Contact effects by mitochondria: biological destruction of cultivated B16 - F10 melanoma cells
Автор: Kit Oleg I., Frantsiyants Elena M., Filippova Svetlana Y., Neskubina Irina V., Mezhevova Irina V., Shikhlyarova Alla I., Kaplieva Irina V., Trepitaki Lidia K., Pogorelova Yulia A., Gusareva Marina A., Bykadorova Oksana V., Serdyukova Elizaveta V., Khokhlova Olga V., Kuchkina Lyudmila P., Gurnak Viktor V., Surikova Ekaterina I.
Журнал: Cardiometry @cardiometry
Статья в выпуске: 24, 2022 года.
Бесплатный доступ
In recent years therapeutic effects produced by mitochondria, transplanted to pathogenic regions in an organism, which demonstrate their high migration activity and tropicity with respect to filling of energetic vacuum, have been inspiring a renewed interest in the scientific community. In this context, this raises the question of the source of metabolically active mitochondria and their histological compatibility required for the mitochondrial transplantation. Aim. The aim of our research work is to study characteristics/ quality of mitochondria from cells of the liver and the heart in rats, which in the in vitro system have produced their impact on different biological features of the B16-F10 murine melanoma cell culture. Materials and methods. In our research work we have used cells of the B16-F10 murine melanoma cell line culture. In the framework of the study, an experiment with mitochondria harvested from the liver and the heart of a rat has been conducted. Mitochondria have been isolated using differential centrifugation with a high-speed refrigerated centrifuge. With the B16-F10 culture cells, we have designed the following variants of our experiments: 1) use of cardiac mitochondria (1 mg/mL, in terms of total protein); 2) use of cardiac mitochondria (1 mg/mL) + succinic acid (10-4%); 3) mitochondria of the liver (1 mg/mL, in terms of total protein); 4) use of mitochondria of the liver (1 mg/mL) + succinic acid (10-4%); 5) use of succinic acid (10-4%) solely; 6) the reference specimen with no use of mitochondria and the above agents. An assessment of the impact made by mitochondria on the migration of the B16-F10 cells has been performed with the scratch wound healing test. For the purpose of an analysis of the effect produced by mitochondria on the energetic metabolism of the B16-F10 cell culture we have measured main parameters of the cell respiration and glycolysis in stress tests with adding some toxic chemicals. The rate of the cellular respiration has been assessed by measuring the amount of oxygen taken in (oxygen consumption rate, OCR), and the glycolysis level has been evaluated by the extracellular acidification rate (ECAR). Results. Adding cardiac and hepatic mitochondria to the cultivated B16-F10 cells has produced a pronounced cytopathic effect, which has become more remarkable upon expiration of two days of the cell cultivation and which has consisted in cytoplasm granulation and partial detaching of the cells. Introducing mitochondria of the heart to the cultivated B16-F10 cells has induced a considerable decrease both in the background-related and the maximum level of oxygen consumption by the B16-F10 cells as against the reference samples without adding of mitochondria. Adding the cardiac mitochondria has led to a statistically significant decrease in the base level of ECAR by 14,36 mpH/min (t = 3,12, df = 10) as compared with the reference values. Introducing hepatic mitochondria has also resulted in a reduction of the average value of ECAR as against the background by 4,8 mpH/min. Conclusion. Metabolically active mitochondria are capable of reformatting energetic fluxes in tumor cells and change their cellular respiration that leads to the most effectively realized death of the cultivated B16-F10 tumor cells.
Mitochondria, b16-f10 cell culture, oxygen consumption rate, extracellular acidification rate, cytotoxic effect
Короткий адрес: https://sciup.org/148326576
IDR: 148326576 | DOI: 10.18137/cardiometry.2022.24.106114
Текст научной статьи Contact effects by mitochondria: biological destruction of cultivated B16 - F10 melanoma cells
Oleg I. Kit, Elena M. Frantsiyants, Svetlana Y. Filippova, Irina V. Neskubina, Irina V. Mezhevova, Alla I. Shikhlyarova, Irina V. Kaplieva, Lidia K. Trepitaki, Yulia A. Pogorelova, Marina A. Gu-sareva, Oksana V. Bykadorova, Elizaveta V. Serdyukova, Olga V. Khokhlova, Lyudmila P. Kuchkina, Viktor V. Gurnak, Ekaterina I. Surikova. Contact effects by mitochondria: biological destruction of cultivated B16 – F10 melanoma cells. Cardiometry; Issue 24; November 2022; p. 106-114; DOI: 10.18137/cardiome-try.2022.24.106114; Available from: issues/no24-november-2022/contact-effects-mitochondria
Mitochondria form the “energetic engine” in eukaryotic cells, which is of primary importance in the processes of aerobic respiration and production of adenosine triphosphate (ATP). Actually, functioning of mitochondria determines homeostasis in the cells, disorders of which may result in development of pathological processes, cancer among them. In recent years therapeutic effects, produced by mitochondria, transplanted to pathogenic regions in an organism, which demonstrate their high migration activity and tropicity with respect to filling of energetic vacuum, have been inspiring a renewed interest in the scientific community.
