COVID-19 и сопутствующая онкологическая патология: терапевтические возможности и сложности
Автор: Патания А.С., Пратипати Ф., Абдул Б.А.А., Чава Ш., Катта С.С., Гупта С.К., Гангула П.Р., Пандей М.К., Дерден Д.Л., Байраредди С.Н., Чаллагундла К.Б.
Журнал: Juvenis scientia @jscientia
Рубрика: Переводные статьи
Статья в выпуске: 6 т.7, 2021 года.
Бесплатный доступ
Коронавирусная инфекция 2019 года (COVID-19) - это вирусное заболевание, вызываемое новым коронавирусом тяжелого острого респираторного синдрома, SARS-CoV-2, который поражает легкие инфицированных лиц. COVID-19 распространяется от человека к человеку посредством респираторных капель, выделяющихся при кашле или чихании инфицированного. Эпидемия COVID-19 началась в городе Ухань (Китай) в конце 2019 года. По состоянию на 29 сентября 2020 года более 235 стран, районов или территорий по всему миру сообщили в общей сложности о 33 441 919 подтвержденных случаях заболевания и о 1 003 497 подтвержденных смертях от COVID-19. Риску заражения подвержены люди любого возраста, но в большинстве случаев тяжелое течение заболевания наблюдается у людей старшей возрастной группы и при наличии сопутствующей патологии, которая снижает иммунитет, например, онкологических заболеваний. Данные многочисленных исследований позволяют предполагать, что пациенты со злокачественными новообразованиями могут быть подвержены более высокому риску тяжелого течения COVID-19 и летального исхода. Поэтому оказание онкологической помощи в условиях пандемии является сложной задачей и требует совместного междисциплинарного подхода для оптимального лечения онкологических больных в условиях стационара. В этом расширенном обзоре мы обсуждаем влияние пандемии COVID-19 на онкологических больных и оказание им медицинской помощи. Помимо этого, обзор содержит общие сведения о пандемии, вызванной SARS-CoV-2, характеристику генома вируса, описание патофизиологии COVID-19 и связанных с ней сигнальных путей, играющих важную роль при онкопатологии, а также информацию о вариантах использования противоопухолевых средств для лечения COVID-19.
Covid-19, коронавирусы, sars-cov-2, онкологические заболевания, воспаление, сопутствующая патология
Короткий адрес: https://sciup.org/14123689
IDR: 14123689 | DOI: 10.32415/jscientia_2021_7_6_28-70
Текст обзорной статьи COVID-19 и сопутствующая онкологическая патология: терапевтические возможности и сложности
Translated article
DOI: 10.32415/jscientia_2021_7_6_28-70
COVID-19 AND CANCER COMORBIDITY: THERAPEUTIC OPPORTUNITIESAND CHALLENGES (RUSSIAN TRANSLATION)
A. S. Pathania1, P. Prathipati2, B. A. A. Abdul3, S. Chava1,S. S. Katta4, S. C. Gupta5, P. R. Gangula6, M. K. Pandey7,D. L. Durden8, 9, 10, S. N. Byrareddy1, K. B. Challagundla1
-
1 University of Nebraska Medical Center
Omaha, NE 68198, USA
-
2 National Institutes of Biomedical Innovation, Health and Nutrition
Saito-Asagi Ibaraki City, Osaka 567-0085, Japan
-
3 PRIST Deemed University
Vallam, Tamil Nadu 613403, India
-
4 REVA University, Rukmini Knowledge Park Kattigenahalli
Yelahanka, Bangalore, Karnataka 560064, India
-
5 Banaras Hindu University
Varanasi, Uttar Pradesh 221005, India
-
6 Meharry Medical College
Nashville, TN 37208, USA
-
7 Cooper Medical School of Rowan University
Camden, NJ 08103, USA
-
8 Levine Cancer Institute
Atrium Health, Charlotte, NC 28202, USA
-
9 University of California
San Diego, San Diego, CA 92093, USA
-
10 SignalRx Pharmaceuticals
Omaha, NE 68124, USA
Tranlators: A. A. Vakhitova ® 11, K. V. Shekhtman © 11
Editor : I. Yu. Pchelin © 11
-
11 Saint Petersburg State University
Original article: Pathania AS, Prathipati P, Abdul BAA, et al. COVID-19 and Cancer Comorbidity: Therapeutic Opportunities and Challenges . Theranostics. 2021;11(2):731-753. DOI: 10.7150/ thno.51471.
The article was translated into Russian and published under the terms of the Creative Commons Attribution 4.0 license.

This article is licensed under a Creative Commons Attribution 4.0 International License.
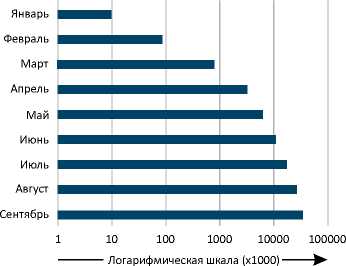
Пандемия COVID-19: Первая вспышка коронавирусной инфекции 2019 года (COVID-19), которая затем привела к пандемии, возникла в городе Ухань (Китай) в 2019 году. В декабре 2019 года у группы людей, работающих на оптовом рынке морепродуктов Хуанань в китайской провинции Хубэй, городе Ухань, была диагностирована COVID-19-ассоциированная пневмония [1]. В Соединенных Штатах Америки (США) первый подтвержденный случай заражения новой коронавирусной инфекцией (SARS-CoV-2) был зарегистрирован 20 января 2020 года у 35-летнего мужчины, который приехал из города Ухань (Китай) [2]. Вскоре во многих других странах было зарегистрировано растущее число пациентов с положитель- ными тестами на коронавирусную инфекцию, и данное заболевание стало серьезной угрозой для здоровья людей во всем мире (Рисунок 1) [3-5]. В марте 2020 года Всемирная организация здравоохранения (ВОЗ) охарактеризовала распространение COVID-19 как пандемию и объявила о чрезвычайной ситуации в области общественного здравоохранения, имеющей международное значение [6].
Вирус распространяется преимущественно от человека к человеку. Передача вируса происходит с выделениями из носа или рта, в том числе воздушно-капельным путем и через слюну, когда инфицированный человек кашляет, чихает, разговаривает или поет [79]. Заражение может также произойти, если
А Количество подтвержденных случаев в мире
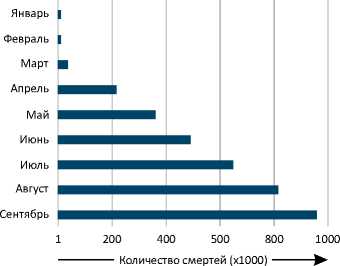
С Количество смертей в десяти наиболее пострадавших государствах
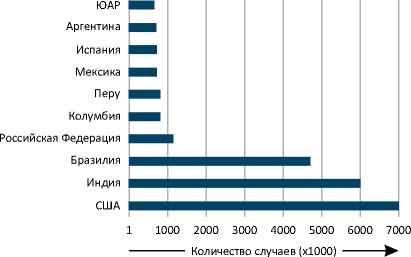
B Количество смертей в мире
D Количество смертей в десяти наиболее пострадавших государствах
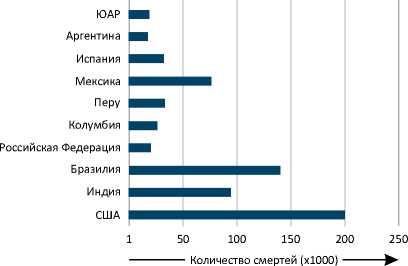
Рисунок 1. Подтвержденные случаи COVID-19 и летальные исходы заболевания в мире по состоянию на 30 сентября 2020 года. Представлено количество подтвержденных случаев COVID-19 и смертей от данного заболевания в мире по месяцам (A, B), а также в отдельных государствах (C, D) [185, 186].
человек сначала прикоснулся к поверхности, на которой находится вирус, а затем к собственному рту, носу или, возможно, глазам [10]. Недавние исследования показали, что многие коронавирусы летучих мышей (CoVs) заражают людей без участия дополнительного переносчика. SARS-CoV и коронавирус ближневосточного респираторного синдрома (MERS-CoV), которые также возникли впервые у летучих мышей, привели к наиболее значительным вспышкам распространения инфекции среди людей до пандемии, вызванной SARS-CoV-2. Симптомы, наблюдаемые у пациентов с COVID-19, включают проявления тяжелого респираторного заболевания, лихорадку, кашель, одышку, боль в горле, заложенность носа, усталость, боли в различных частях тела, потерю вкуса или обоняния, а у некоторых также желудочно-кишечные расстройства [11, 12]. У части пациентов происходит развитие двусторонней пневмонии и полиорганной недостаточности, что в ряде случаев приводит к летальному исходу [13-16]. Люди с выраженной сопутствующей патологией, такой как онкологические и сердечно-сосудистые заболевания, диабет и хронические заболевания легких, подвержены более высокому риску при заражении SARS-CoV-2 [17-21]. В настоящее время не существует ни вакцины, ни специфических противовирусных препаратов, одобренных для лечения COVID-19, но некоторые лекарственные средства были разрешены для применения в экстренных ситуациях у определенных пациентов. К ним относятся кортикостероиды (например, дексаметазон, гидрокортизон, метилпреднизолон, преднизолон) и противовирусный препарат ремдесивир [22].
Характеристика генома SARS-CoV-2. Организация генома SARS-CoV-2 представлена на рисунке 2. SARS-CoV-2 (2019-nCoV HKU-SZ-005b, регистрационный номер GenBank MN975262) имеет одноцепочечный РНК-геном размером 29891 нуклеотид, который кодирует 9860 аминокислот [23].Он состоит из: 5’-нетранслируемой области (UTR); открытой рамки считывания (ORF) 1a/b, которая кодирует неструктурные белки (nsp), репликазы; генов структурных белков, расположенных в следующем порядке: спайковый (S), оболочечный (E), мембранный (M) и нуклеокапсид-ный (N); вспомогательных белков, включая ORF 3, 6, 7a, 7b, 8 и 9b, за которыми следует 3’-нетранслируемая область (3’-UTR) [23, 24]. Спайковый белок SARS-CoV-2, который обеспечивает связывание вируса с рецепторами клетки, состоит из двух субъединиц, S1 и S2. Трансмембранная субъединица S2 характеризуется высокой консервативностью, ее функция заключается в обеспечении проникновения вируса в клетки-мишени [25, 26]. Субъединица S1 состоит из сигнального пептида (СП), N-концевого домена (N-КД) и рецептор-связывающего домена (РСД). Для РСД SARS-CoV-2, как и других патогенных коронавирусов человека, характерна низкая консервативность. Данный домен отвечает за связывание с ангиотензинпревращающим ферментом 2 (AПФ2) на клетках человека, что облегчает проникновение вируса в клетки-мишени [25, 27]. Большинство различий в последовательности аминокислот наблюдаются во внешнем субдомене, тогда как центральная часть РСД очень консервативна. Шесть аминокислот (L455, F486, Q493, S494, N501 и Y505) РСД являются основными детерминантами эффективного связывания SARS-CoV-2 с AПФ2, присутствующим на поверхности клеток организма человека. Кроме того, спайковый белок SARS-CoV-2 имеет участок, включающий последовательность из четырех определенных аминокислот, который расположен на границе между субъединицами S1 и S2. Эта уникальная последовательность (SPRRAR↓S) содержит сайт расщепления фурином, который играет важную роль в активации спайкового белка SARS-CoV-2 [25, 28, 29]. Присутствие многочисленных остатков аргинина в участке на границе S1/S2, также известном как многоосновный участок расщепления фурином, обнаружено во всех секвенированных к настоящему времени вариантах SARS-CoV-2 [30].
A
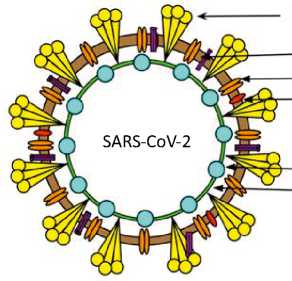
Гликозилированный спайковый белок (S)
Оболочечный белок (Е)
Мембранный белок (М)
Димерный белок гемагглютинин-эстераза (HE)
Нуклеопротеин (N)
РНК
B
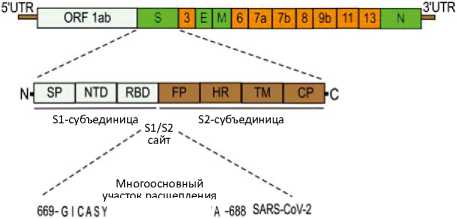
участок расщепления
669-G I CASYQTQTNS- ■ ■ - RSVA -688 RaTG13 669-GVCASY- - - - NS?- AAR- VG-688 RmYN02
Рисунок 2. Структура (А) и организация генома (В) SARS-CoV-2. Геном SARS-CoV-2 состоит из 5’-нетрас-лируемой области; открытой рамки считывания (orf) Ia/b, которая кодирует неструктурные белки (nsp) — репликазы; генов структурных белков, включая спайковый (S), оболочечный (Е), мембранный (М) и нуклеопротеин (N); генов вспомогательных белков, таких как orf 3, 6, 7a, 7b, 8, 9b, 11, 13, и 3’-нетранслируемой области. Спайковый (S) белок имеет 2 функциональных домена — S1 (для прикрепления) и S2 (для слияния), а также многоосновный участок расщепления в месте соединения S1/S2. Приведена молекулярная характеристика сайта расщепления S1/S2 SARS-CoV-2 и наиболее близких к нему родственных вирусов, RaTG13 и RmYN02 [23, 25, 28, 30, 67, 137, 187, 188].
Данный участок необходим для эффективного протеолитического расщепления спайково-го белка, что является обязательным этапом опосредованного S-белком слияния и проникновения вируса в клетки легких человека [28]. Многие коронавирусы проникают и доставляют свой геном в клетки с помощью эндоцитоза [31, 32]. Например, коронавирус гепатита мышей проникает в клетки с помощью клатрин-опосредованного эндоцитоза и позже перемещается в лизосомы благодаря слиянию эндосом с лизосомами. Лизосомальные ферменты расщепляют вирусный белок S в участке непосредственно перед фрагментом, обеспечивающим слияние, который находится в субъединице S2 (см. рис. 2). Этот этап необходим для слияния вирусных мембран с эндоцитарными везикулами, в результате чего происходит высвобождение вируса внутри клетки. Напротив, такие вирусы, как MERS-CoV, не требуют переноса в лизосомы и воздействия лизосомальных ферментов, так как у них присутствует участок расщепления фурином, расположенный в S-белке непосредственно перед фрагментом, обеспечивающим слияние. Более того, показано, что при внедрении сайта расщепления фурином в S-белок коронавируса гепатита мышей пе- ред последовательностью, обеспечивающей слияние, эффективное инфицирование клеток становится возможным без расщепления белка лизосомальными протеазами. Однако, при этом вирус все еще нуждается в ранних эндосомах для эффективного проникновения, и это позволяет предположить, что в них происходит расщепление и активация S-белка фурином [31]. Таким образом, коронавирусы, содержащие участок расщепления фурином непосредственно перед обеспечивающим слияние фрагментом S-белка, попадают в цитоплазму из ранних эндосом фурин-зависи-мым способом, тогда как те, у которых этого участка нет, с большей вероятностью проникают в нее из лизосом после воздействия лизосомальных ферментов [31].
Сообщается, что многие протеазы, включая фуриновую, трансмембранную сериновую протеазу II типа (TMPRSS2), PC1, трипсин, ма-триптазу, катепсин в и катепсин L, расщепляют сайт S1/S2 и потенциально могут активировать S-белок SARS-CoV-2 [28, 33].
