpose of the presented work is to investigate the effect of copolymer compatibilizer with grafted functional groups on the properties of composites obtained from mixtures of mass waste and successfully amenable to sampling and sorting of thermoplastics – PET and PP.
2. MATERIALS AND METHODS
In our study, we used household waste, namely a mixture of rPET transparent and coloured bottles) and rPP food containers, which were crushed into particles up to 50 mm in size and dried before melting.
An Etatilen EVA-g-GMA (EVA-g-GMA) modified ethylene-vinyl acetate copolymer with grafted chemically active polar acrylate and epoxy groups (New Polymer Technologies, Unecha, Bryansk region, Russia) was used as a copolymer compatibilizer. The content of vinyl acetate groups in the copolymer is 22%. The grafting rate of 2,3-epoxypropyl methacrylate (Glycidyl Methacrylate, GMA) was 3%. According to the manufacturer, this brand of compatibilizer is effective for polymer blends based on polyesters, especially when processing recycled PE, PET, and their mixtures.
The polymer mixture (rPET, rPP, EVA-g-GMA) was melted. Then, a mineral filler, limestone flour with the specific surface area of 1000 cm2/g determined by the gas per-
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS meability method, was introduced into the resulting melt. The degree of filling of the compositions varied from 50 to 85 wt.%. The prepared mixture was loaded into a mold. The samples were pressed at a specific pressure of 25 MPa for 2 min and cooled under ambient conditions for 3 hours.
Infrared (IR) spectroscopy was used to study the composition and the presence of functional groups in polymers; the spectra were recorded on an InfralumFT-801 device. Differential thermal analysis (DTA) was carried out on a DerivatographQ-1500D device to track temperature transitions and thermal effects.
Physical and mechanical properties were determined as follows. First, the ultimate compressive strength (R) was determined using the samples with dimensions of 80x40x40 mm made and tested using a method described in GOST 310.4 s. Second, the samples with dimensions of 80x40x10 mm were made for determining the ultimate tensile strength in bending (Rtb) and tested using a method described in GOST 27180.
3. RESULTS AND DISCUSSION
3.1 Melting temperature selection
3.2 Selection of the ratio between polymer components in the melt
According to the manufacturer's recommendations, EVA-g-GMA can be processed at the same temperatures as the polymers of base grades for which it is used. As follows from the DTA curves for EVA-g-GMA copolymer (see Fig. 1), the endothermic effect of melting was observed in the temperature range from 40 to 110оC with a maximum at 75оC. The processes of thermal-oxidative destruction started at 240оC, and they were accompanied
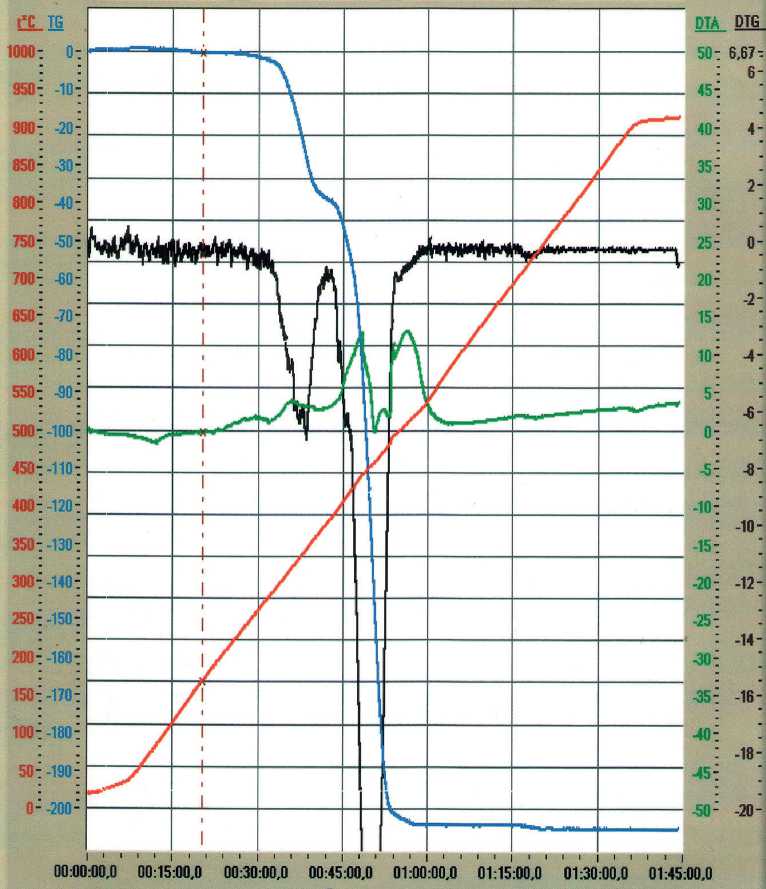
Fig. 1. Derivatogram and thermogram of EVA-g-GMA
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS by the exothermic effects and the onset of active mass loss. Based on these facts, we have chosen the temperature for preparing the melt of polymer components of the composition: 240±5оС. It would not be possible to obtain the rPET melt at lower temperatures because it was previously shown [9] that the endothermic effects of melting for it occurred within the range of 235–265оC with a maximum at 255оC. Therefore, the indicated temperatures are sufficient to obtain the rPP melt [9].