More than two decades passed since Clark and Shay pioneered the artificial mitochondria transfer (AMT). In 2006 Spees J.L et al. reported on the first evidence for horizontal mitochondrial transfer between the cells (from cell to cell) in mammals [1]. It is known that spontaneous transfer of mitochondria from cell to cell has a certain therapeutic potential that has been demonstrated in transplantation of isolated, metabolically active, mitochondria using several experimental pathological models in experiments in vitro and in vivo [2]. Berridge M.V. et al in their in vitro experiments with malignant cells have succeeded in determining the processes of mitochondrial transfer not only immediately to the malignant cells, but also between them [3]. The presented evidence data on horizontal transfer of mitochondria and mtDNA between cells in mammals suggest that these phenomena, no matter what precise mechanisms are involved, may be the basis for some fundamental physiological processes, which merit further careful study, but in the context of the entire organism as a whole. So, in some experiments on mice it has been established that therapy of malignant tumors with the use of mitochondria isolated from the liver inhibits the tumor growth due to reduction in glycolysis and oxidative stress [4]. Besides in our previous research works we have demonstrated a successful application of metabolically active mitochondria to produce an effect of suppression of the primary tumor focus of melanoma with blocking metastazing; we have also presented the respective morphological substantiation of the mechanism of this sort of actions in experimental animals [5,6]. In some other experimental studies, upon determination of the dysfunction of mitochondria in cardiomyocytes against the background of chronic neurogenic pain, followed by transplantation of metabolically active mitochondria of the heart, elimination of the occurrence of myocardial infarction events has been shown [7, 8, 9]. By this means expanding experimental studies to address impacts made by metabolically active mitochondria on pathological processes, including the malignant process, in the context of the sound organism, is a topical, promising, issue, which requires further investigations.
This brings up the question: what can be a source of metabolically active mitochondria? Undoubtedly, the source of mitochondria to be supplied for mitochondrial therapy is of crucial importance, and that is dictated by easiness in organelle extraction, the age of the tissue, the health status of the donor, the respective metabolic characteristics of the organ to be used for mitochondria release as well as histological compatibility. So, some researchers note that in case of deciding on use of the liver as a mitochondria donor, we should take into account the following parameters: high density of these organelles, an easy access to the tissue, a high regenerative potential and relatively high values of the P/O ratios of mitochondrial oxidative phosphorylation [10].
Another team of researchers, when studying variations of bio-energetic characteristics of mitochondria like high membrane potential in a number of the organs and tissues, has shown that there is no qualitative difference in the membrane potential between mitochondria found in the muscles, the brain, the white adipose tissue (WAT) and brown adipose tissue (BAT) [11]. Latest research has demonstrated that mitochondria in young health mice are able to more effectively retard proliferation of tumor cells, as against the old mitochondria, that may be attributed to their higher membrane potential and greater anti-oxidant capabilities [4]. Mitochondria in rats with diabetes contain less ATP as against that found in the reference rats after heat global ischemia [12], and it follows that the source of donor mitochondria shall be free of any pathology. These reports indicate that biological conditions of the donor tissue, which produce an effect on the bio-energetic characteristics of the organelles, may explain their therapeutic potential. Therefore the above evidence obtained in the experimental studies demonstrates that autologous transplantation can be considered to be not the best decision in mitochondrial therapy in case of some diseases, since its therapeutic potential can be limited by the actual health condition of the patient in question.