Кроме того, участок S1/S2 в SARS-CoV-2 содержит в своем начале пролин (ключевая последовательность SPRRAR↓S), который может влиять на протеолиз и конформационные изменения в спайковом белке, что, в свою очередь, изменяет его взаимодействие с рецептором клетки и влияет на патогенность вируса [33]. Предполагается, что наличие в начале остатка пролина позволяет добавлять О-свя-занные гликаны к остаткам соседних аминокислот, которые могут создавать муциноподобный домен. Эти домены могут защищать вирусные эпитопы от иммунной системы хозяина [34]. Некоторые вирусы, в том числе коронавирусы, используют такие муциноподобные домены для уклонения от иммунной системы хозяина. Можно предполагать использование данного механизма повышения вирулентности и в случае с SARS-CoV-2 [35]. Однако, Tse и соавт. показали, что устранение в вирусе птичьего гриппа H9N2 участка гликозилирования, который находится очень близко к участку расщепления фурином, уве- личивает эффективность его трансдукции [36]. Поэтому экспериментальное определение роли предполагаемого гликозилирования вблизи участка S1/S2 важно для понимания механизма инфицирования SARS-CoV-2 и патогенеза COVID-19.
Вследствие широкого распространения в ряде стран со временем стало нарастать генетическое разнообразие SARS-CoV-2 [37]. Однако, приобретенное генетическое разнообразие невелико. Средняя разница между двумя вирусными геномами составляет 9,6 однонуклеотидных замен, что указывает на наличие недавнего общего предшественника. Анализ геномных мутаций, присутствующих в глобальной популяции SARS-CoV-2, выявил 198 повторяющихся мутаций, из которых 80% не вызывают изменений на уровне белков [37]. Наиболее часто повторяющиеся мутации обнаруживаются в трех участках в области Orf1ab (нуклеотидные позиции 11083, 13402 и 16887), кодирующей белки Nsp6, Nsp11 и Nsp13, а также в одном участке в области гена спайкового белка (21575) [37]. Роль этих мутаций пока не определена, но сообщается, что область Orf1ab играет важную роль в патогенезе и адаптации вируса в условиях макроорганизма [38, 39]. Вариабельность последовательности гена nsp2 SARS-CoV-2, находящегося в области Orf1ab, по сравнению с SARS и SARS-подобным коронавирусом летучих мышей, может быть в некоторой степени ответственна за селекционное преимущество SARS-CoV-2, который, таким образом, является более контагиозным, чем другие вирусы [40]. Аминокислота в позиции 501, которая соответствует позиции 321 белка Nsp2, находится в его эндосомно-ассоцииро-ванном белковом домене и является остатком глутамина, тогда как аналогичный сайт генома SARS и SARS-подобного коронавируса летучих мышей содержит остатки треонина и аланина, соответственно. Вполне возможно, что присутствие остатка глутамина обеспечивает более высокую стабильность Nsp2 SARS-CoV-2, благодаря длине его боковой цепи, полярности и способности образовывать водородные связи. Кроме того, эта мутация, приводящая к аминокислотной замене на глутамин в белке Nsp2 SARS-CoV-2, аналогична мутации, обнаруженной в области Nsp2a (идентификатор базы данных Protein Data Bank — 3LD1) генома вируса инфекционного бронхита птиц, которая играет существенную роль в патогенности вируса [40]. Pachetti и со-авт. выявили другие точечные мутации в геноме SARS-CoV-2 в различных географических областях, в том числе в Азии, Европе и Северной Америке. Данная группа исследователей сообщила о трех повторяющихся мутациях в позициях 3036 (в гене nsp3), 14408 (nsp12, или RdRp) и 23403 (в гене спайкового белка) в Европе, а также в позициях 17746 (nsp13), 17857 (nsp13) и 18060 (nsp14) в Северной Америке. Эти мутации не были обнаружены в Азии, что говорит о том, что вирус с течением времени приобретает различные мутации в разных географических регионах [41]. Кроме того, в европейской популяции была обнаружена мутация в гене RdRp (РНК-зависимой РНК-полимеразы) SARS-CoV-2 в положении 14408, которая ассоциирована с увеличением общего количества точечных мутаций в геноме коронавируса [41]. Это немаловажно, поскольку RdRp играет ключевую роль в цикле репликации и транскрипции вируса, а мутации в его гене могут способствовать нарушению корректирующей активности данного фермента [42]. Таким образом, функциональная роль этих мутаций нуждается в дальнейшем изучении, чтобы понять, влияют ли они на патогенность вируса и его устойчивость к лекарственным препаратам. Эти вопросы крайне актуальны с учетом ускоряющегося развития пандемии новой коронавирусной инфекции во всех регионах мира.
COVID-19 и онкологические заболевания: Онкопатология характеризуется аномальной пролиферацией клеток различных локализаций, с возможностью распространения процесса на другие органы и ткани. Механизм действия наиболее часто используемых ме- тодов лечения онкологических заболеваний имеет в своей основе уничтожение или торможение деления быстро размножающихся опухолевых клеток и предотвращение метастазирования. Однако некоторые методы лечения в онкологии подавляют и другие быстро обновляющиеся клетки, такие как лейкоциты, включая Т-и В-лимфоциты в костном мозге, и тем самым могут ослабить иммунную систему [43]. Злокачественные опухоли могут оказывать и непосредственное действие на иммунную систему, метастазируя в костный мозг [44]. Как следствие, пациенты с ослабленной иммунной системой имеют более высокий риск частых инфекций и с большей вероятностью могут заболеть COVID-19. Исследования показывают, что COVID-19 увеличивает риск осложнений и общий риск смерти пациентов с онкопатологией [19, 45-47]. По сравнению с общей популяцией у пациентов с онкопатологией риск смерти от COVID-19 в три раза выше, поскольку их иммунная система может быть ослаблена как опухолевым процессом, так и его лечением [19]. В описанное ниже исследование было включено 105 онкологических больных и 536 сопоставимых по возрасту пациентов без онкопатологии, которые заболели COVID-19. Было показано, что пациенты с онкопатологией имеют относительно высокие уровни летальности, госпитализации в отделение интенсивной терапии (ОРИТ), вероятности использования инвазивной искусственной вентиляции легких (ИВЛ) и риска возникновения критических состояний по сравнению с неонкологическими пациентами при развитии COVID-19. Пациенты с гемобластозами, включая лейкозы, лимфомы и множественную миелому, имеют самый высокий уровень летальности. За ними следуют больные раком легкого и пищевода. Также было показано, что пациенты с IV стадией рака (с метастазами) и COVID-19 подвержены высокому риску смерти, госпитализации в ОРИТ, тяжелого течения заболевания и использования механической вентиляции легких. Было обнаружено, что механическая вентиляция ухудшает исход, поскольку обладает повреждающим действием и не способна эффективно доставлять кислород в пораженные легкие. Кроме того, различные варианты противоопухолевого лечения могут оказывать влияние на течение COVID-19. Пациенты, получавшие иммунотерапию или хирургическое лечение, как правило, имеют более высокий риск смерти и развития критических состояний по сравнению с теми, кто получал химиотерапию или лучевую терапию [19].
Общенациональный анализ онкологических больных с инфекцией, вызванной SARS-CoV-2, в Китае (18 из 1590 случаев COVID-19) показал, что пациенты, перенесшие химиотерапию или хирургическое вмешательство, имели более высокий риск развития тяжелых осложнений, чем пациенты, не получавшие такого лечения по поводу онкопатологии [48]. Однако результаты этого исследования основаны на группе онкологических больных небольшого размера (n=18), что могло быть лимитирующим фактором для формулировки однозначных выводов. Кроме того, ретроспективный анализ 355 пациентов, умерших после заражения SARS-CoV-2 в Италии, показал, что 36% из них страдали сахарным диабетом, 30% — ишемической болезнью сердца, 25% — прогрессирующей онкопатологией, в то время как лишь 0,8% не имели других заболеваний [49]. Аналогичный анализ Trapani и соавт., включавший 909 пациентов, умерших от COVID-19 в Италии, показал, что 17% из них страдали онкологическими заболеваниями, как в фазе ремиссии, так и в периоде активного лечения [50]. Ретроспективный анализ 1878 пациентов с COVID-19, поступивших в стационар в Мадриде, показал, что 2,4% из них были больны злокачественными новообразованиями, из которых 37,7% приходилось на рак легкого. Половина этих больных раком легкого умерли (52,3%), при среднем уровне летальности 10,2% (из 1878 пациентов) [45]. Примечательно, что медиана возраста умер- ших больных раком легкого составила 72 года, при этим медиана возраста выживших равнялась 64,5 года.
Zang и соавт. провели аналогичный анализ, в котором были изучены 28 онкологических больных, получавших ранее противоопухолевую терапию, из общего числа 1276 пациентов с COVID-19, поступивших в три стационара в городе Ухань (Китай). Они обнаружили, что рак легкого является наиболее часто встречающимся типом рака в этой группе, за ним следуют рак пищевода и рак молочной железы [46]. Из всех онкологических больных у 53,6% наблюдались тяжелые осложнения (с потребностью в госпитализации в ОРИТ или в проведении искусственной вентиляции легких), 28,6% пациентов умерли. Это очень высокий процент по сравнению с общей популяцией пациентов, инфицированных SARS-CoV-2, в которой уровень госпитализации составляет 0,16%, при наиболее высоких показателях у людей в возрасте 65 лет и старше (0,3%) [51]. В Китае только 4,7% случаев требовали интенсивной терапии, а 2,3% случаев заканчивались летальным исходом [46]. Количество летальных исходов от COVID-19 у онкологических больных по сравнению с пациентами без онкопатологии показано на рисунке 3. Факторы риска тяжелого течения COVID-19 у онкологических больных представлены на рисунке 4.
Факторы риска тяжелого течения COVID-19 у онкологических больных: Тяжелое течение COVID-19 и смертность среди онкологических больных в значительной степени связаны с возрастом, тяжестью основного заболевания, множественными сопутствующими заболеваниями и вредными привычками, такими как курение. Mehta и соавт. провели исследование с включением 218 онкологических больных с COVID-19 и показали, что пожилой возраст в значительной степени ассоциирован с увеличением риска смерти от COVID-19 [52]. Для пациентов с тяжелым течением заболевания, включая тех, кто нуждался в искусственной вентиляции
|
A 4 3 2 f 1 0 |
B %летальности Количество Количество № Авторы DOI с онко- без смертей у смертей № Авторы DOI патоло- онкопа- пациентов с он- у пациентов без гией тологии копатологией онкопатологии 1 Dai et al. 10.1158/2159-8290.CD-20-0422 11 5 12 (105) 21 (536) 2 Tian et al. 10.1016/S1470-2045(20)30309-0 20 11 46 (232) 56 (519) 3 Rugge et al. 10.1038/s43018-020-0104-9 17,7 4,5 106 (723) 385 (8552) 4 Mehta et al. 10.1158/2159-8290.CD-20-0516 28 15 61 (218) 149 (1090) 5 Miyashita et al. 10.1016/j/annonc/2020.04.006 5,7 1,1 3 (53) 22 (2035) 6 Rogado et al. 10.1007/s12094-020-02381-z 42,2 10,2 19 (45) 192 (1878) 7 Docherty et al. 10.1136/bmj.m1985 35,4 25,2 617 (1743) 3938 (15611) |
||||
|
* 1 |
|||||
|
Без онко- с онко- 8 Shruti et al. 10.1001/jamainternmed.2020.3596 53,6 34,4 60 (112) 724 (2103) патологии патологией 9 Sanchej-Pina et al. 10.1111/ejh.13493 35,9 13,2 14 (39) 7 (53) 10 Meng et al. 10.1186/s13045-020-00907-0 29,4 10,2 32 (109) 260 (2556) |
|||||
Рисунок 3. Сравнение ассоциированной с COVID-19 летальности у пациентов с онкологической патологией и без нее (А) и исходные данные, использованные для сравнения ассоциированной с COVID-19 летальности в этих группах (В). Общее количество пациентов приведено в скобках [19, 52, 58, 99, 181, 189-193].
Сопутствующие заболевания: заболевания сердца (артериальная гипертензия, ИБС, ХСН), заболевания легких (ХОБЛ)



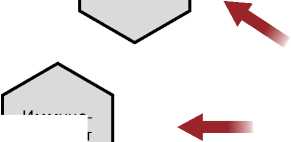
Иммунодефицит

Факторы риска тяжелого течения COVID-19 у пациентов с онкологическими заболеваниями
Тяжесть онкологического заболевания






Локализация опухоли (при раке легкого — высокий риск)
Рисунок 4. Факторы риска, ассоциированные с тяжестью течения COVID-19 у пациентов с онкологическими заболеваниями. ИБС — ишемическая болезнь сердца, ХСН — хроническая сердечная недостаточность, ХОБЛ — хроническая обструктивная болезнь легких.
легких или интенсивной терапии, была характерна высокая летальность. Данная группа авторов не обнаружила статистически значимой связи между наличием прогрессирующего онкологического заболевания с метастазами и риском смерти от COVID-19. Кроме того, пациенты, проходившие химиотерапию или лучевую терапию, не имели существенных отличий по риску COVID-ассоциированной смерти по сравнению с пациентами, не получавшими такого лечения на момент развития инфекции. Сопутствующие заболевания, включая кардиологические заболевания (гипертоническая болезнь, ишемическая болезнь сердца (ИБС), хроническая сердечная недостаточность (ХСН)) и хроническую патологию легких, повышали риск смерти от COVID-19 у онкологических больных. Также при сравнении выживших и умерших от COVID-19 онкологических больных выяснилось, что пациенты, которые в конечном итоге умерли, имели более низкий гемоглобин и более высокие уровни лейкоцитов и нейтрофилов, а также повышенные уровни маркеров воспаления, включая D-димер, лактат и лактатдегидрогеназу [52]. Кроме того, по сравнению со всеми случаями COVID-19 или с сопоставимой по половому и возрастному составу группой неонкологических пациентов с COVID-19, для больных с сопутствующими злокачественными новообразованиями во всех возрастных группах была характерна более высокая летальность. Это еще раз свидетельствует о том, что онкологические больные гораздо хуже переносят данную инфекцию, чем исходно здоровые люди [52-54].
Тяжелое течение COVID-19 у больных с раком легкого было ассоциировано с возрастом, курением в анамнезе, хронической обструктивной болезнью легких (ХОБЛ), артериальной гипертензией и ХСН [55]. Пациенты без ХОБЛ или ХСН в анамнезе, а также те, кто стал меньше курить, имели более высокие шансы на выздоровление. Повышение уровня креатинина у онкологических больных было ассоциировано с тяжелым течением заболевания
(интенсивная терапия, интубация или летальный исход). Однако, у пациентов, недавно получавших химиотерапию или лечение ингибиторами тирозинкиназы, не было выявлено различий в тяжести заболевания (по таким показателям, как потребность в госпитализации, лечение в ОРИТ, интубация и летальный исход) по сравнению с другими больными раком легкого [55].
У пациентов со злокачественными новообразованиями органов грудной полости (включая немелкоклеточный рак легкого [НМРЛ], мелкоклеточный рак легкого, мезотелиому, опухоли тимуса и нейроэндокринные опухоли) возраст (>65 лет), курение, лечение с применением только химиотерапии и наличие каких-либо сопутствующих заболеваний были ассоциированы с повышенным риском смерти от COVID-19 [56]. Факторами риска, связанными с наиболее низкой общей выживаемостью у пациентов с гематологическими злокачественными новообразованиями и COVID-19 (n=536), являлись: пожилой возраст, прогрессирование онкологического заболевания, диагноз острого миелобласт-ного лейкоза, индолентной неходжкинской лимфомы, агрессивной неходжкинской лимфомы или плазмоклеточного новообразования, а также тяжелое или очень тяжелое течение COVID-19. Кроме того, пациенты были подвержены более высокому риску смерти вне зависимости от того, находились ли они в дебюте заболевания или на специфической терапии, или и то, и другое. Летальность у пациентов с гематологическими злокачественными новообразованиями и COVID-19 была более высокой по сравнению с больными COVID-19 в общей популяции и с пациентами с гемобластозами без COVID-19 [57]. Об аналогичных результатах сообщили Sanchez-Pina и соавт., в проведенном ими исследовании пациенты с COVID-19 и гематологическими злокачественными новообразованиями (n=39) имели значительно более высокий уровень летальности по сравнению с пациентами без онкологических заболева- ний. Факторами риска, ассоциированными с летальным исходом, были возраст (>70 лет) и концентрация С-реактивного белка (>10 мг/ дл). Активное химиотерапевтическое лечение и вирусная нагрузка при постановке диагноза не были предикторами неблагоприятного исхода у этих пациентов [54, 58].