The rPET:rPP ratio of 70:30 wt.% was chosen based on previously obtained data [9, 10].
The amount of EVA-g-GMA, from 1.5 to 5%, was initially selected based on the manufacturer's recommendations and the results of previous studies [30–32]. The two-phase morphology of the rPET+rPP mixture, with low adhesion between the phases, adversely affected the mechanical properties of the products prepared from the mixture. We conducted a preliminary series of experiments to study the impact of the EVA-g-GMA content in the rPET+rPP mixture (EVA-g-GMA was added to the mixture to replace part of rPP) on the strength properties of filled polymer composites. It was found that the compatibilizer introduced in the composition in an amount ranged from 1.5 to 10% of the total weight of the rPET and rPP polymer components reduced the compressive strength and increased the flexural strength of the resulting filled samples. The greatest increase in flexural strength was observed when the EVA-g-GMA content was 5 wt.%. When the compatibilizer was introduced in smaller amounts, the effect of enhancing the flexural strength decreased. The introduction of a larger amount of compatibilizer left the flexural strength of the specimens at the same level and reduced the compressive strength. As a result, the ratio between the polymer components of the composition was chosen as follows:
rPET:rPP:EVA-g-GMA = 70:25:5 wt.%.
3.3 Study of the effect of compatibilizer on the strength properties of composites with various filling ratios
The experimental data on the strength of the samples represented as a function of the filling ratio for compositions with and without the EVA-g-GMA compatibilizer (see Fig. 2 and 3) indicate the following patterns. First, the dependencies are extreme, and the highest values of strength indicators were found when the filling ratio was within the range of 60–70% by weight of the composition. Second, the presence of the compatibilizer led to a decrease in the compressive strength of the composite samples, and the flexural strength was higher over the entire range of filling ratio.
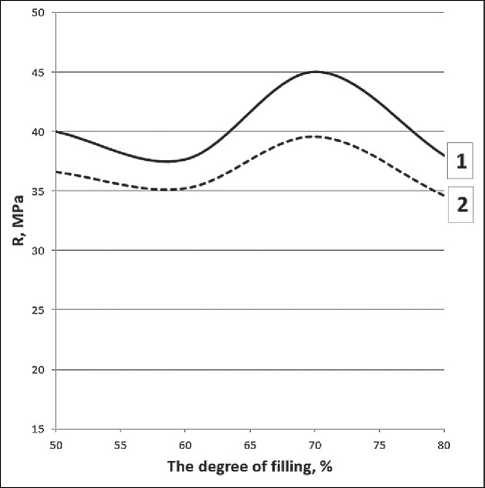
Fig. 2. Dependence of ultimate strength in compression on the filling ratio of the composition
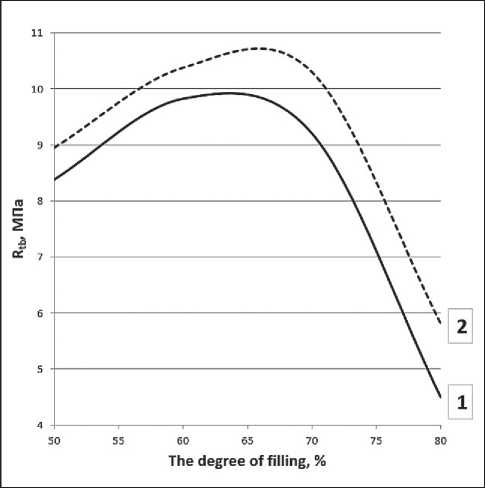
Fig. 3. Dependence of ultimate strength in bending on the filling ratio of the composition
The composition includes:
1 – rPET, rPP in a ratio of 70:30 wt.%; 2 – rPET, rPP, EVA-g-GMA in a ratio of 70:25:5 wt.%
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
3.4 Background of compatibilization in terms of physical processes
The effect of the compatibilizer on the properties of the samples can be explained by morphological changes in the structure of the polymer mixture. If the volume fraction of another polymer in the main polymer is small, a dispersed phase of the second polymer is formed. The morphology of this phase depends on the degree of mixing, volume fractions, and interactions between the polymers. For example, according to [30], the dispersed rPP phase was identified in the rPET+rPP mixture (the rPET:rPP ratio was 80–95:20–5 by weight), and the average size of rPP particles increased with an increase in its volume fraction in the mixture. The introduction of a compatibilizer into the mixture leads to a more uniform distribution and a decrease in the average particle size of the rPP phase. In work [23], on the contrary, the volume fraction of PP significantly exceeded that of PET (85:15 by weight, respectively), and the dispersed phase of PET was identified using scanning electron microscopy. Moreover, in the binary PET+PP mixtures, the PET phase was poorly dispersed, and the average particle size of the dispersed PET phase was reduced in the presence of a compatibilizer, and the particles were distributed quite uniformly.