At present, the issue on histological compatibility in mitochondrial transplantation is topical on the agenda, since it requires its finalization. By assuming that there are considerable limitations on inclusion of exogenous mitochondria into cells, an in vitro experiment was conducted, which has demonstrated that upon an introduction of murine mitochondria into the human cell line no resistance by the host cells has been appeared [13]. However the xenogeneic transplant has been found to be less effective than the allogeneic one in the long term [14], and in this case neither syngeneic nor allogeneic injections has induced alloreactivity, allorecognition or some other responses associated with damage of certain molecular patterns (DAMPs) [15]. Considering all the above data collected, we can derive that the source of mitochondria is of prime importance in certain scenarios. However since the tissue-related, incompatible, autologous, allogeneic and xenogeneic transplants initiate the protection of cells or tissues in different experimental models, the selection thereof in each case can be primarily based on decision on what is the best source of the donor tissue for the purpose of the effective therapeutic outcome and an identification of the key biological levers of damaging actions of transplanted mitochondria.
The aim of our research work has been to study characteristics/quality of mitochondria of the liver and the heart, which in the in vitro system has made an impact on different biological features of the cultivated B16-F10 murine melanoma cells.
Materials and methods
The B16-F10 cells were incubated in a standard growth environment, consisting of the DMEM medium (Gibco, USA), with adding of 10% FBS (HyClone, USA) and 1% antibiotic-antimycotic agent, at a temperature of 37°С and with a 5,0% content of СО2. In total, we have designed three experiments, which have been aimed at an identification of an impact made by mitochondria, obtained from the rat liver and the rat heart, on different biological features of the murine melanoma cells. Mitochondria were isolated using the differential centrifugation with high-speed refrigerated centrifuge according to the method by Egorova M.V. and Afanasiev S.A. (2011) [16]. The harvested tissues on ice were perfused with ice-cold sterile 0.9% KCl solution. To destroy the intercellular junctions, the cell walls and plasma membranes, mechanical processing of the tissues was used with grinding with scis- 108 | Cardiometry | Issue 24. November 2022
sors and homogenization in a glass homogenizer with a Teflon pestle (the Potter-Elveheim homogenizer). Per gram of tissue, 10 ml of sterile isolation medium (0.22 M mannitol, 0.3 M sucrose, 1 mM EDTA, 2 mM TRIS-HCL, 10 mM HEPES, pH 7.4) were added. The tissues were centrifuged for the first time for 10 min at a speed of 1000 g, at a temperature of 0-2°C; the second and third centrifugations were carried out at 20000 g, for 20 min, at a temperature of 0-2°C. Between the centrifugations, the mitochondrial pellet was resuspended in the isolation medium. Mitochondria were further extra purified from lysosomes, peroxisomes, melanosomes, etc. by centrifugation in a 23% Percoll gradient. The suspension of the sub-cellular structures was layered on a Percoll gradient, centrifuged for 15 min at 21000 g, after which the separation into 3 phases was observed; the lower layer of mitochondria was left and resuspended in the isolation medium. The next washing of mitochondria was carried out by centrifugation for 10 min at 15000 g, at a temperature of 0–2°C. The obtained mitochondrial specimens were used for seeding in the cultivated B16-F10 melanoma cells.
In order to test the effect produced by mitochondria on viability of the B16-F10 culture cells, we have seeded the cells at a concentration of 5 × 104 cells per well in the 24-well design microplate. Upon expiration of 2 hours, when the adherent cells had taken to attach on the culture plate bottom, the cultivated medium was replaced with the standard medium with the tested objects to realize the following variants of the experiments: 1) use of cardiac mitochondria (1 mg/mL, in terms of total protein); 2) use of cardiac mitochondria (1 mg/mL) + succinic acid (10-4%); 3) mitochondria of the liver (1 mg/mL, in terms of total protein); 4) use of mitochondria of the liver (1 mg/mL) + succinic acid (10-4%); 5) use of succinic acid (10-4%) solely; 6) the reference specimen with no use of mitochondria and the above agent. Each test variant was designed to be reproduced 4 times. Later on, every 24 hours we renewed the medium with the tested objects and completed cell imaging.
