Content of apoptosis factors and self-organization processes in the mitochondria of heart cells in female mice C57BL/6 under growth of melanoma B16 / F10 linked with comorbid pathology
Автор: Frantsiyants E.M., Neskubina I.V., Shikhlyarova A.I., Yengibaryan M.A., Vashchenko L.N., Surikova E.I., Nemashkalova L.A., Kaplieva I.V., Trepitaki L.K., Bandovkina V.A., Pogorelova Y.A.
Журнал: Cardiometry @cardiometry
Статья в выпуске: 18, 2021 года.
Бесплатный доступ
The aim is to study some mechanisms of regulation of apoptosis and self-organization in the mitochondria in the heart cells in female mice during the growth of experimental melanoma B16/ F10 linked with chronic neurogenic pain as comorbid pathology.
Mitochondria, apoptosis, self-organization processes, heart, chronic neurogenic pain, melanoma в16/f10, female mice
Короткий адрес: https://sciup.org/148321604
IDR: 148321604 | DOI: 10.18137/cardiometry.2021.18.121130
Текст научной статьи Content of apoptosis factors and self-organization processes in the mitochondria of heart cells in female mice C57BL/6 under growth of melanoma B16 / F10 linked with comorbid pathology
Elena M. Frantsiyants, Irina V. Neskubina, Alla I. Shikhlyarova, Marina A. Yengibaryan, Larisa N. Vashchenko, Ekaterina I. Surikova, Lyudmila A. Nemashkalova, Irina V. Kaplieva, Lidia K. Trepitaki, Valeria А. Bandovkina, Yulia А. Pogorelova. Content of apoptosis factors and self-organization processes in the mitochondria of heart cells in female mice C57BL/6 under growth of melanoma B16 / F10 linked with comorbid pathology. Cardiometry; Issue 18; May 2021; p.121130; DOI: 10.18137/cardiometry.2021.18.121130; Available from:
In the myocardium, that is one of the most energy-consuming tissue types in the body, mitochondria occupy 30-40% of the cell volume against 6-8% found in slow and 2-3% in fast muscle fibers. Mitochondria in oxidizable tissues, such as the heart, are numerous, located mostly near myofilaments, and are capable to use fatty acids for energy production. They are adjusted to the continuous supply of energy needed to provide the muscle endurance and the heart function
-
[1] . This operation can be reflected in a complex of functional, morphological, molecular-genetic, and biochemical parameters, most of which are signal markers of multi-level mechanisms of the homeostasis regulation in biological systems, governed by the unified, commonly shared, principle of the causal determination from the genome to the organism as a whole [2]. This integrated approach is the basis for advanced digital technologies for detecting cardiopathology at high functional loads in professional sports, under various diseases, including oncological processes and comorbid pathology linked with mitochondrial dysfunction of cardiomyocytes (the digital technology has been implemented in PC-assisted hemodynamic analyzer CARDIOCODE, STC LLC "Cardiocode", Certificate of Registration RU No. FSR 2011/12126, Taganrog, Russia).
Mitochondria are a well-known regulator of the endogenous cardiomyocyte apoptosis. In response to signals of death and accumulation of reactive oxygen species (ROS), the mitochondria facilitate the release of apoptogenes, such as cytochrome C, caspases and apoptosis-inducing factor (AIF) into the cytosol from the mitochondria [3]. Once in the cytosol, these apoptogenes bind to the activator of the apoptotic program, protease-1 (Apaf1), to provide assembly of the apoptosome for the recruitment and activation of procaspase-9 [4].
The integrity of the outer mitochondrial membrane is regulated both by the anti-apoptic (Bcl-2, Bcl - XL) and pro-apoptic loci (Bax, Bak) of the Bcl-2 protein family. In general, the ratio between the levels of the apoptotic and proapoptotic proteins in the cell is determined by the permeability of the outer mitochondrial membrane and the release of cytochrome C into the cytosol and the activation of caspases with the cleavage of various cellular proteins, causing rapid cell death [5].