Кроме того, у больных раком молочной железы возраст (>70 лет) и артериальная гипертензия были значимо ассоциированы с тяжелым течением COVID-19 (госпитализацией в ОРИТ или летальным исходом) [59]. В когортном исследовании, включившем 1035 онкологических больных с COVID-19 (средний возраст 66 лет), наиболее распространенными злокачественными новообразованиями оказались рак молочной железы и рак предстательной железы [47]. 13% пациентов умерли в течение четырех недель после постановки диагноза COVID-19. В данной группе пациентов с онкопатологией было выявлено большое количество факторов, ассоциированных с риском смерти от COVID-19. К таким факторам относились: возраст (риск летального исхода увеличивался с возрастом), мужской пол (умерло или было госпитализировано в ОРИТ больше мужчин, чем женщин), курение (среди умерших было больше курильщиков или бывших курильщиков), количество сопутствующих заболеваний (большее количество сопутствующих заболеваний было ассоциировано с более высоким риском смерти), тип злокачественных новообразований (пациенты с солидными опухолями умирали чаще, чем больные онкогематологи-ческими заболеваниями) и статус онкологического заболевания. При этом, однако, раса, этническая принадлежность, ожирение и вид проводимого лечения онкологического заболевания не были ассоциированы с уровнем летальности [46, 47]. В таблице 1 показано влияние специфического лечения на тяжесть COVID-19 у онкологических больных. На рисунке 4 представлены факторы риска, связанные с тяжестью COVID-19 у онкологических больных.
Таким образом, онкологические больные более уязвимы к COVID-19 и имеют множество факторов риска, ассоциированных с тяжелым течением заболевания [60]. Кроме того, вероятно, противоопухолевое лечение не связано с тяжестью течения COVID-19, что может помочь лечению онкологических больных во время этой пандемии.
COVID-19, АПФ2, TMPRSS2 и онкологические заболевания: Сходство последовательностей между RBD-доменами SARS-CoV-2 и SARS-CoV предполагает, что SARS-CoV-2 может использовать АПФ2 для проникновения в клетки человека [61]. По данным Hoffmann и соавт., как и в случае с SARS-CoV, проникновение SARS-CoV-2 в клетки зависит от связывания спайкового белка вируса (S) с АПФ2, присутствующим на поверхности клеток [62]. АПФ2 — это цинк-зависимая металлопротеа-за, которая в первую очередь регулирует ре-нин-ангиотензин-альдостероновую систему (РААС) [63]. РААС представляет собой гормональную систему, которая обеспечивает регуляцию артериального давления и баланса жидкости в организме. АПФ2 присутствует на поверхности многих типов клеток по всему организму, но уровень его экспрессии варьирует у разных людей и на различных типах клеток [64-66]. Присутствие АПФ2 в легких, куда вирус SARS-CoV-2 попадает в первую очередь, способствует проникновению вируса в клетки [62, 67]. Домен S1 спайкового белка вируса опосредует связывание с рецептором, тогда как домен S2 обеспечивает слияние мембраны вируса с мембраной клетки. Для слияния SARS-CoV-2 требуется расщепление спайково-го белка эндоцитарной протеазой в сайте S1/ S2 [25, 62, 68]. Множественные остатки аргинина в участке S1/S2 подвергаются действию протеолитических ферментов на поверхности клеток человека, при этом раскрываются аминокислотные последовательности спайкового белка, необходимые для слияния с мембраной клетки. Эту протеолитическую активацию обеспечивают эндосомные цистеиновые протеазы клеток, включая TMPRSS2 и катепсин
Таблица 1
|
Исследования |
Тип лечения |
% исходов (С=смерть, ТТ ― тяжелое течение) NS: статистически не значимо |
|
[19] |
Иммунотерапия |
С: 33,33 против 5 (без онкопатологии), ТТ: 66,8 против 16 (без онкопатологии) |
|
Хирургическое лечение |
С: 25 против 5 (без онкопатологии),ТТ: 62,5 против 16 (без онкопатологии) |
|
|
Химиотерапия |
С: 12 против 5 (без онкопатологии), NS и ТТ: 41 против 16 (без онкопатологии) |
|
|
Лучевая терапия |
С: 8 против 5 (без онкопатологии), NS ТТ: 23 против 16 (без онкопатологии), NS |
|
|
[99] |
Иммунотерапия или таргетная терапия |
ТТ: 81, не-ТТ: 19 |
|
Хирургическое лечение |
ТТ: 40, не-ТТ: 60 |
|
|
Химиотерапия или лучевая терапия |
ТТ: 34, не-ТТ: 66 |
|
|
[135] |
Иммунотерапия |
ТТ при раке легких: 58 против 35 (лечение без иммунотерапии) ТТ при других солидных опухолях: 26 против 15 (лечение без иммунотерапии) |
|
Хирургическое лечение |
Различия между группами NS (наличие / отсутствие хирургического лечения) |
|
|
Химиотерапия |
Различия между группами NS (получавшие / не получавшие химиотерапию) |
|
|
[56] |
Иммунотерапия |
С: 33 против 27 (без лечения), NS |
|
Терапия ингибиторами тирозин-киназы |
С: 29 против 27 (без лечения), NS |
|
|
Химиотерапия |
С: 48 против 27 (без лечения) |
|
|
[194] |
Лечение в течение 4 недель до появления симптомов |
|
|
Иммунотерапия |
С: 1 против 6 (выжили), NS |
|
|
Хирургическое лечение |
С: 3 против 0 (выжили), NS |
|
|
Химиотерапия |
С: 11 против 44 (выжили) |
|
|
Лучевая терапия |
С: 4 против 9 (выжили), NS |
|
|
Таргетная терапия |
С: 4 против 18 (выжили) |
|
|
[195] |
Химиотерапия |
Пациенты с онкогематологическими заболеваниями, недавно получавшие химиотерапевтическое лечение, имеют более высокую вероятность летального исхода во время госпитализации по поводу COVID-19 (отношение шансов 2,09, 95% ДИ 1,09-4,08; p=0,028) |
|
[47] |
Нецитотоксическая терапия: иммунотерапия, лучевая терапия, таргетная терапия и гормональная терапия |
Проводилось / не проводилось лечение в течение 4 недель до постановки диагноза COVID-19 С: 11 против 14, NS ТТ: 24 против 28, NS |
|
Хирургическое лечение |
Проводилось / не проводилось в течение 4 недель до постановки диагноза COVID-19 С: 19 против 13, NS ТТ: 38 против 26, NS |
|
|
Химиотерапия |
Проводилась / не проводилась в течение 4 недель до постановки диагноза COVID-19 С: 14 против 14, NS ТТ: 22 против 28, NS |
|
|
[196] |
Иммунотерапия, хирургическое лечение, химиотерапия, лучевая терапия, гормонотерапия и таргет-ная терапия |
Отсутствие существенного влияния на риск летального исхода у пациентов, получавших терапию в течение предшествующих 4 недель, по сравнению с пациентами, не получавшими лечения |
|
[55] |
Иммунотерапия (анти-PD-1), химиотерапия и терапия ингибиторами тирозинкиназы |
Отсутствие значимых различий в показателях риска С и ТТ по сравнению с пациентами, не получавшими соответствующего лечения |
Влияние специфического лечения на течение COVID-19 у онкологических больных
B и L [33, 62]. В отличие от SARS-CoV, спайко-вый белок SARS-CoV-2 также расщепляется и предварительно активируется во время упаковки вируса в сайте расщепления фурином в участке S1/S2, что снижает его зависимость от протеаз клетки человека при проникновении [68]. Предварительная активация спай-кового белка в месте расщепления фурином и протеолитическая активация протеазами на поверхности клеток оказывают кумулятивное воздействие на развитие вирусной инфекции, что, возможно, и сделало SARS-CoV-2 более заразным по сравнению с родственными ему вирусами (SARS-CoV и вирус гриппа).
Считается, что инфекция, вызванная SARS-CoV-2, снижает активность АПФ2, поскольку вирусы занимают этот фермент как рецептор, ограничивая выполнение им других функций. Одной из важных функций АПФ2 является деградация белка ангиотензина II, приводящая к образованию вазодилататора ангиотензина 1-7 (Ang 1-7) [69]. Накопление ангиотензина II индуцирует воспаление, активируя моноциты, повышая уровень С-реактивного белка и способствуя генерации активных форм кислорода (АФК) [70]. Использование АПФ2 вирусом SARS-CoV-2 вызывает выраженный воспалительный ответ, который приводит к тяжелым последствиям в организме и обусловливает развитие жизнеугрожающих сипмтомов. Исследование на ранее здоровых детях и подростках, инфицированных SARS-CoV-2, показывает, что мультисистемный воспалительный синдром, обусловленный инфекцией, ассоциирован с развитием серьезного жизнеугрожающего состояния [71]. У пациентов со многими видами онкологической патологии была обнаружена низкая активность АПФ2 по сравнению со здоровыми людьми [72-74]. Гиперэкспрессия АПФ2 ингибирует пролиферацию опухолевых клеток, инвазию, эпителиально-мезенхимальный переход (ЭМП) и метастазирование, что свидетельствует о его противоопухолевой роли при различных типах злокачественных новообразований [75, 76]. Кроме того, экспрессия
АПФ2 коррелирует с низкими показателями ЭМП в линиях опухолевых клеток желудочно-кишечного тракта и дыхательной системы, а также у здоровых людей и пациентов с онкопатологией [77]. Снижение экспрессии АПФ2 у онкологических больных с COVID-19 может играть существенную роль в ускорении размножения опухолевых клеток, что в итоге еще больше утяжеляет состояние пациента. Инфицирование SARS-CoV-2 клеточных линий рака легкого снижает экспрессию в них АПФ2 и повышает экспрессию цинк-содержащего транскрипционного фактора ZEB1. ZEB1 способствует индукции ЭМП в этих клетках, отрицательно регулируя активность АПФ2. Это позволяет предположить, что инфицирование SARS-CoV-2 может способствовать изменению фенотипа клеток на более мезенхимальный [77]. Зараженные клетки демонстрируют снижение зависимости от синтеза глутамина, что является важным признаком ЭМП [77]. Экспрессия АПФ2 коррелирует с опухолевой инфильтрацией и благоприятным прогнозом при раке тела матки и папиллярном раке почки [78].
При этих злокачественных опухолях промотор АПФ2 гипометилирован, причем более низкий уровень метилирования характерен для опухолей высокой степени злокачественности и серозных опухолей [78]. Гипометилирование ДНК при злокачественных опухолях представляет собой распространенное явление, связанное с активацией определенных генов при прогрессировании опухолевого процесса [79]. Снижение экспрессии АПФ2 из-за инфицирования SARS-CoV-2 может препятствовать его опухолесупрессивному и иммуноактивирующему эффектам при раке тела матки и папиллярном раке почки, что потенциально ухудшает прогноз COVID-19 у этих пациентов. Кроме того, высокая экспрессия протеазы TMPRSS2 способствует слиянию SARS-CoV-2 с клетками как при локализованном, так и при метастатическом раке предстательной железы. TMPRSS2 регулируется андрогеновым рецептором в ходе развития предстательной железы, но его аберрантная активация приводит к возникновению рака предстательной железы [80, 81].
Андроген-депривационная терапия (АДТ) является основным методом лечения рака предстательной железы. АДТ снижает уровень TMPRSS2 у больных этим заболеванием [82]. Montopoli и соавт. сообщили в своих работах, что пациенты с раком предстательной железы, получающие АДТ, частично защищены от инфекции, вызванной SARS-CoV-2 [83]. Группа ученых изучила 5273 пациентов, получавших АДТ, и обнаружила, что у них был значительно более низкий риск инфицирования SARS-CoV-2 по сравнению с пациентами, не получавшими АДТ (n=37161, отношение шансов (ОШ) 4,05; 95-процентный доверительный интервал (95% ДИ) 1,55-10,59), или пациентами с другими типами опухолей (n=84934, ОШ 4,86; 95% ДИ 1,88-12,56). Полученные результаты могли быть связаны с более низкой экспрессией TMPRSS2 у этих пациентов, что снижает уязвимость к SARS-CoV-2 [83]. Это также может объяснять, почему мужчины более уязвимы к COVID-19 по сравнению с женщинами и детьми [84]. Генетические варианты андрогенового рецептора могут быть причиной расовых различий показателей заболеваемости и смертности от COVID-19. АПФ2 и TMPRSS2 обильно экспрессируются в эпителиальных клетках кишечника и могут способствовать развитию инфекции SARS-CoV-2 в энтероцитах тонкой кишки человека [85, 86]. Кроме того, высокая экспрессия АПФ2 в органах мужской мочеполовой системы, включая простату, и сверхэкспрессия ТMPRSS2 у больных раком предстательной железы могут повысить их восприимчивость к SARS-CoV-2 [8789]. У пациентов с синдромом раздраженного кишечника (СРК) экспрессия АПФ2 и TMPRSS2 в слизистой оболочке толстой кишки не повышена по сравнению с контрольными пациентами без воспалительных заболеваний кишечника (ВЗК). Аналогичным образом, экспрессия двух генов не влияет на наличие воспаления в подвздошной или толстой кишке, что позво- ляет предположить, что пациенты с ВЗК не подвержены повышенному риску инфицирования SARS-CoV-2 желудочно-кишечного тракта [85].
Воспаление и иммунитет у онкологических больных с COVID-19: SARS-CoV-2 распространился практически повсеместно в мире, и общее число случаев COVID-19 продолжает расти [90]. Число случаев заболевания и летальных исходов среди пожилых людей (>65 лет) выше, и 8 из 10 человек, умерших от COVID-19 в США, были в возрасте 65 лет и старше [91, 92]. Более слабая иммунная система и наличие множественных сопутствующих заболеваний у пожилых пациентов повышают риск тяжелого течения COVID-19. Пожилой возраст также является важным фактором риска многих типов онкологических заболеваний, и 25% новых случаев онкопатологии диагностируются у людей в возрасте от 65 до 74 лет. Еще 24% и 19,6% приходится на возрастные группы 55-64 и 75-84 года, соответственно [93]. Это означает, что онкопатология в большинстве случаев является возраст-ассоциированной, и риск заболеть ей увеличивается с возрастом. COVID-19 негативно влияет на уязвимых онкологических больных в большей степени, по сравнению с остальной частью населения, из-за их возраста и состояния иммуносупрессии на фоне проводимой цитотоксической терапии или собственно онкологического заболевания.