According to the designed experiment conditions, on day four of the cell cultivation it was scheduled to stain the cells with DAPI, count nuclei and the number of the cells, but however even upon expiration of one day we observed some signs of cell stress, and on day three the cells in the wells with added mitochondria were found dead and detached from the micro- plate bottom, so that it became impossible to count the cells and measure an impact by mitochondria made on the viability of the B16-F10 line cells. Due to a pronounced cytotoxic effect produced by mitochondria on the B16-F10 line cells, we had to conduct our experiments, aimed at identifying of how mitochondria make their effect on energy metabolism and cell migration of the tested culture line, using a reduced exposure and a smaller amount of mitochondria. An impact made by mitochondria on migration of the B16-F10 cells was assessed in the scratch wound healing assay. We introduced 15*104 cells in each well in the 24-well wound healing assay microplate, and upon expiration of 2 hours replaced with the medium with the tested objects, similar to experiment one, but at the same time we halved the amount of mitochondria added. Each variant of the experiment was designed to be reproduced 4 times. Upon expiration of 24 hours, the monolayer was scratched, and the medium was replaced with the standard-type cultivation medium followed by the controlled cultivation in the Lionheart FX Automated Live Cell Imager (BioTek) at a temperature of 37°С with a СО2 content of 5,0% and with automated measuring of the gap area every 5 hours. The wound closure was assessed by calculation of the change in wound area upon 15 hours of the cultivation as a percentage of gap closure referred to the original wound size. For the purpose of estimation of the effect exerted by mitochondria on energy metabolism of the cultivated B16-F10 line cells, we seeded 2*104 cells per well in a cartridge of the SeaHorse XFp Analyzer (Agilent, USA); after the cell attachment the medium was replaced with the medium with tested objects, similar to experiment one, but the amount of mitochondria was halved. Each variant of the experiment was designed to be reproduced 6 times. One day later, the main parameters of the cell respiration and glycolysis in stress tests with adding metabolic toxic agents were measured. The intensity of the respiration was assessed according to oxygen consumption rate (OCR), and the level of glycolysis was evaluated according to extracellular acidification rate (ECAR).
Results
Adding of cardiac and hepatic mitochondria to the cultivated B16-F10 line cells resulted in a pronounced cytopathic effect that was very much more marked upon expiration of two days of the cultivation and consisted in granulation of cytoplasm and partial cell detaching (see Figure 1 herein). On day three, the tested cells, experienced mitochondria adding, were found fully dead that made impossible to quantitatively assess the cytotoxic activity of mitochondria and compare the two sources of allogeneic mitochondrial material, namely the rat heart and the rat liver.
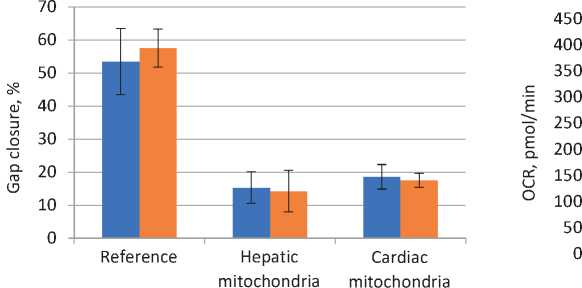
Since mitochondria obtained from both sources have induced pronounced stress and death of the melanoma cells, for the purpose of an assessment of the effect made on the cell migration we have reduced the amount of mitochondria to be added and decreased the exposure up to the levels, when the death of the cells might be expected to be minimal and could not make an influence on the experiment results. Upon a reduction in the mitochondria amount by one half and a decrease in the 24 hour-exposure time, we did obtain a considerable retardation of the B16-F10 cell migration within the next 15 hours, and in that case we didn’t observe any morphological signs of cell stress. Specifically, upon expiration of 15 hours after scratching, the gap area in the reference samples has been reduced by more than 50%, while in both variants with adding mitochondria the gap closure has been recorded to be less than 25% (see Figure 2 herein). It should be mentioned that the use of succinic acid has not have any statistically significant influence on the above effect.
Under the same conditions on dosing and exposure, we have estimated the effect made by mitochondria on energetic metabolism of the B16-F10 cell line culture. For the purpose of an assessment of the main parameters of the cell respiration, we measured OCR at the initial stage (the background) and at the stage upon adding of carbonyl cyanide m-chlorophenyl hydrazone (CCCP). The use of CCCP has resulted in a drop of the proton gradient on the inner membrane of mitochondria that leads to the oxygen consumption growth up to its possible greatest values, which are determined by the number of proteins in the electron transport chain and their state. In this case, a new level of OCR defines a margin in the mitochondrial respiration capacity. Our analysis of the collected data has shown that the use of cardiac mitochondria has resulted in a substantial decrease both in the background and minimal levels of the oxygen consumption by the B16-F10 cells as against the reference samples with no adding of mitochondria (see Figure 3 herein).