Unlike other cell types, cardiomyocytes are highly resistant to the caspase-dependent apoptosis, hence, the death of cardiomyocytes shall involve some caspase-independent apoptotic mechanisms. This is due to the high level of expression of the endogenous apoptosis inhibitors in cardiomyocytes, which prevents Apaf1 from its activity and caspase from its activation even in the presence of the apoptic stimuli [6]. Changes in the mitochondrial morphology also contribute to apoptotic responses in the heart. For example, under the hypertensive heart conditions caused by angiotensin II, the cytoprotective factor SIRT1 is suppressed that allows the acetylation of 122 | Cardiometry | Issue 18. May 2021
p53 and the Drp1-dependent mitochondrial division, due to which mitochondrial fragmentation ultimately leads to the cardiomyocyte apoptosis [7]. In addition, the suppression of protein phosphatase 1 in acute ischemic injury activates mitochondrial division and cardiomyocyte apoptosis [8]. Damage to the heart muscle during reperfusion can also cause a collapse in the heart microcirculation, facilitating the expression of mitochondrial division factor Mff, which promotes the apoptosis of the endothelial cells in the heart microcirculation through increased opening of the mitochondrial transition pore and release of cytochrome C into the cytosol [9]. Taken together, the morphological changes in the mitochondria are important reasons for the stimulation of the cardiomyocyte apoptosis in heart diseases. Studies of some pathophysiological features of cardiomyopathies at the subcellular level can be conducted with the involvement of experimental models, which are beneficial for resolving issues in the context of translational medicine [10, 11, 12].
The aim hereof is to study the parameters of apoptosis in the mitochondria in the heart cells of female mice of the C57BL/6 strain with the growth of experimental melanoma B16/F10 linked with chronic neurogenic pain as comorbid pathology.
Materials and methods
-
2018) . Manipulations with animals were performed in the box in compliance with the generally accepted rules of asepsis and antisepsis.
We used the line of mouse melanoma B16/F10 supplied by the Russian National Medical Research Center of Oncology named after N.N.Blokhin, Ministry of Health, Russia. The material for transplantation was obtained from donor mice on day 12-16 of the tumor growth. The B16/F10 melanoma transplantation was performed by a standard subcutaneous injection of the tumor suspension under the right scapula in a volume of 0.5 ml of the cell suspension in a 1:10 dilution with saline solution. Tools, dishes, and hands were disinfected in a generally accepted way in compliance with the generally accepted rules of asepsis and antisepsis.
The model of chronic neurogenic pain (CNP) was reproduced by applying a ligature to the sciatic nerve on both sides under xyl-zoletil anesthesia [13]. The anesthesia medication was employed as follows: xyl-zo-letil, 10 minutes before the main anesthesia; premedication: xylazine (Xyl preparation) intramuscularly, at a dose of 0.05 ml/kg of body mass (according to the instructions), then after 10 minutes, Zoletil-50 was administered at a dose of 10 mg per 100 g of body mass.
The animals were randomly assigned to the following experimental groups: the intact group (n=21), the control group (n=21) to reproduce the model of chronic neurogenic pain (CNP), the comparison group (n=63) with mice upon standard subcutaneous B16/F10 melanoma transplantation, and the main group (n=63) with mice which were reproduced with a model of CNP and which 3 weeks after that were transplanted with the B16/F10 melanoma.
Decapitation of the animals has been performed with the guillotine, in the main and the comparison group, in the following periods of time: week 1 is day 7 of the melanoma growth, week 2 is day 14 of the melanoma growth, and week 3 is day 21 of the melanoma growth. After decapitation, the heart was quickly extracted with the use of refrigerants; mitochondria were isolated by the method of Egorova M. V., Afanasiev S. A. [14] (using refrigerants and differential centrifugation on a high-speed refrigerated centrifuge Avanti J-E, BECMAN COULTER, USA). The tissues were washed with an icy 0.9% KCl solution. To destroy the intercellular bonds, the cell wall and plasma membranes, we used mechanical treatment of tissues with grinding with scissors and homogenization in a glass homogenizer with a Teflon pestle (Potter-Elveh- jem homogenizer). We have added per gram of tissue, 10 ml of the isolation medium (0.22 M mannitol, 0.3 M sucrose, 1 mm EDTA, 2 mM TRIS-HCL, 10 mm HEPES, pH 7.4). The tissues were homogenized and centrifuged for the first time for 10 minutes at a speed of 1000 g, at a temperature of 0 - 2 °C, the second and third centrifugation was carried out at 20000 g, for 20 minutes, at a temperature of 0 - 2 °C. Between the centrifugation procedures, the mitochondrial sediment was resuspended in the isolation medium. Mitochondria were further purified from lysosomes, peroxisomes, melanosomes, etc., with the use of a 23% Percoll gradient. The suspension of subcellular structures was layered on the Percoll gradient, centrifuged for 15 min at 21000 g, after which the separation into 3 phases was observed; the lower layer of mitochondria was left and resuspended with the isolation medium. The next washing of the mitochondria was performed by centrifugation for 10 minutes at 15000 g, at a temperature of 0 - 2 °C. The mitochondrial samples (protein concentration 4-6 g / l) were stored at -80 ° C in the isolation medium before their analysis. By the ELISA method determined were the following concentrations: cytochrome C (ng/mg protein), caspase-9 (ng/mg protein) (Bioscience, Austria), Bcl-2 (Thermo Fisher Scientific, Austria), AIF (ng/mg protein) (RayBiotech, USA); the concentration of calcium (Ca2+) (mmol/g protein) was determined by the method of arsenazo III (Abris+, Russia), and protein (mg/ml) was estimated by the Biuret method (Olvex Diagnosticum, Russia) with the ChemWell automatic analyzer (Awareness Technology INC, USA).