In our case, the volume fraction of rPP in the polymer mixture was less but comparable with that of rPET. Therefore, one could expect both the formation of an rPET matrix structure with a dispersed rPP phase and an interpenetrating network (IPN) structure. However, in both cases, the compatibilizer is distributed along the interphase boundaries [30]. Therefore, an increase in bending strength and a simultaneous decrease in compressive strength can be partly explained by physical processes, including the morphological changes in the structure and plastic deformation of the interfacial layer of the compatibilizer.
The tensile and tear strength is known to be a more structure-sensitive property when analyzing polymer compatibility. According to [30], the introduction of rPP into rPET leads to a decrease in tensile strength, and the introduction of a compatibilizer into a polymer mixture leads to a significant increase in this indicator. The elastic modulus of the rPET+rPP mixture is lower than that of each of the polymers [30], and the introduction of the compatibilizer into their mixture leads to an even greater decrease. This fact can be attributed to the deformability of the layer of the EVA copolymer-based compatibilizer, the elastic modulus of which is significantly lower than that of PP. However, with a large proportion of rPP in the polymer mixture, the compatibilizer insignificantly reduces the elastic modulus of the mixture. In [31], an increase in bending and tensile strength was also marked when recycled polyethylene and rPET compatibilizer were added to the mixture. All these facts indicate an increased adhesion between the two polymer phases, which is difficult to explain only by physical reasons.
In addition, the authors of [23] showed that the average particle size of the dispersed PP phase in the PP+PET mixture increased with the increasing melt temperature. This property is known for incompatible mixtures since the enthalpy of mixing (which negatively affects miscibility) will increase with increasing temperature [33]. Nevertheless, on the contrary, in the presence of a compatibilizer, the average particle size of the dispersed PET phase decreases with an increase in temperature [23]. This fact indicates a more complex mechanism of com-patibilization, which is caused not only by changes in the morphology of the phases. Note that in the studies discussed here, GMA-modified copolymers were also used as compatibilizers.
3.5 Justification of compatibilization by physicochemical processes
The EVA-g-GMA compatibilizer grafted with the bifunctional monomer 2,3-epoxypropyl methacrylate contains an unsaturated group for polymerization or free-radical grafting and an epoxy group that can react with terminal functional groups such as hydroxyls and carboxyls.
The presence of functional groups in the EVA-g-GMA compatibilizer was investigated using IR spectroscopy (Fig. 4).