Основной путь заражения SARS-CoV-2 — воздушно-капельный [94]. Наличие АПФ2 на поверхности эпителиоцитов дыхательных путей человека облегчает проникновение SARS-CoV-2 внутрь клеток [62, 95]. Проникновение SARS-CoV-2 в респираторный эпителий приводит к репликации вируса и распространению инфекции. Хотя общая экспрессия АПФ2 в респираторном эпителии невысока, он экспрессируется большим количеством типов эпителиальных клеток дыхательных путей и альвеолярными эпителиоцитами II типа — основными защитниками от чужеродных патогенов в легких [96]. Примечательно, что наиболее высокая экспрессия АПФ2 среди всех клеток дыхательной системы характерна для эпителиальных клеток носа [96]. Инфицирование SARS-CoV-2 приводит к интенсивной воспалительной реакции, которая фактически является реакцией иммунной системы [97]. Воспаление альвеол легких обусловливает развитие пневмонии, которая проявляется одышкой, кашлем, слабостью и лихорадкой. Патоморфологическое исследование ткани легкого двух больных раком легкого (84 и 73 года), перенесших лобэктомию и инфицированных SARS-CoV-2, выявило отек, венозное полнокровие и очаговые скопления фибрина с мононуклеарными воспалительными клетками и гигантскими многоядерными клетками в альвеолах [98]. У пациентов с онкологическими заболеваниями (n=232), по сравнению с пациентами без онкопатологии (n=519), чаще наблюдалась одышка (затруднение дыхания, n=63 [27%] против n=89 [17%], соответственно, p=0,0022) и продуктивный кашель (отхождение мокроты или слизи, n=52 [22%] против n=83 [16%], соответственно, p=0,044), но они реже жаловались на боль в горле и насморк (воспаление слизистой оболочки полости носа) при развитии COVID-19 [99]. Другие распространенные симптомы, включая лихорадку, сухой кашель и слабость, существенно не отличались по частоте возникновения у больных с онкопатологией и без нее. Кроме того, при компьютерной томографии (КТ) онкологических больных, по сравнению с пациентами без злокачественных новообразований, чаще выявлялся симптом матового стекла (148 из 195 [76%] против 183 из из 301 [61%], соответственно, р=0,0007) и очаговые тени (126 из 195 [65%] против 152 из 301 [50%], соответственно, р=0,0027). У онкологических больных по сравнению с другими пациентами были выше уровни провоспали-тельных цитокинов, в том числе фактора некроза опухоли, ФНО (n=89 против n=336, 8,7 против 6,9 пг/мл, р=0,004, соответственно), интерлейкина-6, ИЛ-6 (n=138 против n=350, 12,8 против 4,9 пг/мл, р<0,0001, соответствен- но), рецептора интерлейкина-2 (n=79 против n=340, 615 против 535 Ед/мл, р=0,012, соответственно), а также связанных с инфекцией биомаркеров, таких как прокальцитонин (n=161 против n=251, 0,3 против 0,1 нг/мл, р=0,0041) и С-реактивный белок (n=91 против n=246, 46,4 против 40,7 мг/л, р=0,047). Кроме того, у онкологических больных наблюдалось значительное снижение количества лимфоцитов, в том числе CD4+ Т-клеток (n=37 против n=82, 370 против 625,5 клеток/мкл, р<0,0001, соответственно) и CD8+ Т-клеток (n=43 против n=82, 206 против 305,0 клеток/мкл, р<0,0081, соответственно) [99]. Это вполне объяснимо, поскольку опухолевые клетки вызывают дисфункцию иммунной системы, которая характеризуется нарушением опосредованной Т-клетками цитотоксичности и торможением пролиферации Т-клеток. Снижение количества лейкоцитов может быть фактором риска повышенной восприимчивости к инфицированию SARS-CoV-2 у онкологических больных.
Исследование, проведенное Diao и соавт., показало, что у пациентов с COVID-19 количество Т-клеток было значительно снижено, а их функциональный резерв — истощен [100]. Количество CD8+ и CD4+ Т-клеток отрицательно коррелировало с выживаемостью пациентов с COVID-19. Кроме того, у более высокой доли CD8+ и CD4+ Т-клеток наблюдалась повышенная экспрессия белка 1 программируемой клеточной гибели (PD-1) и молекулы 3, содержащей домен Т клеточного иммуноглобулина и муцина (Tim-3). Некоторые опухолевые клетки содержат большое количество PD-L1, трансмембранного белка, который действует как лиганд для PD-1. Связывание PD-L1 с PD-1 активирует передачу сигнала от рецептора PD-1 в Т-клетках, что ингибирует их пролиферацию и цитотоксическую активность [101]. Следовательно, высокая экспрессия PD-L1 у онкологических больных может сделать их более восприимчивыми к патогенам, таким как SARS-CоV-2.
Кроме того, у онкологических больных по сравнению с другими пациентами при разви- тии инфекции, вызванной SARS-CoV-2, с большей вероятностью развивается полиорганное поражение [99]. У пациентов с онкопатологией наблюдались значительно более высокие уровни аланинаминотрансферазы, лактатдегидрогеназы и альбумин-глобулинового соотношения [99]. Для пациентов с гемобластозами повышенный уровень С-реактив-ного белка и гипоксия были предикторами неблагоприятных исходов при COVID-19, тогда как концентрация гемоглобина, уровень тромбоцитов и соотношение нейтрофилы/ лимфоциты не имели значимой связи с тяжестью заболевания [60]. Анализ уровня тревоги, связанной с COVID-19, у больных раком молочной железы показал, что новая коронавирусная инфекция может влиять на процесс принятия решений пациентками [102]. Vanni и соавт. проанализировали процесс принятия решений у пациенток с неметастатическим раком молочной железы, делающих выбор в отношении медикаментозного лечения и операции [102]. Ученые разделили пациенток с подозрением на опухолевое поражение молочной железы (n=82) или рак молочной железы (n=78) на две группы: одна из них обратилась за медицинской помощью в период до начала пандемии COVID-19 (n=43 и n=41 для подозрения на поражение молочной железы и рак молочной железы, соответственно), а другая — после начала пандемии COVID-19 (n=39 и n=37 для подозрения на поражение молочной железы и рак молочной железы, соответственно). Частота отказов от лекарственного и хирургического лечения среди онкологических больных после начала пандемии была значительно выше, чем до этого. Данный факт позволяет предположить, что страх и тревога из-за возможности инфицирования SARS-CoV-2 могут влиять на решения онкологических больных в пользу отказа от лечения [102].
Влияние лечения COVID-19 на пациентов со злокачественными новообразованиями: Не существует специфического противовирусного лечения COVID-19, и большинство паци- ентов с данным заболеванием выздоравливает в домашних условиях. Методы лечения или медикаментозной терапии, которые назначаются или исследуются, включают неинвазивную или инвазивную механическую вентиляцию легких для предотвращения нарушения дыхания [103], препараты, такие как кортикостероиды (например, дексаметазон) [104], противомалярийные (например, гидроксихлорохин [ГХХ]) [105] и противовирусные (например, ремдесивир, фавипиравир, лопинавир или ритонавир) [14, 106], терапию реконвалесцентной плазмой [107] и противовоспалительные антитела (например, антитела к рецептору интерлейкина-6 — тоцилизу-маб) [108]. Стратегии лечения, применяемые у онкологических больных, при развитии COVID-19, приведены в таблице 2.
Разногласия вокруг ГХХ: ГХХ (гидроксихлорохин) — это одобренный FDA (Управлением по санитарному контролю качества пищевых продуктов и медикаментов США) препарат для лечения или профилактики малярии, а также аутоиммунных заболеваний, таких как хроническая дискоидная красная волчанка, системная красная волчанка у взрослых и ревматоидный артрит [109]. Точный механизм действия препарата неизвестен, однако очевидно, что ГХХ действует как лизосомотропный агент, повышая рН внутри лизосом и нарушая функционирование пути аутофагии / лизосомальной деградации [110]. ГХХ может подавлять иммунитет, влияя на процессинг и презентацию антигенов, а также на продукцию цитокинов [111, 112]. Кроме того, ГХХ способен ингибировать репликацию SARS-CoV-2 in vitro [113]. Некоторые наблюдательные исследования не выявили преимуществ использования ГХХ для лечения COVID-19, тогда как другие показали улучшение исходов. Исследование, проведенное Luo и соавт., показало, что использование ГХХ у пациентов с COVID-19 и раком легкого (n=35) не снижало риск неблагоприятных исходов, включая потребность в госпитализации в ОРИТ, интубацию и летальный исход.
Таблица 2
Стратегии лечения, применявшиеся для терапии COVID-19 у онкологических больных
Хотя для больных раком легкого характерно более тяжелое течение COVID-19, большинство пациентов (65%) выздоровели [55]. Другое исследование, направленное на изучение эффекта применения ГХХ и азитромицина у онкологических больных с тяжелым течением COVID-19, показало, что комбинированное лечение этими препаратами увеличивает летальность [47]. 89 онкологических больных получали ГХХ, из которых 11 (12%) умерли, 18 (20%) были госпитализированы в отделение интенсивной терапии и 14 (16%) нуждались в искусственной вентиляции легких. При лечении только азитромицином (n=93), умерло 12 (13%) человек, 15 (16%) были госпитализированы в отделение интенсивной терапии и 14 (15%) нуждались в искусствен- ной вентиляции легких. Среди пациентов, получавших комбинированную терапию ГХХ и азитромицином (n=181), 45 (25%) — умерло, 53 (29%) были госпитализированы в отделение интенсивной терапии и 51 (28%) нуждался в искусственной вентиляции легких, по сравнению с группой пациентов, не получавших такого лечения (n=486), в которой умер только 41 (8%) человек, 39 (8%) были госпитализированы в отделение интенсивной терапии и 29 (6%) нуждались в искусственной вентиляции легких.
Результаты исследования показали, что как по отдельности, так и в комбинации эти препараты не улучшают клинические исходы и могут быть токсичными для уязвимых онкологических больных [47]. Однако, следует отметить, что в данном исследовании препараты назначались пациентам с более тяжелым течением COVID-19, по сравнению с контрольной группой, что вызывает сомнения относительно выводов о возможной токсичности такого лечения для пациентов. Тем не менее, применение ГХХ, азитромицина или их комбинации у онкологических больных не облегчает течение коронавирусной инфекции. Обсервационное исследование 2186 онкологических больных с COVID-19 показало, что пациенты, получавшие ГХХ в комбинации с азитромицином (n=203, 23%), или азитромицин с высокими дозами кортикостероидов (n=24, 3%), или тоцилизумаб (n=18, 2%), или тоцилизумаб с азитромицином (n=18, 2%), имели повышенный риск 30-дневной летальности от всех причин по сравнению с сопоставимыми или не сопоставимыми между собой положительными контрольными группами (лечение без ГХХ) и отрицательными контрольными группами (не получавшими лечения). Однако лечение только ГХХ (n=179, 21%) не было ассоциировано с повышенным риском по сравнению с положительным или отрицательным контролем. У большинства пациентов в этом исследовании были солидные опухоли (n=1781, 81%), наиболее частой из которых являлся рак молочной железы (n=455, 21%), следующими по частоте были опухоли предстательной железы (n=368, 17%) и желудочно-кишечного тракта (n=290, 13%) [114]. Аналогичные результаты были получены и в другом обсервационном исследовании, включившем 1376 пациентов с COVID-19, в котором изучалось влияние ГХХ на риск необходимости интубации или летального исхода [115]. Из 1376 пациентов 811 (109 были онкологическими больными, 13,4%) получали ГХХ (600 мг дважды в 1-й день, затем 400 мг в сутки в течение 5 дней) со средним сроком наблюдения 22,5 дня. Пациенты, получавшие ГХХ, находились в тяжелом состоянии и не имели значимых различий по потребности в интубации и частоте летальных исходов (отношение рисков 1,04; 95% ДИ 0,82-1,32) по сравнению с теми, кто не получал ГХХ [115]. Кроме того, было проведено ретроспективное многоцентровое когортное исследование с включением 1438 пациентов с COVID-19 из 25 больниц Нью-Йорка и пригородов для определения клинических преимуществ ГХХ в сочетании с азитромицином или без него. В этом исследовании не было обнаружено существенных различий в летальности пациентов, получавших эти препараты, по сравнению с пациентами, не получавшими ни одного из этих препаратов [116]. Из 1438 пациентов с COVID-19 735 получали ГХХ и азитромицин, 271 — только ГХХ, 211 — только азитромицин, остальные 221 — не получали ни один из этих препаратов. После корректировки на демографические данные, различия больниц, сопутствующие заболевания и тяжесть течения не было выявлено значимых различий в показателях летальности между пациентами, получавшими ГХХ + азитромицин (скорректированное отношение рисков 1,35 [95% ДИ 0,76-2,40]), только ГХХ (скорректированное отношение рисков 1,08 [95% ДИ 0,63-1,85]) или только азитромицин (скорректированное отношение рисков 0,56 [95% ДИ 0,26-1,21]) по сравнению с пациентами, не получавшими ни одного из этих препаратов. Более того, у пациентов, получавших ГХХ + азитромицин, вероятность остановки сердца была выше, чем у пациентов из других групп [116]. Эти результаты подтверждаются данными многоцентрового рандомизированного контролируемого исследования, включившего 4716 пациентов с COVID-19, из которых 1561 получали ГХХ и 3155 получали стандартную медицинскую помощь. В группе ГХХ летальный исход в течение 28 дней развился у 26,8% пациентов, в то время как в группе стандартного лечения — у 25% пациентов. Различия между двумя группами не были статистически значимыми (р=0,18), соотношение вероятности неблагоприятного исхода (лечение ГХХ / стандартное лечение) составило 1,09 (95% ДИ 0,96-1,23). Кроме того, для группы пациентов, получавших ГХХ, была характерна более длительная госпитализация, по сравнению с группой стандартного лечения (в течение 28 дней выписано n=941, 60,3% против n=1982, 62,8%, соответственнно) [95% ДИ 0,85-0,99] (Идентификатор исследования ClinicalTrials.gov: NCT04381936) [117]. Общество клинических исследований по профилактике и раннему лечению острого повреждения легких (PETAL) Национального института сердца, легких и крови (NHLBI) Национального института здоровья (NIH) провело клиническое исследование для оценки безопасности и эффективности применения ГХХ у госпитализированных пациентов с COVID-19 (NCT04332991). Исследование получило название «Клинические исходы COVID-19 среди стационарных пациентов с симптомным заболеванием, получавших ГХХ» или «Исследование ORCHID». В него планировалось включить более 500 госпитализированных взрослых пациентов с COVID-19. Исследование показало, что применение ГХХ не дает дополнительных клинических преимуществ по сравнению с плацебо при лечении COVID-19. Национальный институт здоровья прекратил это исследование, придя к выводу, что лечение ГХХ не вредит и не приносит пользы госпитализированным пациентам c COVID-19. К моменту прекращения в него было включено более 470 пациентов [118].
Другое исследование, напротив, показало, что комбинированное лечение ГХХ и азитромицином значительно улучшает исход COVID-19 у больных раком легкого (1 умерший из 8 пациентов, ОШ 0,04, ДИ 0,01-0,57, р=0,018), что позволяет предполагать о возможной предпочтительности данного варианта терапии [45]. Возраст умершего был 72 года, при этом средний возраст выживших составил 64,5 года (р=0,12). Другие отчеты о результатах исследований подтверждают это аналогичными данными о снижении летальности у пациентов с COVID-19, получавших только ГХХ (162/1202, 13,5% [95% ДИ: 11,6%-15,5%]) или ГХХ в комбинации с азитромицином (157/783, 20,1% [95% ДИ: 17,323,0%]) по сравнению с теми, кто не получал
ГХХ (108/409, 26,4% [95% ДИ: 22,2-31,0%]) или получал только азитромицин (33/147, 22,4% [95% ДИ: 16,0%-30,1%]). Риск смерти в группе ГХХ и группе ГХХ + азитромицин снижался на 66% и 71%, соответственно [105]. Во Франции продолжается исследование, изучающее применение ГХХ и азитромицина при лечении COVID-19 у онкологических больных. В первый день лечения пациенты получают ГХХ (800 мг) + азитромицин (500 мг), затем в течение четырех дней — ГХХ 400 мг/сут + азитромицин 250 мг/сут (NCT04341207). Таким образом, большинство исследований показали, что ГХХ не снижает риск заражения SARS-CoV-2 или риск смерти среди госпитализированных пациентов с COVID-19. Поэтому Национальный институт здоровья не рекомендует использовать хлорохин (ХХ) или ГХХ отдельно или в комбинации с азитромицином для лечения COVID-19 у госпитализированных и негоспи-тализированных пациентов, за исключением клинических исследований [119].
Ингибиторы интерлейкинов (ИЛ): Одним из отличительных признаков инфекции, вызванной SARS-CoV-2, является патологическое воспаление, которое ассоциировано с тяжелым течением заболевания и риском смерти пациентов [120]. У пациентов с COVID-19 повышено содержание в сыворотке провос-палительных цитокинов, включая ИЛ-6, ИЛ-1, интерферон (ИФН) γ, IP10 и MCP1. Более высокие концентрации GCSF, IP10, MCP1, MIP1A и фактора некроза опухоли α обнаруживаются у пациентов с COVID-19, которым требуется госпитализация в ОРИТ. Это позволяет предполагать, что цитокиновый шторм, при котором организм начинает атаковать собственные клетки, ассоциирован с тяжестью заболевания [15]. Исследования методов лечения, направленных на предотвращение цитоки-нового шторма, проводятся по всему миру, и в некоторых из них была показана клиническая эффективность такой терапии [121, 122]. Ингибиторы ИЛ-6, включая тоцилизумаб и са-рилумаб, которые блокируют рецептор ИЛ-6, используются в качестве экспериментальных методов лечения COVID-19. Тоцилизумаб продемонстрировал существенное положительное влияние на течение COVID-19, осложненного развитием цитокинового шторма [121], тогда как испытания сарилумаба еще не завершены (NCT04315298 и NCT04327388). Ретроспективное когортное исследование Guaraldi и соавт. установило, что внутривенное или подкожное лечение тоцилизумабом может снизить риск инвазивной искусственной вентиляции легких и летального исхода у пациентов с COVID-19. Однако этот эффект не наблюдался у пациентов с онкопатологией, заболевших COVID-19. В исследовании сравнивалось влияние тоцилизумаба (n=2) и отсутствия лечения тоцилизумабом (n=8) на онкологических больных. Авторы не обнаружили каких-либо значимых клинических различий в данных группах (р=0,38). Обе группы получали стандартное лечение, включавшее кислородотерапию, гидроксихлорохин, азитромицин, антиретровирусные препараты и низкомолекулярный гепарин. Авторы не уточнили, какой тип стандартного лечения был назначен каждому из онкологических больных [123].