In parallel with the study of the apoptosis factors, a visual examination of the supramolecular processes in the mitochondrial self-organization in native mitochondrial suspension smears made on slide glasses was performed for the general assessment of the state of the mitochondrial energy. Smears were stained according to Pappenheim, and we used also an alcoholic solution of fuchsin with the addition of a drop of ammonia applied to the smear under microscopy, photo and video recording. Applied were the techniques of light, dark and polarizing microscopy with the Leica DM LS2 microscope with the x40, x90,x100 magnification, by capturing photo and video images utilizing digital camera OLYMPUS Camedia C-5050 (Germany) and image input device OLYMPUS C-3040 ADU (Japan).
The Statistica 10.0 software was applied for statistical analysis of the obtained data. The data were
Issue 18. May 2021 | Cardiometry | 123
analyzed for the compliance of the features distribution with the normal distribution law using the Shapiro-Wilk test (for small samples). The comparison of the quantitative data in the groups (independent sampling) was carried out using the Kruskal-Wallis test (multiple comparisons). The table data are presented in the M±m form, where M is the arithmetic mean, and m is the standard error of the mean; p<0.05 was taken as the level of statistical significance. The obtained results were statistically processed in compliance with the general recommendations for medical research.
Results
The results have shown that the reproduction of chronic neurogenic pain (CNP) caused by bilateral ligation of the sciatic nerves makes a stimulating effect on the growth of experimental melanoma in female mice and promotes early tumor formation and metastasizing, as well as shortening of the life expectancy span of animals and canceling the genetically determined inhibition of the malignant process [15].
In the dynamics of two variants of the melanoma growth, i.e. an independent melanoma growth and that with comorbid pathology (CNP), the state of the heart muscle in female mice was examined and assessed macroscopically and morphologically; the results thereof are presented in Table 1 herein.
The morphological examination of the areas of the heart muscle damage has demonstrated that in the myocardium there are hemorrhage, foci of necrosis, ruptures of some individual cells, focal infiltration by leukocytes, fibrinous necrosis of the vascular walls, enlarged heart cavities, blood clotting in the lumen.
Firstly, it was of interest to study changes in the dynamics of the apoptosis factors in the mitochondria of the heart cells during the standard growth of melanoma (1, 2, 3 weeks) (see Table 2 herein). It was found that the level of calcium in the mitochondria of the heart after 1 week of the tumor growth decreased by 2.9 times compared with the same indicator in the intact mice; after 2 weeks it decreased by 6.7 times, after 3 weeks it had no statistically significant differences from the indicators recorded in the previous study period. The level of AIF in the heart cell mitochondria in the dynamics of standard melanoma growth changed as follows: after 2 weeks of the tumor growth it decreased by 1.4 times ( p<0.05 ) compared with the respective indicator in the intact animals, and after 3 weeks thereof it was 2 times lower. The level of Bcl-2
Table 1.
Heart state in female mice with independent growth of the B16/F10 melanoma versus that against the background of chronic neurogenic pain
|
Groups of animals |
% of animals with heart muscle damage |
||
|
week 1 n=21 |
2 weeks n=21 |
3 weeks n=21 |
|
|
Standard melanoma growth B16/F10 |
- |
||
|
Stimulated melanoma growth (CNP + B16/F10) |
14.3% |
57.1% |
71.4% |
Note: The table shows % of the animals with heart muscle damage.
after 1 week decreased, if to compare with the values in the intact animals, by 1.7 times, and after 2 weeks it was recorded to be 2.9 times lower, remaining the same upon expiration of 3 weeks. The level of cytochrome C and caspase 9 in the mitochondria of the heart cells did not have statistically significant changes in the dynamics of the standard melanoma growth.
In addition, it was important to study the indicators of apoptosis in the mitochondria of the heart cells after the reproduction of CNP before the tumor inoculation. It was found that under the influence of CNP, in the mitochondria of the heart, the level of calcium decreased by 3.2 times, the level of Bcl-2 by 1.3 times ( p <0.05 ) and caspase 9 by 1.5 times ( p<0.05 ) as compared with the respective indicators in the intact mice. At the same time, the AIF content, on the contrary, increased by 2.3 times, and the level of cytochrome C did not significantly differ from the intact level (see Table 2 herein).