The difference in the OCR averaged values between the reference specimen values without adding of succin-
Issue 24. November 2022 | Cardiometry | 109
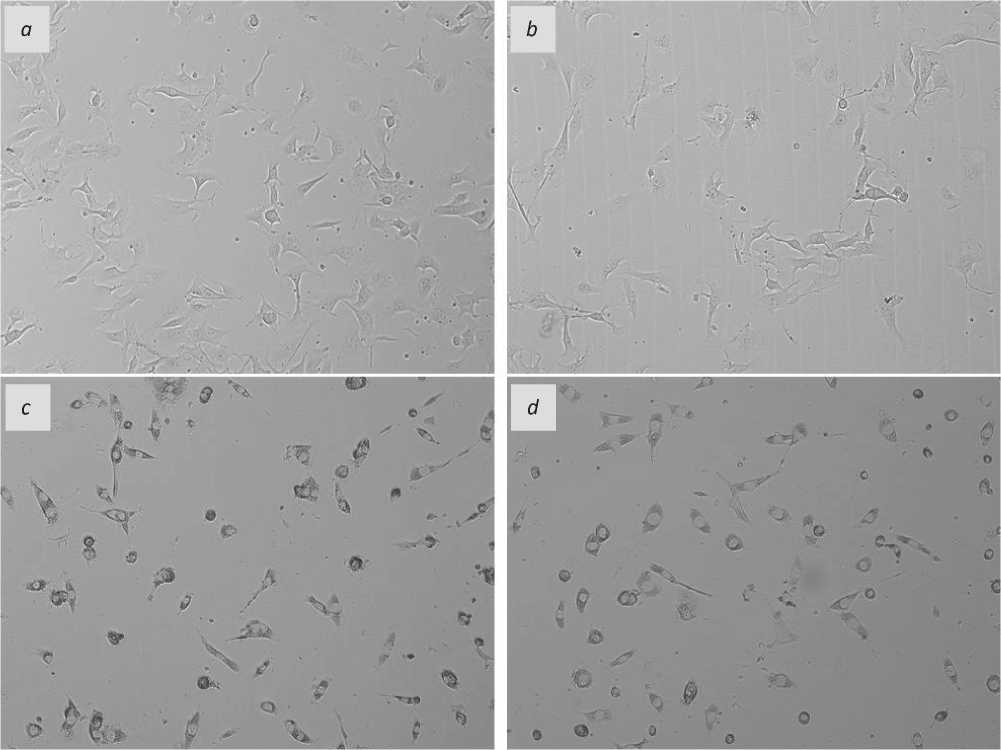
Figure 1. Images of the cultivated B16-F10 cells after the 48 hour-cultivation: a – the reference specimen cells, with no use of mitochondria and agents; b – with adding succinic acid; c – adding of mitochondria of the liver; d – adding of mitochondria of the heart. Magnification x10.
Список литературы Contact effects by mitochondria: biological destruction of cultivated B16 - F10 melanoma cells
- Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial Transfer between Cells Can Rescue Aerobic Respiration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1283-1288. doi:10.1073/pnas.0510511103.
- Nascimento-Dos-Santos G., de-Souza-Ferreira E., Linden R., Galina A., Petrs-Silva H. Mitotherapy: unraveling a promising treatment for disorders of the central nervous system and other systemic conditions. Cells. 2021;10(7):1827. doi:10.3390/cells10071827.
- Berridge MV, Dong LF, Neuzil J. Mitochondrial DNA in tumor initiation, progression and metastasis: role of horizontal mtDNA transfer. Cancer Res. 2015; 75: 3203-3208.
- Fu A, et al. Healthy mitochondria inhibit the metastatic melanoma in lungs. Int. J. Biol. Sci. 2019;15:2707–2718. doi: 10.7150/ijbs.38104.
- Kit OI, et al. Mitochondrial therapy of melanoma B16/F10, pathophysiological parameters of tumor regression. Cardiometry. 2022; 22:56-61. doi: 10.18137/cardiometry.2022.22.5661.
- Kit OI, et al. Biological effects of mitochondrial therapy: preventing development of myocardial infarction and blocking metastatic aggression of B16/ F10 melanoma. Cardiometry. 2022; 22:50-55. doi: 10.18137/cardiometry.2022.22.5055.