Аналогичное исследование, сравнившее влияние тоцилизумаба на проявления COVID-19 у двух онкологических больных, не продемонстрировало улучшения состояния пациентов по сравнению с пациентами, не получавшими тоцилизумаб [124]. Michot и со-авт., напротив, сообщили об успешном лечении 42-летнего мужчины с COVID-19, у которого незадолго до этого был диагностирован метастатический саркоматоидный светлоклеточный рак почки. Сначала ему назначили ло-пинавир-ритонавир (400-100 мг) перорально на 7-й день заболевания (когда он поступил в стационар с симптомами COVID-19), сроком на 5 дней. Его состояние не улучшилось, и на 8-й день он получил две дозы тоцилизумаба по 8 мг/кг с интервалом 8 часов. После этого состояние пациента начало улучшаться, у него нормализовалась температура тела, на 19-й день частично регрессировали легочные инфильтраты, концентрация С-реактивно-го белка снизилась с 225 мг/л до 33 мг/л за 4 дня. Количество циркулирующих субпопуляций лимфоцитов и CD4+CD25+ клеток после лечения тоцилизумабом не изменилось. Пациент клинически полностью выздоровел от COVID-19 [108]. Следует еще раз отметить, что очень небольшой размер выборок ставит под сомнение выводы, сделанные по результатам вышеописанных исследований. Для оценки эффективности терапии COVID-19 у онкологических больных ингибиторами ИЛ-6 необходимо проведение когортного исследования. Ингибиторы ИЛ-6 также изучались в качестве таргетной терапии злокачественных новообразований. Однако отсутствие эффекта от такой терапии из-за адаптивности опухолевых клеток и возможности активации путей, регулируемых ИЛ-6, другими факторами роста, заставляет усомниться в возможностях данного вида лечения. Кроме того, ингибиторы интерлейкинов защищают от COVID-19 пациентов с хроническими воспалительными заболеваниями, такими как астма, также как противовоспалительные препараты способствуют обезболиванию при онкопатологии [125, 126]. У пациентов с COVID-19 на фоне тяжелой эозинофильной астмы, получавших лечение моноклональными антителами против ИЛ-5, включая реслизумаб и бенрализумаб, симптомы COVID-19 были менее выражены [127, 128]. Renner и соавт. описали 41-летнего пациента с диагнозом COVID-19, который в течение девяти лет страдал тяжелой эозинофильной астмой. Пациент проходил лечение моноклональными антителами против ИЛ-5, включая реслизумаб и бенрализумаб, в течение последних четырех лет. При этом у него не развилось тяжелого обострения астмы из-за COVID-19, и через неделю симптомы были полностью купированы. Примечательно, что этому пациенту ранее всегда требовались пероральные кортикостероиды при вирусных инфекциях, пока не было начато лечение антителами к ИЛ-5/ИЛ-5R [127]. Об аналогичных результатах в отношении COVID-19
у пациентов (N=2) с тяжелой эозинофильной астмой, получавших лечение бенрализума-бом, сообщили Ismael García-Moguel и соавт. После постановки диагноза COVID-19 одному пациенту назначили системные кортикостероиды, а другому — азитромицин, ГХХ и амоксициллин/клавулановую кислоту. Оба пациента хорошо отреагировали на терапию и выздоровели от COVID-19. Примечательно, что, поскольку SARS-CoV-2 является респираторным вирусом, было бы логично ожидать более тяжелого течения заболевания у пациентов со среднетяжелой и тяжелой астмой. Однако, пациенты, получавшие лечение антителами к ИЛ-5/ИЛ-5R по поводу астмы, напротив, хорошо отвечали на терапию COVID-19. Вышеописанные данные позволяют предполагать, что лечение антителами к ИЛ-5/ИЛ-5R может иметь некоторый защитный эффект от COVID-19, однако нельзя исключить, что благоприятное течение заболевания было связано с другими препаратами, которые пациенты получали в связи с развитием COVID-19. Несмотря на это, приведенные данные свидетельствуют в пользу продолжения использования антител к ИЛ-5/ИЛ-5R у пациентов с астмой во время пандемии COVID-19 [128].
Противовирусные препараты из группы ингибиторов протеаз: Еще одним методом лечения COVID-19 является применение комбинации ингибиторов протеаз лопинави-ра и ритонавира, одобренных для лечения ВИЧ-инфекции [129, 130]. Однако, по результатам одного из исследований (n=199), эта комбинация не приносит существенной пользы пациентам с COVID-19.в данном исследовании пять онкологических больных получали лечение лопинавиром и ритонавиром в дозах 400 мг и 100 мг, соответственно, два раза в день в течение 14 дней в дополнение к стандартному лечению. Контрольную группу составил пациент, получавший стандартную терапию (n=1). Лечение не дало клинического улучшения, не снизило вирусную нагрузку или летальность [130]. При этом комбинация лопинавира-ритонавира с интерфероном- бета-1b и противовирусным препаратом рибавирином значительно снижала вирусную нагрузку SARS-CoV-2 и уменьшала медиану времени от начала лечения до получения отрицательного результата теста (мазок из носоглотки) с 12 до 7 дней по сравнению с лечением лопинавиром-ритонавиром [131].
Исследование Zhang и соавт. показало, что онкологические больные, которые получали противоопухолевую терапию, с меньшей вероятностью отвечали на терапию COVID-19 и имели более высокий риск развития осложнений [46]. В этом ретроспективном когортном исследовании, включившем 28 онкологических больных, инфицированных SARS-CoV-2, 20 пациентам (71,4%) было назначено по крайней мере одно противовирусное средство, включая арбидол (n=14, 200 мг перорально, три раза в день), комбинацию лопи-навира и ритонавира (n=10, 400 мг и 100 мг перорально, два раза в день), ганцикловир (n=9, 500 мг внутривенно капельно, два раза в день) и рибавирин (n=1, 500 мг внутривенно капельно, два раза в день), 9 пациентам (32,1%) назначались комбинации противовирусных препаратов, 15 пациентам — системные кортикостероиды (n=15, 53,6%), 10 пациентам — лечение иммуноглобулинами. Системное лечение кортикостероидами чаще назначалось пациентам с тяжелыми осложнениями, под которыми понимали поступление в отделение интенсивной терапии или использование искусственной вентиляции легких. 8 пациентов (28,6%) умерли во время лечения, а 10 пациентов (35,7%) были выписаны из стационара без симптомов COVID-19 с медианой продолжительности госпитализации 19 дней (межквартильный размах — 16,0-28,5). Тяжесть заболевания была ассоциирована со стадией опухолевого процесса и противоопухолевым лечением. Из пациентов, которые получали противоопухолевое лечение в течение 14 дней, включая химиотерапию (n=3), лучевую терапию (n=1), тар-гетную терапию (n=2) и иммунотерапию (n=1), у 5 из 6 (83,3%) развились тяжелые ослож- нения. В группе пациентов, не получавших такого лечения, они отмечались лишь у 10 из 22 (44,4%) (отношение рисков — 4,079, 95% ДИ 1,086-15,322, p = 0,037). Кроме того, у 7 из 10 (70%) пациентов с IV стадией онкологического заболевания, лечившихся от COVID-19, развились тяжелые осложнения, по сравнению с 8 из 18 (44,4%) пациентов с другой стадией заболевания. Также было отмечено, что у 11 из 13 (84,6%) пациентов с наличием очагов уплотнения по данным компьютерной томографии развились тяжелые осложнения, по сравнению с 4 из 15 (26,7%) пациентов без очагов уплотнения (отношение рисков — 5,000, 95% ДИ 1,576-15,861, р=0,006) [46]. Суммируя результаты этих исследований, мы можем заключить, что онкологические больные, инфицированные SARS-CoV-2, хорошо отвечают на одни виды лечения и не отвечают на другие. Однако на эти различия могут оказывать критическое влияние некоторые факторы, включая тип опухоли, стадию опухолевого процесса, противоопухолевое лечение, позднюю госпитализацию, возраст, сопутствующие заболевания и наличие очагов уплотнения легочной ткани.
Кортикостероиды: Вполне вероятно, что при лечении COVID-19 у онкологических больных необходимо принимать особые меры предосторожности, поскольку они подвержены более высокому риску развитию осложнений, которые могут привести к смерти. Аналогичным образом некоторые методы лечения COVID-19 могут быть эффективны в общей популяции, но требуют особой осторожности при их применении у онкологических больных. Например, лечение дексаметазоном рекомендовано Национальным институтом здоровья, поскольку применение данного препарата показало многообещающие результаты в отношении снижения 28-дневной летальности у пациентов с тяжелым течением заболевания, включая тех, кто получал инвазивную искусственную вентиляцию легких или кислородную поддержку [132]. Дексаметазон рекомендован и онкологическим больным для уменьшения воспаления и подавления иммунного ответа [133]. Исследование Cook и соавт. показало, что добавление к терапии дексаметазона у онкологических больных истощает популяцию CD4+ и CD+ Т-клеток и активирует иммуносупрессорные регуляторные Т-клетки. Снижение численности популяции CD4+ и CD+ Т-клеток также наблюдается у пациентов с COVID-19 [134]. Кроме того, Т-клетки пациентов с COVID-19 имеют гораздо более высокую экспрессию PD-1, что ассоциировано с истощением пула Т-клеток и их апоптозом [100]. Поэтому для онкологических больных, особенно тех, кто получает противоопухолевую терапию, лечение COVID-19 препаратами, обладающими иммуносупрессивным действием, может увеличить риск тяжелых осложнений и оппортунистических инфекций. Кроме того, онкологические больные, получавшие лечение ингибиторами контрольных точек иммунного ответа, могут иметь более тяжелые осложнения на фоне лечения COVID-19 [135]. Ингибиторы контрольных точек иммунного ответа предотвращают передачу сигналов Т-клеткам и гиперактивируют их для борьбы с онкологическим заболеванием. К сожалению, гиперактивация иммунной системы ассоциирована с цитокиновым штормом, который часто наблюдается у пациентов с COVID-19, приводя к острому респираторному дистресс-синдро-му и полиорганной недостаточности [136]. Следовательно, нельзя исключить синергизм между ингибиторами контрольных точек иммунного ответа и COVID-19 у онкологических больных, и к выбору лекарственных препаратов необходимо подходить с особой осторожностью. Эту гипотезу поддерживают Robilotti и соавт., которые обнаружили, что лечение ингибиторами контрольных точек иммунного ответа было ассоциировано с госпитализацией и тяжелой дыхательной недостаточностью при развитии COVID-19 у онкологических больных [135].
АДТ: АДТ или связанные с ней методы лечения рака предстательной железы, по-видимо- му, играют защитную роль против SARS-CoV-2. Для пациентов с раком предстательной железы, которые получали АДТ, было характерно значительное (в 4 раза) снижение риска заболевания COVID-19 по сравнению с пациентами, не получавшими АДТ [83]. С учетом этих данных проводятся клинические исследования применения АДТ при COVID-19 (NCT04446429). Кроме того, также исследуется применение при COVID-19 препаратов, действующих на экспрессию TMPRSS2, таких как нафамостат (NCT04473053) и бромгексин (NCT04355026).
Ведение онкологических больных во время пандемии COVID-19: Рассмотренные выше данные показывают, что пациенты, находящиеся на противоопухолевом лечении, подвергаются более высокому риску развития осложнений COVID-19, которые могут привести к смерти. Онкологическим больным часто требуется посещение клиники для наблюдения за их состоянием, что может увеличить риск внутрибольничных инфекций в период пандемии COVID-19 и делает этих пациентов с ослабленным иммунитетом более уязвимыми для инфекции.
Основные рекомендации: Центры по контролю и профилактике заболеваний (CDC) в США рекомендуют соблюдение некоторых мер защиты от COVID-19 онкологическим больным, которые считаются группой высокого риска, и работникам, оказывающим им медицинскую помощь. Основные рекомендации совпадают с теми, которые даются широкой публике. Они включают наблюдение за температурой тела для выявления лихорадки (38°C или выше), знание признаков и симптомов инфекции, частое мытье рук, избегание прикосновений к лицу, использование защитных масок, максимально возможное ограничение контактов с другими людьми и соблюдение дистанции не менее 6 футов (2 м) [137]. Онкологическим больным рекомендуется получить необходимые им лекарственные препараты с запасом, если им нужно оставаться дома в течение длительного времени, и по- звонить своему лечащему врачу за несколько дней до приема, чтобы убедиться в том, что доктор может их принять [138]. Кроме того, онкологическим больным рекомендуется свести к минимуму посещения клиник, чтобы уменьшить риск инфицирования, и предлагается рассмотреть возможность отсрочки лечения, если их заболевание хорошо отвечает на текущую терапию [139]. Пациенты должны связаться со своими врачами для получения советов и рекомендаций относительно лечения их заболевания во время пандемии. Врачам следует обсудить преимущества и риски текущей терапии со своими пациентами во время пандемии COVID-19. Как уже обсуждалось выше, исследования показывают, что онкологические больные, которые получали противоопухолевое лечение и заболели COVID-19, имели более тяжелые исходы по сравнению с пациентами, не получавшими лечения [19, 99, 135]. Для пациентов, получающих различные виды противоопухолевого лечения, характерна различная тяжесть течения COVID-19. Важно, чтобы пациенты и те, кто обеспечивает уход за ними, знали факторы риска, ассоциированные с тяжестью течения COVID-19. Врачи должны быть в курсе исследований вопросов лечения COVID-19, чтобы принимать более обоснованные решения относительно рекомендаций по лечению и ведению пациентов. Эффективное общение между медицинскими работниками, пациентами и лицами, осуществляющими уход за ними, является ключом к перестройке системы здравоохранения и сопоставлению преимуществ лечения с «ценой социальных контактов» во время пандемии.
Трудности лечения: Из-за высокой кон-тагиозности COVID-19 его распространение среди населения происходит очень быстро. Это приводит к переполнению больниц и повышенной нагрузке на медицинских работников [140]. Весьма вероятно, что онкологические больные, которым недавно поставлен диагноз, и те, которые уже находятся на противоопухолевой терапии, могут не полу- чить медицинскую помощь вовремя. Кроме того, стресс из-за риска заражения во время вспышки этого инфекционного заболевания может серьезно повлиять на решение онкологических больных обратиться в клинику для дальнейшего наблюдения и лечения. Риск несвоевременного получения лечения, психологический стресс и дистресс, связанный с онкологическим заболеванием, неопределенность и социальная изоляция без возможности узнать срок окончания пандемии могут негативно сказаться на психическом и физическом благополучии онкологических больных [141-143]. В то же время онкологам и другим медицинским работникам приходится принимать нелегкое решение о госпитализации онкологических больных, сопряженное с возможностью заражения этой уязвимой группы COVID-19. Нехватка медицинского персонала и нехватка ресурсов из-за возросшей потребности в них еще больше усугубляют проблемы, с которыми онкологи сталкиваются в период пандемии. Поэтому многие учреждения здравоохранения изменили рекомендации по онкологической помощи [144, 145]. Рекомендуемой стандартной стратегией является расстановка приоритетов, при которой анализируется и сопоставляется риск госпитализации и польза терапевтического вмешательства [146, 147]. Факторы, которые могут повлиять на анализ соотношения риска и пользы, включают состояние здоровья пациента, статус онкологического процесса, тяжесть заболевания, факторы риска, ассоциированные с тяжелым течением COVID-19, и предпочтения пациента. Кроме того, некоторые виды противоопухолевой терапии обладают высокой иммуносупрессивной активностью [43, 148]. Поэтому решения, касающиеся отсрочки или начала лечения, должны быть тщательно взвешены. Согласно рекомендациям Американского общества клинической онкологии (ASCO), при принятии решения о переносе или изменении системной противоопухолевой терапии следует учитывать индивидуальную оценку риска и пользы, которая включает в себя общие цели лечения, риски прогрессирования заболевания, переносимость лечения пациентом и общее состояние пациента [149].