-
1 week after the inoculation and the growth of melanoma against the background of CNP as the comor-bid process, the level of calcium in the mitochondria in the heart cells in the female mice increased by 5.3 times as compared with the control values. In the subsequent periods of the study, the level of calcium in the mitochondria in the heart cells during the tumor process against the background of CNP decreased to almost undetectable values. The level of the AIF concentration in the mitochondria in the heart cells in the dynamics of the melanoma growth linked with CNP changed abruptly: 1 week after the tumor transplantation, it increased by 3.7 times, then after 2 weeks it fell to the level of the control values, and after 3 weeks it became 5.2 times lower than that recorded in the control animals and 2.3 times lower as against that in the mitochondria of the heart cells in the intact ani-
Table 2
Content of apoptosis factors in the mitochondria in the heart cells in female mice with standard versus stimulated by chronic neurogenic pain growth of melanoma B16/F10
Са2+ mmol / g protein
AIF ng / mg protein
BCL-2. ng / mg protein
Cytochrome C ng / mg protein
Caspase 9 ng / mg protein
Intact group
0.194±0.015
40.53±1.25
62.00±2.85
4.61±0.57
0.266±0.028
Control group (CNP)
0.061±0.0041
91.24±3.721
46.66±3.871
6.18±0.59
0.174±0.0181
B16/F10 melanoma growth (comparison group)
week 1
0.067±0.0151
36.62±1.74
36.85±1.921
5.11±0.59
0.238±0.019
week 2
0.029±0.0021
29.51±1.381
21.38±1.561,3
3.81±0.47
0.204±0.02
week 3
0.024±0.0021
19.91±1.481,3,4
21.6±1.551,4
4.92±0.43
0.221±0.018
CNP + B16/F10 melanoma growth (main group)
week 1
0.326±0.0162
340.8±11.642
80.99±1.62
7.62±0.47
0.388±0.0222
week 2
0.01±0.0012,3
107.03±4.262,3
24.03±1.382,3
2.93±0.472,3
0.457±0.0342
week 3
0.01±0.0012,4
17.68±1.52,3,4
19.35±1.092,4
2.74±0.362,4
0.420±0.0262
Note: statistically significant differences: 1 - in relation to the level in the intact group; 2 - in relation to the level in the CNP groups
(control); 3 - in relation to the level at the previous period of the study; 4 - in relation to the level in week 1.
mals. The levels of Bcl-2 and cytochrome C were similarly altered. Thus, the level of Bcl-2 after 1 week of the melanoma growth against the background of CNP increased by 1.7 times (p<0.05 ) as compared with the control level, and then after 2-3 weeks it decreased and became on average 2.2 times lower than that in the mitochondria of the control group. The level of cytochrome C after 1 week had no statistically significant differences from the values in the control group, and after 2-3 weeks it decreased by 2.2 times. The level of caspase 9 in the mitochondria in the mouse heart cells during the growth of melanoma linked with CNP was high, on average, 2.4 times higher than the control values recorded in all the study periods.
The investigation of molecular factors of the apoptosis regulation mitochondrial mechanism was supplemented by morphological studies of the isolated mitochondria in the heart cells in the C57BL/6 female mice with the growth of the B16/F10 melanoma linked with chronic neurogenic pain.
Under microscopy of the heart cells mitochondria suspension smears in week 2-3 of the melanoma growth against the background of CNP, a process of high-brightness cold light (chemiluminescence) was recorded. During the photo and video examination, the phenomenon of luminescence was manifested both in the form of a spot-type chemiluminescent reaction by the substrate, representing vast areas of small granules of mitochondrial associates, and in the form of the formation of enlarged complexes-associates of the linear filamentous shape, located in the field of view. The luminescence of the heart mitochondrial samples was accompanied by bright flashes, as well as 10-15 sec- onds of intense white glow with its gradual fading and sedimenting a large filamentous aggregation of mitochondria on the substrate layer (see Figure 1 herein).