A strong absorption band of the epoxy group and ether bond is observed at 1240–1250 cm–1, a weak absorption band of the epoxy group is seen at 870 cm–1. In [34], possible chemical reactions between the compatibilizer and polybutylene terephthalate were investigated through changes in the characteristic epoxy groups in GMA-modified copolymers of ethylene bands at 846, 909, and 995 cm–1. In our case, these bands are either not expressed or shifted. The functional groups are difficult to identify, probably due to the low degree of GMA grafting to the
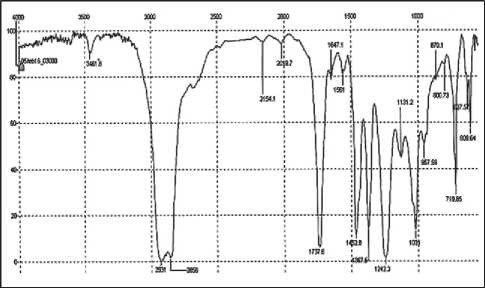
Fig 4. IR spectrum of EVA-g-GMA
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
EVA copolymer, which was 3% in our case versus 8% in [23, 30, 34]).
It is known that at temperatures of the order of 150– 180оC, epoxy cycles are capable of opening and participating in chain elongation reactions. Both epoxy and acrylate functional groups are active in the formation of intramolecular and intermolecular hydrogen bonds leading to the formation of polyassociates. An intensive broad absorption band at 3420–3590 cm–1 in the IR spectra of a mixture of polymers can indirectly indicate this type of reaction (Fig. 5), but it is difficult to identify chemical reactions from the spectra since the same band also indicates the presence of terminal hydroxyl groups in secondary polymers.
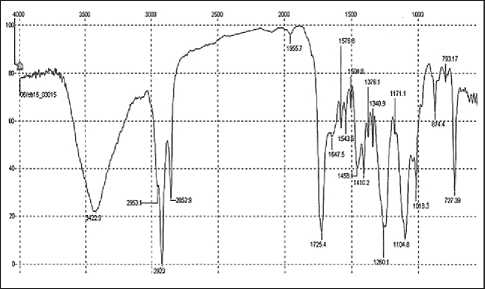
Fig. 5. IR spectrum of a mixture of rPET, rPP, EVA-g-GMA in a ratio of 70:25:5 wt.%
Figure 6 schematically represented possible reactions between functional groups of the EVA-g-GMA compatibilizer and the rPET phase in the melt. The reactions are reversible. As noted in many studies [12, 23, 31, 35], the increased reactivity of the functional groups of GMA-modified copolymers with respect to the PET phase may be a result of the reaction of thermal activation with an equilibrium shift to the right and an increase in the number of reactive PET groups generated by destructive processes. In general, there is an increase in the compatibility effect of PP and PET due to GMA-modified compatibilizers with increasing melt temperature. Therefore, in these studies, the melt temperatures were chosen in the range of 270–300оC. As shown above, in our case, the temperature should not exceed 245оC due to the onset of thermal destruction of EVA-g-GMA.
4. СONCLUSIONS
-
1. When processing mixed thermoplastic polymer waste into construction products, technological compatibility can be improved directly in the melt by introducing compatibilizers into the mixture. The modified compati-
- Fig. 6. Schemes of possible chemical reactions between the epoxy group of GMA and the end groups of rPET: hydroxyl (a); carbonyl (b)
-
2. Introduction of the modified EVA-g-GMA compatibilizer into a mixture of polyethylene terephthalate and polypropylene waste in an amount of 5% of the total weight of the polymer components of the composition leads to an increase in flexural strength and a decrease in the compressive strength of filled samples. This fact is explained by morphological changes in the structure of the polymer mixture and plastic deformation of the interfacial layer of the compatibilizer under loading. In addition, chemical reactions in the melt with the opening of epoxy rings are also probable.
-
3. It is rather difficult to assess the actual efficiency of the compatibilizer and its grafted functional groups in the studied mixtures of polymer waste since its effect on the flexural and compressive strength of the filled samples is not significant. Therefore, additional mechanical tests for tensile strength and impact strength are needed, which will make it possible to formulate practical recommendations for managing the properties of products for more efficient processing of this waste.