Еще одна проблема, с которой столкнутся врачи, — это этические соображения и рациональность медицинской помощи с учетом определения групп пациентов, которые, вероятно, получат наибольшую пользу от лечения [150]. Например, в переполненных больницах, если пациент с COVID-19 имеет позднюю стадию хронического заболевания или прогрессирующие нарушения, такие как сердечная или легочная недостаточность, и нуждается в аппарате жизнеобеспечения, в частности, ИВЛ, его шансы на выживание невелики. Концепция распределения ограниченных ресурсов во время пандемии не является новой. Во время Второй мировой войны бóльшая часть производимого в США пенициллина использовалась для солдат, и его было недостаточно для лечения всех пациентов [151]. Поступали сообщения о внедрении этических норм для распределения дефицитных ресурсов здравоохранения в некоторых странах, поскольку количество госпитализированных пациентов с COVID-19, нуждающихся в ИВЛ, превысило количество соответствующих аппаратов [152, 153]. Чтобы справиться с подобными этическими дилеммами, когда врачам приходится выбирать между пациентами и учитывать соображения безопасности при оказании помощи инфицированным пациентам, крайне важно проводить совместное обсуждение этих вопросов онкологами, специалистами по медицинской этике и медицинскими работниками, оказывающими паллиативную помощь.
Кроме того, рекомендуется расширить использование телемедицины в онкологической помощи. Телемедицина подразумевает использование средств коммуникации для оказания медицинской помощи [154]. Например, онкологический больной и онколог могут обмениваться любыми данными пациента и обсуждать их с помощью мобильных телефонов или с использованием компьютеров через интернет. Посредством телемедицины онколог может вести пациентов или давать рекомендации, что позволяет свести к минимуму визиты пациентов в клинику во время пандемии. Это может повысить доступность и качество помощи, снизить затраты и защитить онкологических пациентов от риска инфекций. Кроме того, основным способом принятия решений, связанных с лечением онкологических больных, в период пандемии COVID-19 должно быть междисциплинарное обсуждение вопросов их ведения комиссией, в состав которой входят врачи различных специальностей [155]. Этим комиссиям следует рассмотреть возможность сотрудничества с зарубежными коллегами для улучшения качества помощи пациентам и преодоления проблем, возникающих во время пандемии.
Рекомендации Европейского общества медицинской онкологии (ESMO): ESMO выпустило руководство по ведению онкологических больных во время пандемии COVID-19 [144]. Согласно руководству ESMO, решение о начале или продолжении противоопухолевого лечения должно приниматься и обсуждаться врачом и пациентом, после надлежащего объяснения пациенту соотношения риска и пользы. Руководству больниц следует подумать о приоритетном подходе при оказании онкологической помощи. Высокий уровень приоритета следует отдавать пациентам, чье состояние является жизнеугрожающим, клинически нестабильным, и/или для которых предполагается максимальная польза от планируемого вмешательства (например, повышение общей выживаемости и/ или качества жизни). Для пациентов со средним уровнем приоритета срочное лечение не требуется, но задержка вмешательства более чем на шесть недель потенциально может повлиять на общий исход и/или результат лечения. Пациенты с низким уровнем приоритета находятся в стабильном состоянии, и вмешательство для них не принесет существенной пользы (в виде повышения общей выживаемости и/или качества жизни). У этих пациентов лечение может быть отложено на время пандемии. Предположим, планируется местное лечение (хирургическое или лучевое) опухоли на ранней стадии. В этом случае врачам рекомендуется выжидательный подход (“wait and see”), который означает, что опухоль, не вызывающая никаких симптомов или проблем, тщательно контролируется, но не лечится. Также можно провести анализ ожидаемых позитивных и негативных результатов лечения (сравнение проводимых мероприятий и их последствий), с учетом возраста пациента, сопутствующих заболеваний и возможных последствий различных вариантов хирургических вмешательств. Кроме того, при наличии соответствующей возможности, следует рассмотреть замену противоопухолевого или паллиативного лечения препаратами для внутривенного введения аналогичными средствами для перорального приема [144].
Хотя такие изменения в стандартах лечения онкологических больных уместны и необходимы, весьма вероятно, что это приведет к увеличению заболеваемости или даже смертности пациентов с онкопатологией.
Перепрофилирование противопухоле-вых препаратов для лечения COVID-19: Разработка лекарств и вакцин — это медленный, сложный и дорогостоящий процесс [156]. Альтернативный подход заключается в выявлении существующих препаратов, которые можно использовать для новых терапевтических целей, например для лечения COVID-19. Это называется перепрофилированием или репозиционированием препаратов, и около 30% одобренных для применения в 2017 году препаратов являются перепрофилированными [157, 158]. Если этот подход будет успешным, то он будет очень полезен, тем более с учетом существующей срочной необходимости в препаратах для лечения COVID-19. В настоящее время проводится ряд клинических исследований применения перепрофилированных препаратов для лечения COVID-19 [159-162]. Между симптомами онкологических заболеваний и COVID-19 есть некоторое сходство, поэтому использование противоопухолевых препаратов для лечения COVID-19 может помочь пациентам. Например, некоторые случаи тяжелого течения COVID-19 ассоциированы с развитием состояния, аналогичного острому респираторному дистресс-синдрому [163]. Оно возникает вследствие чрезмерного высвобождения провоспалительных цитокинов, выделяемых иммунными клетками, из-за повреждения легких, вызванного SARS-CoV-2 [164]. Этот ци-токиновый шторм является одной из самых распространенных причин смерти, ассоциированных с COVID-19 [165]. Он вызывает обширное повреждение тканей и затем, в процессе их восстановления, развитие фиброза, который может привести к стойкой дисфункции органов. Некоторые противоопухолевые препараты, одобренные для применения в клинической практике, подавляют воспаление и, следовательно, могут помочь пациентам с тяжелым течением COVID-19. Одним из примеров таких препаратов является ингибитор Янус-киназы руксолитиниб, используемый для лечения миелофиброза, опухолевого поражения костного мозга, при котором нарушается нормальная продукция клеток крови [166]. Руксолитиниб подавляет активацию широкого спектра провоспалительных цитокинов и факторов роста, включая ФНО α, ИФН γ, ИЛ-1, ИЛ-6, ИЛ-8, ИЛ-12, TGFβ, VEGF, FGF, PDGF, GM-CSF и G-CSF [167]. Руксолити-ниб назначали пациентам с тяжелым течением COVID-19 (n=14), имевшим высокий риск чрезмерной воспалительной реакции, состояние которых ухудшалось на фоне стандартного лечения. Медиана продолжительности лечения составила 9 дней, а медиана кумулятивной дозы — 135 мг. Пациенты, получавшие руксолитиниб, хорошо ответили на лечение, у них значительно уменьшилась выраженность воспаления и снизился уровень ассоциированных с ним лабораторных показателей, включая С-реактивный белок (СРБ), ИЛ-6 и ферритин; кроме того, уменьшилась выраженность лимфопении. Из 14 пациен- тов 10 выздоровели; 4 пациента, у которых не наблюдалось хорошего ответа на лечение, имели серьезные сопутствующие заболевания [168].
Другой противоопухолевый препарат, ака-лабрутиниб, который ингибирует передачу сигналов Брутон-тирозинкиназы (БТК), также оказался эффективным в лечении пациентов с тяжелым течением COVID-19 [169]. Акала-брутиниб используется для лечения неходж-кинской лимфомы, известной как лимфома из клеток мантии [170]. В ответ на определенные вирусные патогены, такие как SARS-CoV-2, Toll-подобные рецепторы, присутствующие на поверхности макрофагов, активируют БТК, обеспечивая врожденный иммунный ответ [169, 171]. БТК активирует сигнальный путь NF-κB (ядерного фактора kB), который запускает продукцию множества провоспалительных цитокинов и хемокинов [172]. Ингибиторы БТК демонстрируют способность уменьшать выраженность чрезмерного воспаления и, следовательно, могут рассматриваться как одна из стратегий лечения пациентов с тяжелым течением COVID-19. Лечение акалабрутини-бом пациентов с тяжелым течением COVID-19 (n=19), получавших кислородную поддержку (n=11) или механическую вентиляцию легких (n=8), значимо повысило оксигенацию и снизило количество интубаций. Перед лечением 18 из 19 (95%) пациентов имели значительно повышенный уровень СРБ (n=15: СРБ>10 мг/ дл, n=4: СРБ=3-10 мг/дл), ферритина сыворотки крови (n=16, ферритин>500 нг/мл, n=3: ферритин <500 нг/мл), фибриногена (>400 мг/ дл, n=10), D-димера (>0,5г/мл: n=15), ИЛ-6 (>15 пг/мл) и низкое абсолютное количество лимфоцитов (n=15: <1000 клеток/л и n=3: >1000 клеток/л). Акалабрутиниб вводили по 100 мг перорально через зонд для энтерального питания 2 раза в день в течение 10 дней пациентам, получавшим кислородную поддержку, и 14 дней пациентам на ИВЛ. В когорте пациентов, получавших кислородную поддержку (n=11), 8 пациентов (75%) перестали нуждаться в дополнительном кислороде и были выписаны, а у остальных 3 пациентов потребность в кислороде снизилась. В когорте пациентов, получавших ИВЛ, 4 пациента (50%) были успешно экстубированы, и 2 из них были выписаны. Три пациента оставались интубированными с колебаниями потребности в кислороде, и один умер после отмены поддержки. В обеих группах у тех, кто хорошо отвечал на терапию, уровни СРБ и ИЛ-6 либо снижались, либо возвращались к нормальным значениям, а показатели абсолютного количества лимфоцитов значительно повышались или приходили к норме. Ex vivo анализ образцов цельной крови этих пациентов (n=3) показал увеличение фосфорилирования БТК (Y223) в CD14+ моноцитах по сравнению со здоровыми людьми (n=5). Кроме того, у пациентов с тяжелым течением COVID-19 (n=4) по сравнению со здоровыми людьми (n=5) увеличивался процент ИЛ-6+ CD14+ моноцитов, что свидетельствует об активации передачи сигналов БТК в моноцитах пациентов при развитии инфекции, вызванной SARS-CoV-2 [169].
Интерферон-альфа-2b, антипролифератив-ное средство, обладающее также противовирусными свойствами, является еще одним препаратом, который способен оказывать положительное влияние на выздоровление пациентов с COVID-19 [173]. Интерферон-аль-фа-2b используется для лечения ассоциированной со СПИДом саркомы Капоши, волосатоклеточного лейкоза и меланомы [174]. Интерферон-альфа-2b связывается с рецепторами интерферона 1-го типа, включая IFNAR1 и IFNAR2c, и вызывает их димеризацию. Димеризованные рецепторы активируют два тирозиновых рецептора Янус-киназы (JAK), Jak1 и Tyk2, которые трансфосфорилируют себя и рецепторы. Фосфорилированные рецепторы связывают и активируют трансдукторы сигналов и активаторы транскрипции (STAT) 1 и 2. Активированные факторы STAT димеризируют и активируют множество белков с иммуномодулирующей и противовирусной активностью [175]. Терапия интерфероном-альфа-2b снижает вирусную нагрузку и уровень ИЛ-6 и СРБ у пациентов с COVID-19. Лечение пациентов (n=77) только интерфероном-альфа-2b или интерфероном-альфа-2b в комбинации с противовирусным препаратом широкого спектра действия арбидолом приводило к элиминации вируса в среднем через 20,3 и 21,1 дня, соответственно, по сравнению со средним значением 27,9 дня в группе, получавшей только арбидол. Уровни циркулирующих ИЛ-6 и СРБ в группе лечения арбидолом были выше, чем у пациентов, получавших только интерферон или интерферон в комбинации с арбидолом. Это говорит о том, что интерфе-рон-альфа-2b может оказывать положительные эффекты при COVID-19, снижая вирусную нагрузку и уменьшая выраженность клинических проявлений. Следует отметить, что у пациентов в данном исследовании не было респираторного дистресс-синдрома, который требовал бы длительной кислородной поддержки или интубации, что свидетельствует о средней степени тяжести COVID-19 [175]. Кроме того, показано, что интерферон-аль-фа-2b в сочетании с арбидолом ускоряет разрешение пневмонии и значительно улучшает картину легких при компьютерной томографии у пациентов с легким течением COVID-19 [176]. Проводятся клинические исследования применения интерферона-альфа-2b в сочетании с ринтатолимодом для лечения COVID-19 легкой или средней степени тяжести у онкологических больных (NCT04379518).
Другой противовирусный препарат рем-десивир, пролекарство, превращающееся в аналог нуклеотидов, который ингибирует вирусные РНК-полимеразы, показал многообещающие результаты использования при COVID-19 в большом количестве исследований [177-181]. Ремдесивир не является противоопухолевым препаратом и также не был одобрен FDA для лечения или профилактики каких-либо заболеваний до пандемии COVID-19. Тем не менее, важно упомянуть ремдесивир в данном обзоре, поскольку FDA выдало разрешение на экстренное использование ремдесивира для лечения всех госпитализированных пациентов с COVID-19 [182]. Применение ремдесивира у пациентов с COVID-19 значительно улучшает клинический исход [177]. Из 53 пациентов, получавших ремдесивир, у 68% потребность в кислороде снизилась в течение периода наблюдения, медиана которого составила 18 дней. У всех пациентов (n=12), получавших низкопоточный дополнительный кислород или неинвазивную кислородную поддержку, наблюдалось улучшение состояния. Более того, на момент завершения исследования, 47% пациентов были выписаны (24% пациентов получали инвазивную ИВЛ, а 89% — неинвазивную кислородную поддержку). После завершения лечения 13% пациентов умерли, летальность была высокой у пожилых пациентов (> 70 лет) и у тех, кто находился на инвазивной ИВЛ [177]. Beigel и соавт. сообщили об аналогичных результатах в своем клиническом исследовании внутривенного применения ремдесивира у взрослых пациентов (n=538), госпитализированных с COVID-19 [181]. Лечение ремдесивиром значительно сократило время выздоровления у пациентов с COVID-19 по сравнению с контрольной группой, получавшей плацебо. Среднее время выздоровления пациентов, получавших ремде-сивир, составило 11 дней, а летальность — 7% к 14 дням после включения в исследование [181]. Кроме того, клиническое исследование, проведенное Gilead Sciences, показало, что у пациентов с COVID-19, получавших ремде-сивир, клиническое выздоровление наступало быстрее, а риск смерти снизился на 62% по сравнению с больными, получавшими стандартное лечение [178]. В настоящее время проводятся многочисленные клинические исследования, посвященные изучению эффектов ремдесивира при лечении COVID-19 [183, 184].
В заключение следует отметить, что применяемые на момент написания данной публикации в клинической практике подходы к лечению включают профилактику инфекции и обеспечение поддерживающей терапии, такой как кислородная поддержка и искусственная вентиляция легких, а также некоторые экспериментальные препараты. Противоопухолевые препараты могут быть использованы для лечения воспаления, дисфункции иммунной системы и подавления размножения вирусов; они являются безопасными и эффективными. Поэтому перепрофилирование лекарств, применяющихся при онкопатологии, может быть разумным выбором при лечении COVID-19 — заболевания, которое быстро распространяется и создает хаос во всем мире. В таблице 3 приведены противоопухолевые препараты, которые используются при COVID-19 в качестве экспериментального лечения.