The luminescence effect was not observed in preparations of other organs: it was detected only in the associates of the mitochondria of the heart in animals showing the tumor growth against the background of chronic neurogenic pain. Concomitant comorbid pathology, CNP, contributed to the deployment of the processes of disabling of the energy supply systems in the energy systems of cardiomyocytes. Apparently, the disabling process occurred through an elevation of a low-energy shift, an increase in acetic-oxalic- restriction (degree 1-4), and an increase in free-radical activity, as was previously shown in chemical carcinogenesis [16]. It can be assumed that structuring of the mitochondrial associates of cardiomyocytes is associated with the formation of long electron transport systems, the suppression of respiration against the background of a drop in the level of cytochrome C and the depletion of endogenous succinic acid reserves [16]. All of the above factors contributed to the production of the effect of the bright luminescence chemical reaction. Obviously, the revealed effect of the induced chemiluminescence of the heart mitochondria is directly related to a significant increase in the concentration of calcium in week 1, which is also one of the key conditions for the regulation of apoptosis. In this case, we can note a single complex path of self-organization of the mitochondrial processes in the implementation of the program of cardiomyocyte death, including the detected changes in Bcl-2, AIF and caspase content as a natural result from the influence of chronic neuro-
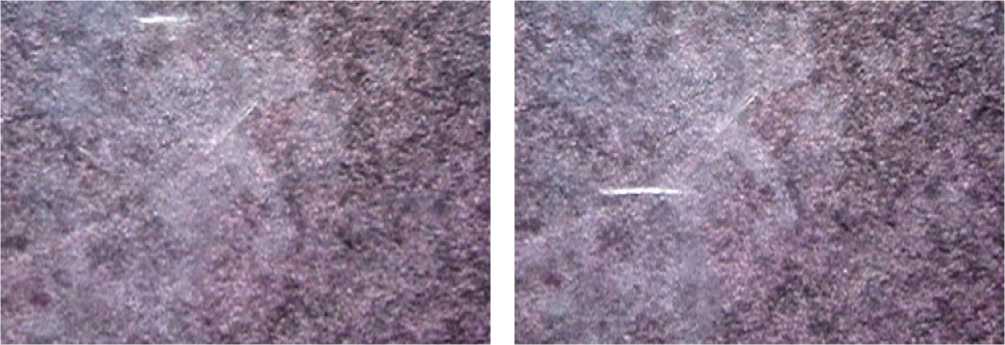
Figure1. Suspension of the heart mitochondria. C57BL/6 strain mice, chronic neurogenic pain, 2-3 weeks of tumor growth. The effects of cold light, chemiluminescence with dislocation of filamentous associates of the heart mitochondria are observed.
genic pain under the growth of melanoma B16 / F10. The common structural and functional features of the degradation in the mitochondria of the heart cells demonstrate the leading role of these organelles in the triggering mechanisms of apoptosis.
Discussion and conclusions
A number of authors have shown that the mitochondria in the heart cells in female rats show their greater capability to retain calcium than it is the case with the mitochondria in the male rodents [17]. The calcium kinetics also differs between the heart mitochondria in the male and female rats [18]. Mitochondria in females are more resistant to mitochondrial swelling at high concentrations of calcium [19]. The influx of calcium into the mitochondria is necessary for their activation [20].
In our study, it was found that in the dynamics of the standard growth of melanoma B16/F10 in female mice, the level of calcium in the mitochondria of the heart cells progressively decreased, but still remained at the level of the determined values of the indicator.
It is known that calcium is an important secondary messenger responsible for linking of the contractile function of the heart and mitochondrial metabolism [21]. The authors hereof have shown a correlation between the mitochondrial calcium content and the cardiac dysfunction under chronic stress. However an exact amount of calcium absorbed by mitochondria in the normal and weakened hearts has not been determined.
Taking into account the above, we can assume there is a disorder in the contractile function of the heart in the dynamics of the standard growth of melanoma, as a manifestation of tumor stress, or rather, a systemic effect produced by the tumor on the organ- 126 | Cardiometry | Issue 18. May 2021
ism. However, our macroscopic and morphological examination revealed no signs of damage to the heart wall. Interestingly, CNP as an independent pathogenic factor also led to a decrease in the level of calcium in the mitochondria of the heart, but without disordering the structural integrity of the myocardial cells.
Only under the conditions, when the initial growth of melanoma occurred against the background of CNP as comorbid pathology, a sharp increase in the content of calcium in the mitochondria in the heart cells was observed (week 1). Only in 14.3% of the cases, myocardial damage was detected (see Table 1 herein).
It should be mentioned that mitochondrial metabolism is stimulated by calcium, but under pathological conditions, calcium overloading can trigger opening of the mitochondrial permeability transition pore (mPTP). The release of the mitochondrial content, such as cytochrome C, causes apoptosis or loss of membrane potential and, as a result, prolonged opening of mPTP, which causes ATP deprivation and necrosis [22]. Most of the evidence data on calcium toxicity in mitochondria comes from experiments using genetic approaches. Indeed, calcium overloading in a number of animal models leads to mitochondrial phenotypes similar to those observed in heart failure, such as mPTP opening, increased mitochondrial oxidative stress, mitochondrial membrane potential collapse, impaired ATP production and cardiomyocyte necrosis [23,24].
At a later stage of the study, the further growth of melanoma against the background of CNP (week 2 and 3) was characterized by a sharp decrease in the level of calcium in the mitochondria of the heart almost to zero that can be regarded as a dramatic disorder in the heart rhythm. Just in these periods of the study we have detected 57.1-71.4% of the cases of heart attacks in female mice (see Table 1 herein).