-
4. It is necessary to optimize the melt temperature and find a balance between the activation of the reaction compatibilization and the minimization of destructive processes in polymers.
bilizers with grafted epoxy groups are effective for waste containing polyethylene terephthalate, since they react in the melt with the terminal hydroxyl and carboxyl groups of polyethylene terephthalate.
In conclusion, it should be added that the processing of polymer waste into building materials saves primary raw materials and energy and ensures the recycling of plastics, which is crucial in light of current trends of increased attention to environmental issues and tightening of legislation on waste disposal.
MANUFACTURING TECHNOLOGY FOR BUILDING MATERIALS AND PRODUCTS
Список литературы Compatibilization of polymer mixtures during processing of waste products from thermoplastics
- Geyer R., Jambeck J., Law K.L. Production, use, and fate of all plastics ever made. Science Advances. 2017; 3(7). Available from: doi: 10.1126/sciadv.1700782.
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste, in: European Commission (Ed.). 2008.
- Sustainable Plastics Strategy. Edition 2, December 2020. Available from: https://www.plasticseurope.org/en/ resources/publications/4352-sustainable-plastics-strategy [Accessed 5th July 2021].
- International Forum of the Union of Plastic Converters. Polymer materials. 2021; 4: 4–13.
- BS 8001:2017: Framework for implementing the principles of the circular economy in organizations.
- Kumi-Larbi Jnr Al., Yunana D., Kamsouloum P., Webster M., Wilson D.C., Cheeseman Ch. Recycling waste plastics in developing countries: Use of low-density polyethylene water sachets to form plastic bonded sand blocks. Waste Management. 2018; 80: 112-118. Available from: doi: 10.1016/j.wasman.2018.09.003.
- Dalhat M.A., Al-Abdul Wahhab H.I. Cement-less and asphalt-less concrete bounded by recycled plastic. Construction and Building Materials. 2016; 119: 206-214. Available from: doi: 10.1016/j.conbuildmat.2016.05.010.
- Dhawan R., Brij Mohan Singh Bisht, Rajeev Kumar, Saroj Kumari, Dhawan S.K. Recycling of plastic waste into tiles with reduced flammability and improved tensile strength. Process Safety and Environmental Protection. 2019; 124: 299-307. Available from: doi: 10.1016/j.psep.2019.02.018.
- Fomina N.N., Polyanskij M.M. Components of solid municipal waste in construction compositions. IOP Conference Series: Earth and environmental Science. 2019; 337(1). Available from: doi: 10.1088/1755-1315/337/1/012022.
- Fomina N.N., Khozin V.G. Thermoplastic binder from polymer wastes. Construction materials. 2021; 1–2: 105–114. Available from: doi: 10.31659/0585-430X-2021-788-1-2-00-00.
- Rzaev K.V. State-of-art and trends of plastic wastes recycling market in Russia. Polymer materials. 2020; 8: 4–10.
- Marisa J., Bourdon S., Brossard J.-M., Cauret L., Fontaine L., Montembault V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polymer Degradation and Stability. 2018; 147. Available from: doi: 10.1016/j.polymdegradstab.2017.11.001.
- Flory P.J. Thermodynamics of high polymer solutions. The Journal of Chemical Physics. 1941; 9(8): 660–661.
- Huggins M.L. Solutions of long chain compounds. The Journal of Chemical Physics. 1941; 9(5): 440.
- Polymer mixtures. Volume 1: Systematics. Paul D.R., Backnell K.B. (eds). Saint-Petersburg: Scientific fundamentals and technologies; 2009.
- Polymer mixtures. Volume 2: Functional properties. Paul D.R., Backnell K.B. (eds). Saint-Petersburg: Scientific fundamentals and technologies; 2009.
- Zaikin A.E., Bobrov G.B. Influence of peroxide on characteristics of mixture of polypropylene and ethylenevinyl- acetate copolymer. The Bulletin of Kazan Technological University. 2012; 15(7): 67–70.
- Zaikin A.E., Bobrov G.B. Threshold extension of technical carbon percolation in polymer mixtures. The Bulletin of Kazan Technological University. 2012; 15(18): 119–125.
- Zaikin A.E., Bobrov G.B. Compatibilization of mixtures of noncompatible polymers by means of filling. Polymer Science. Series A. 2012; 54(8): 1275–1282.