Выводы и дальнейшие перспективы: Пандемия COVID-19 представляет собой угрозу для здоровья людей во всем мире. Как уже было отмечено выше, не существует общепризнанных утвержденных методов лечения COVID-19. Поэтому широкомасштабные исследования могли бы помочь найти молекулы препаратов, которые позволят эффективно бороться с развитием пандемии. До сих пор остается неизвестным, почему определенные пациенты по-разному реагируют на инфекцию, вызванную SARS-CoV-2. По сравнению со здоровыми людьми в общей популяции пациенты с сопутствующей патологией, такой как онкологические заболевания, более подвержены тяжелому течению COVID-19. Поэтому онкологические больные должны быть особенно осторожны, а медицинские учреждения должны совершенствовать планы мероприятий, направленных на смягчение неблагоприятных последствий пандемии COVID-19 для уязвимых групп населения, к которым относятся пациенты со злокачественными новообразованиями. Следует рассматривать возможность рекомендовать отсрочку химиотерапии или хирургического вмешательства, интенсивное лечение, усиление мер личной защиты, использование средств телекоммуникации и особые стратегии лечения онкологических пациентов
Таблица 3
Перепрофилированные противоопухолевые препараты в клинических исследованиях по лечению COVID-19
|
Противоопухолевые препараты |
Онкологические заболевания, являющиеся зарегистрированным показанием |
Механизм действия |
Клинические исследования применения при COVID-19 |
|
Дювелизиб |
Хронический лимфолей-коз и мелкоклеточная лимфома |
Ингибитор дельта- и гамма-изоформ фосфоинозитид-3-киназы (PI3K) [202] |
Доза: 25 мг 2 раза в день в течение 10 дней. ИИ: NCT04372602 |
|
Изотретиноин (13-цис-ретиноевая кислота) |
Нейробластома |
Точный механизм неизвестен. Индуцирует апоптоз и остановку клеточного цикла [203-208] |
Доза: 0,5 мг/кг/сут в 2 приема внутрь в течение одного месяца. ИИ: NCT04361422 |
|
Децитабин |
Миелодиспластический синдром |
Препарат, гипометилирующий ДНК. Является ингибитором ДНК-метил-трансфераз [209] |
Доза: 10 мг/м2 поверхности тела внутривенно в течение 5 дней. ИИ: NCT04482621 |
|
Дексаметазон |
Дексаметазон используется в комбинации с другими лекарственными средствами для лечения лейкозов и лимфом |
Противовоспалительное и иммуносупрессивное. Ингибирует экспрессию медиаторов воспаления [210] |
Доза: 20 мг внутривенно ежедневно в течение 5 дней, затем 10 мг внутривенно ежедневно в течение 4 дней. ИИ: NCT04325061 |
|
Этопозид |
Многочисленные онкологические заболевания, включая рак яичка и легкого, лимфомы, лейкозы, нейробластому и рак яичников |
Образует стабильный комплекс с ДНК и ферментом топоизомеразой II. Предотвращает репарацию топоизомеразой II и индуцирует разрывы двухцепочечной ДНК [211] |
Доза: 150 мг/м2 поверхности тела в сутки внутривенно в 1-й и 4-й день. Если у пациента наблюдается положительный эффект, но развиваются симптомы цитокинового шторма, лечение продолжают на 8-й, 11-й, 18-й и 25-й дни. ИИ: NCT04394416 |
|
Иматиниба мезилат |
Острый лимфобластный лейкоз, хронический миелолейкоз, гастроинтестинальные стромальные опухоли и миелодиспластический синдром |
Ингибитор тирозинкиназы. Ингибирует bcr-abl тирозинкиназу [212] |
Доза: 400 мг в день, перорально в течение 14 дней. ИИ: NCT04394416 |
|
Интерлейкин 2 (ИЛ-2) |
Метастатическая почечно-клеточная карцинома и меланома |
Активирует CD8+ Т- и NK-клетки [213] |
Доза: подкожные инъекции 1 раз в день в течение 10 дней. ИИ: NCT04357444 |
|
Нинтеданиб |
Немелкоклеточный рак легкого |
Связывается с АТФ-связывающим «карманом» рецепторов фактора роста фибробластов, фактора роста тромбоцитов и фактора роста эндотелия сосудов, что приводит к блокированию аутофосфорилирования этих рецепторов и нижестоящих сигнальных каскадов |
Доза: по 1 капсуле (150 мг) 2 раза в день, с интервалом около 12 часов, в течение 8 недель. ИИ: NCT04338802 |
|
Леналидомид |
Миелодиспластический синдром, множественная миелома и лимфома мантийной зоны |
Подавляет пролиферацию клеток, ангиогенез и стимулирует иммунный ответ. Ингибирует циклооксигеназу-2 (ЦОГ-2). Способствует убиквитиниро-ванию транскрипционных факторов IKZF1 и IKZF3 [214, 215] |
Доза: по 1 капсуле (5 мг) в сутки перорально в 1-й, 3-й и 5-й дни в комбинации с профилактической дозой низкомолекулярного гепарина. ИИ: NCT04361643 |
|
Преднизолон |
Острый лимфобластный лейкоз, хронический лимфолей-коз, лимфома Ходжкина и неходжкинские лимфомы |
Ингибирует NF-kB и другие провос-палительные транскрипционные факторы [216] |
Доза: перорально 0,75 мг/кг/сут в течение 5 дней, затем 20 мг/сут еще 5 дней. ИИ: NCT04344288 |
|
Тамоксифен |
Рак молочной железы |
Связывается с рецептором эстрогенов и блокирует пролиферативные эффекты, ассоциированные с его активацией [217] |
Доза: 20 мг перорально 2 раза в день в течение 14 дней. ИИ: NCT04389580 |
|
Занубрутиниб |
Лимфома мантийной зоны |
Связывает и ингибирует активность тирозинкиназы Брутона (BTK) [218] |
Доза: 320 мг (4 x 80 мг) в капсулах перорально один раз в день до 28 дней. ИИ: NCT04382586 |
|
Метотрексат |
Различные виды опухолей, включая рак молочной железы, распространенные формы опухолей головы и шеи, легкого, желудка и системы крови |
Ингибирует ферменты, участвующие в синтезе нуклеотидов, используемых в синтезе нуклеиновых кислот, в том числе дигидрофолатредуктазу, что приводит к истощению запаса нуклеотидов, необходимых для синтеза нуклеиновых кислот [219] |
Доза повышается поэтапно: Фаза 1: 20 мг в неделю в течение 4 недель. Фаза 2: 30 мг в неделю в течение 4 недель. Фаза 3: 40 мг в неделю в течение 4 недель. ИИ: NCT04352465 |
|
Проксалутамид |
Рак предстательной железы |
Антагонист рецепторов андрогенов [220] |
Доза: 200 мг в сочетании со стандартным лечением или в сочетании с 200 мкг/кг ивермектина и 500 мг азитромицина один раз в день. ИИ: NCT04446429 |
|
Дутастерид |
Доброкачественная гиперплазия предстательной железы |
Препарат ингибирует фермент 5α-редуктазу, который превращает тестостерон в дигидротестерон (ДГТ) и снижает его уровень [221] |
Доза: 0,5 мг в сочетании со стандартным лечением или в сочетании с 200 мкг/кг ивермектина и 500 мг азитромицина один раз в день. ИИ: NCT04446429 |
|
Ремдесивир |
Не является противоопухолевым препаратом |
Пролекарство, активное вещество представляет собой аналог нуклеотида, который ингибирует вирусные РНК-полимеразы [222] |
Доза: 100-200 мг в сочетании со стандартным лечением или без него (ИИ: NCT04292899 и NCT04292730) или в сочетании с другими препаратами, включая тоцилизумаб (ИИ: NCT04409262), ГХХ (ИИ: NCT04345419), барицитиниб (ИИ: NCT04401579) |
с COVID-19. Несмотря на все успехи современной медицины, пандемия новой коронавирусной инфекции распространилась по всему миру, и в будущем может появиться много других межвидовых инфекций. Следовательно, также важно наблюдать за другими вирусами, чтобы повысить нашу готовность к возможным новым эпидемиям.
Благодарности : Авторы хотели бы поблагодарить Джеффри Паттерсона из Медицинского центра Университета Небраски, Омаха, Небраска, за помощь в редактировании.
Заявление о конфликте интересов: Авторы заявляют об отсутствии конфликта интересов.
Финансирование: Лаборатория доктора Чал-лагундла полностью или частично поддерживается грантом NIH/NCI 1K22CA197074; грантами Buffet Pilot & Pediatric Cancer Research в UNMC, LB506 (NE state DHHS); Фондом исследований лейкозов, LB506 (NE state DHHS) и стартап-грантами Кафедры биохимии и молекулярной биологии.
С.Н.Б. заявил о получении финансирования в рамках 5P30-CA036727-33.
ЛИТЕРАТУРА [REFERENCES]
-
1. Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019 . N Engl J Med. 2020 ;382(8):727-733. DOI: 10.1056/NEJMoa2001017
-
2. Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States . N Engl J Med. 2020 ;382(10):929-936. DOI: 10.1056/NEJMoa2001191
-
3. Cohen J, Normile D. New SARS-like virus in China triggers alarm . Science. 2020 ;367(6475):234-235. DOI: 10.1126/science.367.6475.234
-
4. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak . J Autoimmun. 2020 ;109:102433. DOI: 10.1016/j.jaut.2020.102433
-
5. Singu S, Acharya A, Challagundla K, Byrareddy SN. Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States . Front Public Health. 2020 ;8:406. DOI: 10.3389/fpubh.2020.00406
-
6. Li P, Kaslan M, Lee SH, et al. Progress in Exosome Isolation Techniques . Theranostics. 2017 ;7(3):789-804. DOI: 10.7150/thno.18133
-
7. Liu J, Liao X, Qian S, et al. Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020 . Emerg Infect Dis. 2020 ;26(6):1320-1323. DOI: 10.3201/eid2606.200239
-
8. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster . Lancet. 2020 ;395(10223):514-523. DOI: 10.1016/S0140-6736(20)30154-9
-
9. Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia . N Engl J Med. 2020 ;382(13):1199-1207. DOI: 10.1056/NEJMoa2001316
-
10. Santarpia JL, Rivera DN, Herrera VL, et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care . Sci Rep. 2020 ;10(1):12732. DOI: 10.1038/s41598-020-69286-3
-
11. Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage . Science. 1984 ;224(4653):1121-1124. DOI: 10.1126/science.6719137
-
12. Cholankeril G, Podboy A, Aivaliotis VI, et al. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California . Gastroenterology. 2020 ;159(2):775-777. DOI: 10.1053/j.gastro.2020.04.008
-
13. Liu F, Zhang Q, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients . Theranostics. 2020 ;10(12):5613-5622. DOI: 10.7150/ thno.45985
-
14. Zhao W, Zhong Z, Xie X, et al. CT Scans of Patients with 2019 Novel Coronavirus (COVID-19) Pneumonia . Theranostics. 2020 ;10(10):4606-4613. DOI: 10.7150/thno.45016
-
15. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet. 2020 ;395(10223):497-506. DOI: 10.1016/S0140-6736(20)30183-5
-
16. Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study . Lancet Child Adolesc Health. 2020 ;4(9):653-661. DOI: 10.1016/ S2352-4642(20)30177-2
-
17. Cao J, Zheng Y, Luo Z, et al. Myocardial injury and COVID-19: Serum hs-cTnI level in risk stratification and the prediction of 30-day fatality in COVID-19 patients with no prior cardiovascular disease . Theranostics. 2020 ;10(21):9663-9673. DOI: 10.7150/thno.47980
-
18. Tersalvi G, Veronese G, Winterton D. Emerging evidence of myocardial injury in COVID-19: A path through the smoke . Theranostics. 2020 ;10(21):9888-9889. DOI: 10.7150/thno.50788
-
19. Dai M, Liu D, Liu M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak . Cancer Discov. 2020 ;10(6):783-791. DOI: 10.1158/2159-8290.CD-20-0422
-
20. Li Y, Han X, Alwalid O, et al. Baseline characteristics and risk factors for short-term outcomes in 132 COVID-19 patients with diabetes in Wuhan China: A retrospective study . Diabetes Res Clin Pract. 2020 ;166:108299. DOI: 10.1016/j.diabres.2020.108299
-
21. Nishiga M, Wang DW, Han Y, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives . Nat Rev Cardiol. 2020 ;17(9):543-558. DOI: 10.1038/s41569-020-0413-9