It should be noted that the most obvious connection between myocardial infarction caused by myocardial ischemia and necrotic cell death is mPTP. Cardiomyocytes, as terminally differentiated cells, have an extremely limited ability to regenerate, and excessive death of heart myocytes caused by stress and their pathological effects leads to the development of various heart diseases, including myocardial infarction [25]. Using a similar probe, Hamilton S. and Terentyev D. [26] recently have shown that increased mitochondrial calcium accumulation increases the mitochondrial ROS production and increases proarrhythmic spontaneous calcium release.
In our experiment, it was recorded that with the standard growth of melanoma, along with a decrease in the calcium content, starting from the second week after the tumor transplantation, a decrease in the level of AIF was observed in the mitochondria of the heart in female mice. This fact should probably be considered as a gradual transition to the hypoxic type of respiration and the production of ATP. A slightly different situation occurs when studying the dynamics of AIF in the mitochondria of the heart during the growth of melanoma stimulated by CNP. First of all, CNP itself induces a more than twofold increase in the level of this factor and an even greater increase therein upon expiration of 1 week after tumor transplantation, which also correlates with the level of calcium.
It should be noted that AIF is an evolutionarily old mitochondrial flavoprotein involved in the embryonic development and survival of the heart cell [27]. Healthy mitochondria contain a mature form of AIF consisting of two FAD-binding domains, the NADH-binding domain and the C-terminal domain. AIF is attached to the inner mitochondrial membrane (IMM), where it plays a bioenergetic role, regulating mainly the activity of complex I of the mitochondrial respiratory chain [28]. It has been found that human or mouse cells deprived of AIF accumulate lactic acid and show an increased dependence on the formation of ATP by glycolysis due to a serious decrease in the activity of the respiratory chain complex I. Further studies have shown that without AIF complex I demonstrates the most serious damage. So, AIF is required for the normal process of oxidative phosphorylation [3]. In addition, AIF deficiency leads to a higher sensitivity to oxidative stress [3]. A rise in the
ROS production may be directly related to an increase in the calcium content. The mitochondrial respiratory chain complexes I-IV transfer electrons to oxygen, producing superoxide radicals as a by-product of this process due to incomplete oxygen reduction. At low concentrations, ROS may mediate some physiological effects, but the ROS overproduction is involved in the pathogenesis of coronary heart disease. Elevated mitochondrial calcium levels were shown in a mouse model of mitochondrial cardiomyopathy [29]. The authors note that this increase in calcium may contribute to a deficiency in oxidative phosphorylation. A deficit of oxygen and respiratory substrates stops oxidative phosphorylation, leading to the collapse of the mitochondrial membrane polarization, calcium overloading, the cytochrome C release, impaired membrane permeability, and, finally, cell necrosis. Thus, mitochondria play a central role in both types of cell death: necrosis and apoptosis [30].
The best-characterized function of the Bcl-2 family consists in controlling the permeability of the mitochondrial outer membrane [5].
In the present study, no increase in the level of Bcl-2 was found in the mitochondria of the heart during any period of the study with standard tumor growth. On the contrary, in the dynamics of the tumor growth, its content in the mitochondria of the heart progressively decreased. With the growth of melanoma linked with comorbid pathology after 1 week of the growth, an increase in the level of Bcl-2 was detected in the mitochondria of the heart, along with an increase in the content of calcium and AIF, and their further decline in the dynamics of tumor growth was recorded.
The drop in the level of AIF could also be interpreted from the standpoint of intracellular stress, which results in the depolarization of the mitochondrial membrane, followed by the release of the apop-togenic form of AIF from the mitochondria into the cell nucleus, where it induces chromatin condensation and large-scale DNA fragmentation through a mechanism independent of caspase activation [27].
As to caspase 9 in the mitochondria of the heart, studied herein, its content in case of the standard growth of melanoma in mice did not have statistically significant differences from the indicators in intact animals throughout the experiment. However, in the mitochondria of the mice heart with the growth of melanoma linked with CNP, the level of caspase 9 remained at high values throughout the study period.
Similarly to AIF, cytochrome C is an important component of the mitochondrial respiratory chain, responsible for the transfer of electrons from complex III to IV, and plays an important regulatory role in oxidative phosphorylation due to its highly dynamic interactions with redox targets [31].
In the context of our study, it was shown that the level of cytochrome C in the mitochondria of the heart with standard growth of melanoma did not experience significant changes during all the study periods, while with the growth of melanoma against the background of comorbid pathology after two weeks, its sharp decline was noted.