- Frantsiyants EM, et al. Content of apoptosis factors and self-organization processes in the mitochondria of heart cells in female mice C57BL/6 under growth of melanoma B16/F10 linked with comorbid pathology. Cardiometry. 2021:18:121-130. doi: 10.18137/cardiometry.2021.18.121130.
- Kit OI, et al. Mitochondrial therapy: direct visual assessment of the possibility of preventing myocardial infarction under chronic neurogenic pain and B16 melanoma growth in the experiment. Cardiometry. 2022; 22:38-49. doi: 10.18137/cardiometry. 2022.22.3849.
- Frantsiyants EM, et al. The functional state of mitochondria of cardiomyocytes in a malignant process against the background of comorbid pathology in the experiment. South Russian Journal of Oncology. 2021;2(3):13-22. doi: 10.37748/2686-9039-2021-2-3-2.[in Russian]
- Nascimento-Dos-Santos G., et al. Neuroprotection from optic nerve injury and modulation of oxidative metabolism by transplantation of active mitochondria to the retina. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165686. doi: 10.1016/j.bbadis.2020.165686.
- Nakamura Y, Park JH, Hayakawa K. Therapeutic use of extracellular mitochondria in CNS injury and disease. Exp. Neurol. 2020;324:113114.doi: 10.1016/j.expneurol.2019.113114.
- Doulamis I.P., Guariento A., Duignan T., Orfany A., Kido T., Zurakowski D., Del Nido P.J., McCully J.D. Mitochondrial transplantation for myocardial protection in diabetic hearts. Eur. J. Cardio-Thoracic Surg. 2020;57:836–845. doi: 10.1093/ejcts/ezz326.
- Clark MA, Shay JW. Mitochondrial transformation of mammalian cells. Nature. 1982;295:605–607.doi: 10.1038/295605a0.
- Chang JC, et al. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: Restoration of mitochondria functions and attenuation of 6-hydroxydopamine–induced neurotoxicity. Transl. Res. 2016;170:40–56. doi: 10.1016/j.trsl.2015.12.003.
- Ramirez-Barbieri G, et al. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion. 2019; 46: 103-115. doi: 10.1016/j.mito.2018.03.002.
- Egorova MV, Afanasiev SA. Isolation of mitochondria from cells and tissues of animals and humans: Modern methodological techniques. Siberian Medical Journal. 2011; 26(1-1): 22-28.
- Béatrice Morioa, Baptiste Panthua, Arthur Bassot, Jennifer Rieusset. Role of mitochondria in liver metabolic health and diseases. Cell Calcium. 2021; 94: 102336. https://doi.org/10.1016/j.ceca.2020.102336.
- Jungermann K. Metabolic zonation of liver parenchyma. Semin. Liver Dis., 8 (1988), pp. 329-341, 10.1055/s-2008-1040554.
- Matsumoto S, et al. Investigation of the hepatic respiration and liver zonation on rat hepatocytes using an integrated oxygen biosensor in a microscale device. Biotechnol. Prog. 2019; 35: e2854, 10.1002/btpr.2854.
- Jaakko L. Pohjoismäki, Steffi Goffart. The role of mitochondria in cardiac development and protection. Free Radical Biology and Medicine. 2017; 106:345-354. https://doi.org/10.1016/j.freeradbiomed.2017.02.032.
- Rong Tian, et al. Unlocking the Secrets of Mitochondria in the Cardiovascular System: Path to a Cure in Heart Failure. Circulation. 2019 Oct 1; 140(14): 1205–1216. doi: 10.1161/CIRCULATIONAHA. 119.040551.
- Shirakabe А., Y. Ikeda, S. Sciarretta, D.K. Zablocki, J. Sadoshima Aging and autophagy in the heart. Circ. Res. 2016; 118 (10): 1563-1576.
- Chen Y., Liu Y., Dorn G.W. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ. Res. 2011;109 (12): 1327-1331.
- Wai J., et al. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science. 2015; 350 (6265): aad0116.
- Lopaschuk G.D., Ussher J.R., Folmes C.D., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010; 90 (1): 207-258.
- Forner F., Foster LJ., Campanaro S., Valle G., Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol Cell Proteomics. 2006;5(4):608-19. doi: 10.1074/mcp.M500298-MCP200.