- Asadsky A.A., Matseevich T.A., Popova M.N. Secondary polymer materials (mechanical and barrier characteristics, plastifying, mixtures and nanocomposites). Moscow: ASV; 2017.
- Mikitaev M.A., Kozlov G.V., Mikitaev A.K. Structural analysis of cosite of polymer mixtures. Plasticheskie massy. 2017; 1–2: 20–23.
- Bruggen E. P. A., Koster R. P., Picken S. J., Ragaert K. Influence of Processing Parameters and Composition on the Effective Compatibilization of Polypropylene–Poly(ethyleneterephthalate) Blends. International Polymer Processing. 2016; 31(2): 179–187. Available from: doi: 10.3139/217.3124.
- Jayanarayanan K., Thomas S., Joseph K. Effect of compatibilizer on the morphology development, static and dynamic mechanical properties of polymer-polymer composites from LDPE and PET. International Journal of Plastics Technology. 2015; 19(1): 84–105. Available from: doi: 10.1007/s12588-015-9108-1.
- Utracki L. Compatibilization of Polymer Blends. The Canadian Journal of Chemical Engineering. 2002; 80: 1008–1016. Available from: doi: 10.1002/cjce.5450800601.
- Ma P., Cai X., Zhang Y., Wang S., Dong W., Chen M., Lemstra P.J. In-situ compatibilization of poly(lactic acid) and poly(butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polymer Degradation and Stability. 2014; 102: 145–151. Available from: doi: 10.1016/j.polymdegradstab. 2014.01.025.
- Wang Y., Li D., Zhang J.-M., Xie X.-M. Compatibilization and toughening of immiscible ternary blends of polyamide 6, polypropylene (or a propylene—ethylene copolymer), and polystyrene. Journal of Applied Polymer Science. 2011; 11(3): 1652–1658. Available from: doi: 10.1002/app.32839.
- Gu J., Xu H., Wu C. Thermal and crystallization properties of HDPE and HDPE/PP blends modified with DCP. Advances in Polymer Technology. 2014; 33(1). Available from: doi: 10.1002/adv.21384.
- Abdel Tawab K., Ibrahim S.M., Magida M.M. The effect of gamma irradiation on mechanical, and thermal properties of recycling polyethylene terephthalate and low density polyethylene (R-PET/LDPE) blend compatibilized by ethylene vinyl acetate (EVA). Journal of Radioanalytical and Nuclear Chemistry. 2012; 295(2): 1313–1319. Available from: doi: 10.1007/s10967-012-2163-6.
- Baccouch Z., Mbarek S., Jaziri M. Experimental investigation of the effects of a compatibilizing agent on the properties of a recycled poly(ethylene terephthalate)/ polypropylene blend. Polymer Bulletin. 2016; 1–18. Available from: doi: 10.1007/s00289-016-1748-6.
- Chen R.S., Ghani M.H., Salleh M.N., Ahmad S., Gan S. Influence of blend composition and compatibilizer on mechanical and morphological properties of recycled HDPE/PET blends. Materials Sciences and Applications. 2014; 5(13): 943–952. Available from: doi: 10.4236/msa.2014.513096.
- Pantyukhov P., Popov A., Kolesnikova N. Рreparation, structure, and properties of biocomposites based on low-density polyethylene and lignocellulosic fillers. Polymer Composites. 2016; 37(5): 1461–1472. Available from: doi: 10.1002/pc.23315.
- Higgins J. S., Lipson J. E. G., White R. P. A Simple Approach to Polymer Mixture Miscibility. Philosophical Transactions of Royal Society A. 2010; 368: 1009–1025. Available from: doi:10.1098/rsta.2009.0215.
- Tsai C.H., Chang F.C. Polymer Blends of PBT and PP Compatibilized by Ethylene-co-Glycidyl Methacrylate Copolymers. Journal of Applied Polymer Science. 1996; 61: 321–332.
- Yildirim E., Yurtsever M. A Comparative Study on the Efficiencies of Polyethylene Compatibilizers by Using Theoretical Methods. Journal of Polymer Research. 2012; 19. Available from: doi: 10.1007/s10965-011-9771-7.