The mitochondrial function is known to be closely related to cardiomyopathies and coronary heart disease. Although the exact underlying mechanisms are not fully understood, it is clear that mitochondrial metabolism is intensively involved in both heart damage and protection [22]. We should also mention a unique discovery in the heart muscles: it is a continuous mitochondrial communication [32]. It is noted that there are between the neighboring mitochondria "kissing molecules" available, which provide the exchange of proteins and ions. In addition, nanotunnels are found between the neighboring organelles to control the mitochondrial response, especially under changes in the calcium dynamics [33]. Indeed, in the preparations of the mitochondria of the heart in the animals with the tumor growth against the background of chronic pain, it was possible to see significant areas of associates forming complexes and a spatially complicated, self-organized, network structure. All the above points to the important role of the surface charge in establishing intermitochondrial contacts. Because of the above, permanent pain stimulation, modifying the pathogenesis of the tumor, determines the only way of energy supply through the self-organization of long electron transport pathways, transmembrane movement of protons and an increase in the potential difference at the membrane, followed by photo effects of luminescence in a reactive medium activated by NH4OH. The enlargement of the sizes of the moving in space luminous aggregations is evidence for their complex multidimensional organization. Obviously, the critically high load on the heart under the conditions of an integration of chronic pain and the tumor growth is realized due to the compensatory reaction of the mitochondriome self-organization, as a key factor of life support under the conditions of prolonged stress, 128 | Cardiometry | Issue 18. May 2021
when protection is provided by destruction and high energy consumption.
Taking into account the above, we consider it possible to draw the following conclusions:
-
1 The standard growth of B16/F10 melanoma and the development of CNP as independent variants of pathology in female mice were accompanied by a decrease in the respiratory and energy function of the heart cell mitochondria.
-
2 The combination of both factors, namely, the growth of melanoma against the background CNP as comorbid pathology, significantly aggravated the mitochondrial dysfunction and led to the development of myocardial infarction in the vast majority of animals.
-
3 The mitochondrial mechanisms of apoptosis and self-organization of subcellular energy structures under the conditions of extreme functional loads linked with the growth of a malignant tumor against the background of CNP as a comorbid state are mediated by disordering the poly-enzyme systems of the apoptosis regulation, by a high level of oxidative stress that induces chemiluminescence of mitochondrial associates in cardiomyocytes.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Content of apoptosis factors and self-organization processes in the mitochondria of heart cells in female mice C57BL/6 under growth of melanoma B16 / F10 linked with comorbid pathology
- Ventura-Clapier R, Piquereau J, Veksler V, Garnier, A. Estrogens, Estrogen Receptors Effects on Cardiac and Skeletal Muscle Mitochondria. Frontiers in endocrinology. 2019; 10: 557. DOI: 10.3389/fendo.2019.00557.
- Alla I.Shikhlyarova, Elena Frantsiyants, Lyubov Yu. Vladimirova, Anna E. Storozhakova, Larisa N. Vashchenco, Emma E. Kechedzhieva, Galina V. Zhukova, Elena A. Sheyko. Signal morphological criteria for cardiotoxicity in brest cancer chemothtrapy. Cardiometry. May 2020;16:67-73.
- Bano D, Prehn J. Apoptosis-Inducing Factor (AIF) in Physiology and Disease: The Tale of a Repented Natural Born Killer. EBioMedicine. 2018; 30: 29-37. DOI: 10.1016/j.ebiom.2018.03.016.
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2015; 7(4): a026716. DOI: 10.1101/cshperspect.a026716.
- Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018; 25(3): 486-541. DOI: 10.1038 / s41418-017-0012-4.
- Potts MB, et al. Reduced Apaf‐1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J Cell Biol. 2005; 171(6): 925-930. DOI: 10.1083 / jcb.200504082.
- Qi J, et al. Mitochondrial fission is required for angiotensin II induced cardiomyocyte apoptosis mediated by a Sirt1 p53 signaling pathway. Front Pharmacol. 2018; 9: 176. DOI: 10.3389 / fphar.2018.00176.
- Jin Q, et al. DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff required mitochondrial fission and Bnip3 related mitophagy via the JNK pathways. Redox Biol. 2018; 14: 576-587. DOI: 10.1016 / j.redox.2017.11.004.
- Zhou H, et al. Mff dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS mediated cardiolipin oxidation and HK2/VDAC1 disassociation involved mPTP opening. J Am Heart Assoc. 2017; 6(3): e005328. DOI: 10.1161 / JAHA.116.005328.
- Kit OI, et al. Method for obtaining liver metastases in an experiment. Bulletin of Experimental Biology and Medicine. 2014. Vol. 157. No. 6. pp. 745-747. [in Russian]
- Kit OI, et al. Expression of markers of neoangiogenesis and the fibrinolytic system in the dynamics of experimental renal ischemia in rats. Experimental and clinical urology. 2015. No. 1. pp. 20-23. [in Russian]
- Zhukova GI, et al. Effects of combined exposure to low-intensity electromagnetic radiation of the millimeter range and complexes of essential amino acids in elderly tumor-bearing rats. South-Russian Journal of Oncology. 2020. Vol. 1 no. 4. p. 38-46. [in Russian]
- Kit OI, et al. Effect of chronic neuropathic pain on the course of malignant melanoma B16/F10 in male mice. News of higher educational institutions. The North Caucasus region. Series: Natural Sciences. 2019; 1(201):106-111. [in Russian]
- Egorova MV, Afanasyev SA. Isolation of mitochondria from animal and human cells and tissues: Modern methodological techniques. Siberian Medical Journal. 2011; 26(1-1): 22-8. [in Russian]
- Frantsiyants EM, et al. The effect of urokinase knockout on the growth of melanoma in the experiment. Siberian Scientific Medical Journal. 2019. Vol. 39. no. 4. pp. 62-70. [in Russian]
- Kit OI, et al. Processes of mitochondrial self-organization in the growth of experimental tumors in conditions of chronic neurogenic pain. News of higher educational institutions. The North Caucasus region. Series: Natural Sciences. 2019. No. 2 (202). pp. 97-105. [in Russian]
- Ribeiro RF, et al. Sex differences in the regulation of spatially distinct cardiac mitochondrial subpopulations. Mol Cell Biochem. 2016; 419: 41-51. DOI: 10.1007/s11010-016-2748-4.
- Chweih H, Castilho RF, Figueira TR. Tissue and sex specificities in Ca2+ handling by isolated mitochondria in conditions avoiding the permeability transition. Exp Physiol. 2015; 100: 1073-1092. DOI: 10.1113/EP085248.
- Milerova M, et al. Sex difference in the sensitivity of cardiac mitochondrial permeability transition pore to calcium load. Mol Cell Biochem. 2016; 412: 147-154. DOI: 10.1007/s11010-015-2619-4.
- Umemoto T, et al. Ca2+ -mitochondria axis drives cell division in hematopoietic stem cells. The Journal of experimental medicine. 2018; 215(8): 2097-2113. DOI: 10.1084/jem.20180421.
- Glancy B, et al. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. 2013; 52(16): 2793-2809. DOI: 10.1021/bi3015983.
- Zhou B., Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. The Journal of clinical investigation. 2018; 128(9), 3716-3726. DOI: 10.1172/JCI120849.
- Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015; 78: 100-106. DOI: 10.1016/j.yjmcc.2014.09.023.
- Santulli G, et al. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015; 112(36): 11389-11394. DOI: 10.1073/pnas.1513047112.
- Zhang J., et al. Programmed necrosis in cardiomyocytes: mitochondria, death receptors and beyond. British journal of pharmacology. 2019; 176(22): 4319-4339. DOI: 10.1111/bph.14363.
- Hamilton S, Terentyev D. Altered Intracellular Calcium Homeostasis and Arrhythmogenesis in the Aged Heart. International journal of molecular sciences. 2019; 20(10): 2386. DOI: 10.3390/ijms20102386.
- Farina B, et al. Structural and biochemical insights of CypA and AIF interaction. Scientific reports. 2017; 7(1): 1138. DOI: 10.1038/s41598-017-01337-8.
- Hangen E., et al. Interaction between AIF and CHCHD4 Regulates Respiratory Chain Biogenesis. Mol. Cell. 2015; 58:1001-1014. DOI: 10.1016/j.molcel.2015.04.020.
- Sommakia S, et al. Mitochondrial cardiomyopathies feature increased uptake and diminished efflux of mitochondrial calcium. Journal of molecular and cellular cardiology. 2017; 113: 22-32. DOI: 10.1016/j.yjmcc.2017.09.009.
- Kuznetsov AV, et al. The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants (Basel, Switzerland). 2019; 8(10): 454. DOI: 10.3390/antiox8100454.
- Pérez-Mejías G, et al. Cytochrome c: Surfing Off of the Mitochondrial Membrane on the Tops of Complexes III and IV. Computational and structural biotechnology journal. 2019; 17: 654-660. DOI: 10.1016/j.csbj.2019.05.002.
- Siasos G, et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Annals of translational medicine. 2018; 6(12): 256. DOI: 10.21037/atm.2018.06.21.
- Lavorato M, et al. Increased mitochondrial nanotunneling activity, induced by calcium imbalance, affects intermitochondrial matrix exchanges. Proc Natl Acad Sci USA. 2017; 114: 849-58. DOI: 10.1073/pnas.1617788113.