-
22. What's New in the Guidelines. URL: https://www.covid19treatmentguidelines.nih.gov/whats-new .
-
23. Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan . Emerg Microbes Infect. 2020 ;9(1):221-236. DOI: 10.1080/22221751.2020.1719902
-
24. Wang H, Li X, Li T, et al. The genetic sequence, origin, and diagnosis of SARS-CoV-2 . Eur J Clin Microbiol Infect Dis. 2020 ;39(9):1629-1635. DOI: 10.1007/s10096-020-03899-4
-
25. Walls AC, Park YJ, Tortorici MA, et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein . Cell. 2020 ;181(2):281-292.e6. DOI: 10.1016/j.cell.2020.02.058
-
26. Wan Y, Shang J, Graham R, et al. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus . J Virol. 2020 ;94(7):e00127-20. DOI: 10.1128/ JVI.00127-20
-
27. Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients . Sci Immunol. 2020 ;5(48):eabc8413. DOI: 10.1126/sciimmunol.abc8413
-
28. Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells . Mol Cell. 2020 ;78(4):779-784.e5. DOI: 10.1016/j. molcel.2020.04.022
-
29. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation . Science. 2020 ;367(6483):1260-1263. DOI: 10.1126/science.abb2507
-
30. Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2 . Nat Med. 2020 ;26(4):450-452. DOI: 10.1038/s41591-020-0820-9
-
31. Burkard C, Verheije MH, Wicht O, et al. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner . PLoS Pathog. 2014 ;10(11):e1004502. DOI: 10.1371/journal.ppat.1004502
-
32. Milewska A, Nowak P, Owczarek K, et al. Entry of Human Coronavirus NL63 into the Cell . J Virol. 2018 ;92(3):e01933-17. DOI: 10.1128/JVI.01933-17
-
33. Jaimes JA, Millet JK, Whittaker GR. Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the Novel S1/S2 Site . iScience. 2020 ;23(6):101212. DOI: 10.1016/j.isci.2020.101212
-
34. Bagdonaite I, Wandall HH. Global aspects of viral glycosylation . Glycobiology. 2018 ;28(7):443-467. DOI: 10.1093/glycob/cwy021
-
35. Cook JD, Lee JE. The secret life of viral entry glycoproteins: moonlighting in immune evasion . PLoS Pathog. 2013 ;9(5):e1003258. DOI: 10.1371/journal.ppat.1003258
-
36. Tse LV, Hamilton AM, Friling T, Whittaker GR. A novel activation mechanism of avian influenza virus H9N2 by furin . J Virol. 2014 ;88(3):1673-1683. DOI: 10.1128/JVI.02648-13
-
37. van Dorp L, Acman M, Richard D, et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2 . Infect Genet Evol. 2020 ;83:104351. DOI: 10.1016/j.meegid.2020.104351
-
38. Graham RL, Sparks JS, Eckerle LD, et al. SARS coronavirus replicase proteins in pathogenesis . Virus Res. 2008 ;133(1):88-100. DOI: 10.1016/j.virusres.2007.02.017
-
39. Forni D, Cagliani R, Mozzi A, et al. Extensive Positive Selection Drives the Evolution of Nonstructural Proteins in Lineage C Betacoronaviruses . J Virol. 2016 ;90(7):3627-3639. DOI: 10.1128/JVI.02988-15
-
40. Angeletti S, Benvenuto D, Bianchi M, et al. COVID-2019: The role of the nsp2 and nsp3 in its pathogenesis . J Med Virol. 2020;92(6):584-588. DOI: 10.1002/jmv.25719
-
41. Pachetti M, Marini B, Benedetti F, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant . J Transl Med. 2020 ;18(1):179. DOI: 10.1186/s12967-020-02344-6
-
42. Wang C, Liu Z, Chen Z, et al. The establishment of reference sequence for SARS-CoV-2 and variation analysis . J Med Virol. 2020 ;92(6):667-674. DOI: 10.1002/jmv.25762
-
43. Kang DH, Weaver MT, Park NJ, et al. Significant impairment in immune recovery after cancer treatment . Nurs Res. 2009 ;58(2):105-114. DOI: 10.1097/NNR.0b013e31818fcecd
-
44. Wu MY, Li CJ, Yiang GT, et al. Molecular Regulation of Bone Metastasis Pathogenesis . Cell Physiol Biochem. 2018 ;46(4):1423-1438. DOI: 10.1159/000489184
-
45. Rogado J, Pangua C, Serrano-Montero G, et al. Covid-19 and lung cancer: A greater fatality rate? Lung Cancer. 2020 ;146:19-22. DOI: 10.1016/j.lungcan.2020.05.034
-
46. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China . Ann Oncol. 2020 ;31(7):894-901. DOI: 10.1016/j. annonc.2020.03.296
-
47. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 ;395(10241):1907-1918. DOI: 10.1016/S0140-6736(20)31187-9
-
48. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China . Lancet Oncol. 2020 ;21(3):335-337. DOI: 10.1016/S1470-2045(20)30096-6
-
49. Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 ;323(18):1775-1776. DOI: 10.1001/jama.2020.4683
-
50. Trapani D, Marra A, Curigliano G. The experience on coronavirus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy Region . Eur J Cancer. 2020 ;132:199-206. DOI: 10.1016/j.ejca.2020.04.017
-
51. Park JR, Bagatell R, London WB, et al. Children's Oncology Group's 2013 blueprint for research: neuroblastoma . Pediatr Blood Cancer. 2013 ;60(6):985-993. DOI: 10.1002/pbc.24433
-
52. Mehta V, Goel S, Kabarriti R, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System . Cancer Discov. 2020 ;10(7):935-941. DOI: 10.1158/2159-8290.CD-20-0516
-
53. Lara Álvarez MÁ, Rogado Revuelta J, Obispo Portero B, et al. COVID-19 mortality in cancer patients in a Madrid hospital during the first 3 weeks of the epidemic . Med Clin (Engl Ed). 2020 ;155(5):202-204. DOI: 10.1016/j.medcle.2020.05.012
-
54. Rugge M, Zorzi M, Guzzinati S. SARS-CoV-2 infection in the Italian Veneto region: adverse outcomes in patients with cancer . Nat Cancer. 2020 ;1(8):784-788. DOI: 10.1038/s43018-020-0104-9
-
55. Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer . Ann Oncol. 2020 ;31(10):1386-1396. DOI: 10.1016/j.annonc.2020.06.007
-
56. Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study . Lancet Oncol. 2020 ;21(7):914-922. DOI: 10.1016/S1470-2045(20)30314-4
-
57. Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study . Lancet Haematol. 2020 ;7(10):e737-e745. DOI: 10.1016/S2352-3026(20)30251-9
-
58. Sanchez-Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, et al. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies . Eur J Haematol. 2020 ;105(5):597-607. DOI: 10.1111/ejh.13493
-
59. Vuagnat P, Frelaut M, Ramtohul T, et al. COVID-19 in breast cancer patients: a cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res. 2020 ;22(1):55. DOI: 10.1186/s13058-020-01293-8
-
60. Aries JA, Davies JK, Auer RL, et al. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients . Br J Haematol. 2020 ;190(2):e64-e67. DOI: 10.1111/bjh.16852
-
61. Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor . Nature. 2020 ;581(7807):215-220. DOI: 10.1038/s41586-020-2180-5
-
62. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor . Cell. 2020 ;181(2):271-280.e8. DOI: 10.1016/j. cell.2020.02.052
-
63. Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system . Trends Endocrinol Metab. 2004 ;15(4):166-169. DOI: 10.1016/j.tem.2004.03.001
-
64. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis . J Pathol. 2004 ;203(2):631-637. DOI: 10.1002/ path.1570
-
65. Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome . J Microbiol Immunol Infect. 2020 ;53(3):425-435. DOI: 10.1016/j.jmii.2020.04.015
-
66. Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation . Aging Cell. 2020 ;19(7):e13168. DOI: 10.1111/acel.13168
-
67. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin . Nature. 2020 ;579(7798):270-273. DOI: 10.1038/s41586-020-2012-7
-
68. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2 . Proc Natl Acad Sci U S A. 2020 ;117(21):11727-11734. DOI: 10.1073/pnas.2003138117
-
69. Deshotels MR, Xia H, Sriramula S, et al. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism . Hypertension. 2014 ;64(6):1368-1375. DOI: 10.1161/HYPERTENSIONAHA.114.03743
-
70. Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging . EMBO Mol Med. 2010 ;2(7):247-257. DOI: 10.1002/emmm.201000080
-
71. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents . N Engl J Med. 2020 ;383(4):334-346. DOI: 10.1056/NEJMoa2021680
-
72. Zong H, Yin B, Zhou H, et al. Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumour Biol . 2015 ;36(7):5171-5177. DOI: 10.1007/s13277-015-3171-2
-
73. Yu C, Tang W, Wang Y, et al. Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry . Cancer Lett. 2016 ;376(2):268-277. DOI: 10.1016/j. canlet.2016.04.006
-
74. Zhou L, Zhang R, Yao W, et al. Decreased expression of angiotensin-converting enzyme 2 in pancreatic ductal adenocarcinoma is associated with tumor progression . Tohoku J Exp Med. 2009 ;217(2):123-131. DOI: 10.1620/tjem.217.123
-
75. Xu J, Fan J, Wu F, et al. The ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Pleiotropic Roles in Cancer . Front Physiol. 2017 ;8:276. DOI: 10.3389/fphys.2017.00276
-
76. Qian YR, Guo Y, Wan HY, et al. Angiotensin-converting enzyme 2 attenuates the metastasis of non-small cell lung cancer through inhibition of epithelial-mesenchymal transition . Oncol Rep. 2013 ;29(6):2408-2414. DOI: 10.3892/or.2013.2370
-
77. Stewart CA, Gay CM, Ramkumar K, et al. SARS-CoV-2 infection induces EMT-like molecular changes, including ZEB1-mediated repression of the viral receptor ACE2, in lung cancer models . bioRxiv. 2020 ;Version 1: Preprint. 2020 May 29
-
78. Yang J, Li H, Hu S, Zhou Y. ACE2 correlated with immune infiltration serves as a prognostic biomarker in endometrial carcinoma and renal papillary cell carcinoma: implication for COVID-19 . Aging (Albany NY). 2020 ;12(8):6518-6535. DOI: 10.18632/aging.103100
-
79. Ehrlich M. DNA hypomethylation in cancer cells . Epigenomics. 2009 ;1(2):239-259. DOI: 10.2217/epi.09.33
-
80. Lucas JM, Heinlein C, Kim T, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis . Cancer Discov. 2014 ;4(11):1310-1325. DOI: 10.1158/2159-8290.CD-13-1010
-
81. Wang Q, Li W, Liu XS, et al. A hierarchical network of transcription factors governs androgen receptordependent prostate cancer growth . Mol Cell. 2007 ;27(3):380-392. DOI: 10.1016/j.molcel.2007.05.041
-
82. Shaw GL, Whitaker H, Corcoran M, et al. The Early Effects of Rapid Androgen Deprivation on Human Prostate Cancer. Eur Urol. 2016 ;70(2):214-218. DOI: 10.1016/j.eururo.2015.10.042
-
83. Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) . Ann Oncol. 2020 ;31(8):1040-1045. DOI: 10.1016/j.annonc.2020.04.479
-
84. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report
-
85. Burgueño JF, Reich A, Hazime H, et al. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD . Inflamm Bowel Dis. 2020 ;26(6):797-808. DOI: 10.1093/ibd/izaa085
-
86. Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes . Sci Immunol. 2020 ;5(47):eabc3582. DOI: 10.1126/sciimmunol.abc3582
-
87. Wu ZS, Zhang ZQ, Wu S. Focus on the Crosstalk between COVID-19 and Urogenital Systems . J Urol. 2020 ;204(1):7-8. DOI: 10.1097/JU.0000000000001068
-
88. Vaarala MH, Porvari K, Kyllönen A, et al. The TMPRSS2 gene encoding transmembrane serine protease is overexpressed in a majority of prostate cancer patients: detection of mutated TMPRSS2 form in a case of aggressive disease . Int J Cancer. 2001 ;94(5):705-710. DOI: 10.1002/ijc.1526
-
89. Bahmad HF, Abou-Kheir W. Crosstalk between COVID-19 and prostate cancer . Prostate Cancer Prostatic Dis. 2020 ;23(4):561-563. DOI: 10.1038/s41391-020-0262-y
-
90. https://covid19.who.int
-
91. Spitz R, Hero B, Simon T, Berthold F. Loss in chromosome 11q identifies tumors with increased risk for metastatic relapses in localized and 4S neuroblastoma . Clin Cancer Res. 2006 ;12(11 Pt 1):3368-3373. DOI: 10.1158/1078-0432.CCR-05-2495
-
92. Xu PP, Tian RH, Luo S, et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study . Theranostics. 2020 ;10(14):6372-6383. DOI: 10.7150/thno.46833
-
93. Spitz R, Hero B, Westermann F, et al. Fluorescence in situ hybridization analyses of chromosome band 1p36 in neuroblastoma detect two classes of alterations . Genes Chromosomes Cancer. 2002 ;34(3):299-305. DOI: 10.1002/gcc.10070
-
94. https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview
-
95. Datta PK, Liu F, Fischer T, et al. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy . Theranostics. 2020 ;10(16):7448-7464. DOI: 10.7150/thno.48076
-
96. Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020 ;26(5):681-687. DOI: 10.1038/s41591-020-0868-6
-
97. Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol Sin. 2020 ;35(3):266-271. DOI: 10.1007/s12250-020-00207-4
-
98. Tian S, Hu W, Niu L, et al. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer . J Thorac Oncol. 2020 ;15(5):700-704. DOI: 10.1016/j.jtho.2020.02.010
-
99. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study . Lancet Oncol. 2020 ;21(7):893-903. DOI: 10.1016/S1470-2045(20)30309-0
-
100. Diao B, Wang C, Tan Y, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) . Front Immunol. 2020 ;11:827. DOI: 10.3389/fimmu.2020.00827
-
101. McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy . Cancer Med. 2013 ;2(5):662-673. DOI: 10.1002/cam4.106
-
102. Vanni G, Materazzo M, Pellicciaro M, et al. Breast Cancer and COVID-19: The Effect of Fear on Patients' Decision-making Process . In Vivo. 2020 ;34(3 Suppl):1651-1659. DOI: 10.21873/invivo.11957
-
103. Ñamendys-Silva SA. Respiratory support for patients with COVID-19 infection . Lancet Respir Med. 2020 ;8(4):e18. DOI: 10.1016/S2213-2600(20)30110-7
-
104. Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives . Nature. 2020 ;582(7813):469. DOI: 10.1038/d41586-020-01824-5
-
105. Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020 ;97:396-403. DOI: 10.1016/j.ijid.2020.06.099
-
106. Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica. 2020 ;44:e40. DOI: 10.26633/RPSP.2020.40
-
107. Piechotta V, Chai KL, Valk SJ, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review . Cochrane Database Syst Rev. 2020 ;7(7):CD013600. DOI: 10.1002/14651858.CD013600.pub2
-
108. Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020 ;31(7):961-964. DOI: 10.1016/j.annonc.2020.03.300
-
109. Wan Y, Shang J, Graham R, et al. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus . J Virol. 2020 ;94(7):e00127-20. DOI: 10.1128/ JVI.00127-20
-
110. Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug . Semin Arthritis Rheum. 1993 ;23(2 Suppl 1):82-91. DOI: 10.1016/s0049-0172(10)80012-5
-
111. Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond . EMBO Mol Med. 2020 ;12(8):e12476. DOI: 10.15252/emmm.202012476
-
112. Piconi S, Parisotto S, Rizzardini G, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders . Blood. 2011;118(12):3263-3272. DOI: 10.1182/blood-2011-01-329060
-
113. Yao TT, Qian JD, Zhu WY, et al. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option . J Med Virol. 2020 ;92(6):556-563. DOI: 10.1002/jmv.25729
-
114. Rivera DR, Peters S, Panagiotou OA, et al. Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study . Cancer Discov. 2020 ;10(10):1514-1527. DOI: 10.1158/2159-8290.CD-20-0941
-
115. Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19 . N Engl J Med. 2020 ;382(25):2411-2418. DOI: 10.1056/NEJMoa2012410
-
116. Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State . JAMA. 2020 ;323(24):2493-2502. DOI: 10.1001/jama.2020.8630
-
117. RECOVERY Collaborative Group, Horby P, Mafham M, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19 . N Engl J Med. 2020 ;383(21):2030-2040. DOI: 10.1056/NEJMoa2022926
-
118. https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine
-
119. https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine-with-or-without-azithromycin/
-
120. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages . Nat Rev Immunol. 2020 ;20(6):355-362. DOI: 10.1038/s41577-020-0331-4
-
121. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab . Proc Natl Acad Sci U S A. 2020 ;117(20):10970-10975. DOI: 10.1073/pnas.2005615117
-
122. Liu J, Wan M, Lyon CJ, Hu TY. Nanomedicine therapies modulating Macrophage Dysfunction: a potential strategy to attenuate Cytokine Storms in severe infections . Theranostics. 2020 ;10(21):9591-9600. DOI: 10.7150/thno.47982
-
123. Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study . Lancet Rheumatol. 2020 ;2(8):e474-e484. DOI: 10.1016/S2665-9913(20)30173-9
-
124. Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study . Eur J Intern Med. 2020 ;76:43-49. DOI: 10.1016/j.ejim.2020.05.021
-
125. Magee DJ, Jhanji S, Poulogiannis G, et al. Nonsteroidal anti-inflammatory drugs and pain in cancer patients: a systematic review and reappraisal of the evidence . Br J Anaesth. 2019 ;123(2):e412-e423. DOI: 10.1016/j. bja.2019.02.028
-
126. Coussens LM, Werb Z. Inflammation and cancer . Nature. 2002 ;420(6917):860-867. DOI: 10.1038/ nature01322
-
127. Renner A, Marth K, Patocka K, Pohl W. COVID-19 in a severe eosinophilic asthmatic receiving benralizumab -a case study . J Asthma. 2021 ;58(9):1270-1272. DOI: 10.1080/02770903.2020.1781165
-
128. García-Moguel I, Díaz Campos R, Alonso Charterina S, et al. COVID-19, severe asthma, and biologics . Ann Allergy Asthma Immunol. 2020 ;125(3):357-359.e1. DOI: 10.1016/j.anai.2020.06.012
-
129. Kaplan SS, Hicks CB. Lopinavir/ritonavir in the treatment of human immunodeficiency virus infection . Expert Opin Pharmacother. 2005 ;6(9):1573-1585. DOI: 10.1517/14656566.6.9.1573
-
130. Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19 . N Engl
J Med. 2020 ;382(19):1787-1799. DOI: 10.1056/NEJMoa2001282
-
131. Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial . Lancet. 2020 ;395(10238):1695-1704. DOI: 10.1016/S0140-6736(20)31042-4
-
132. Group RC, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report . N Engl J Med. 2020 : Published on July 17, 2020.
-
133. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020 ;12(1):8. DOI: 10.1038/s41368-020-0074-x
-
134. Cook AM, McDonnell AM, Lake RA, Nowak AK. Dexamethasone co-medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo-immunotherapy strategies. Oncoimmunology. 2015 ;5(3):e1066062. DOI: 10.1080/2162402X.2015.1066062
-
135. Rubinstein SM, Steinharter JA, Warner J, et al. The COVID-19 and Cancer Consortium: A Collaborative Effort to Understand the Effects of COVID-19 on Patients with Cancer . Cancer Cell. 2020 ;37(6):738-741. DOI: 10.1016/j.ccell.2020.04.018
-
136. Ceschi A, Noseda R, Palin K, Verhamme K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database . Front Pharmacol. 2020 ;11:557. DOI: 10.3389/ fphar.2020.00557
-
137. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding . Lancet. 2020 ;395(10224):565-574. DOI: 10.1016/S0140-6736(20)30251-8
-
138. Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong . J Clin Microbiol. 2006 ;44(6):2063-2071. DOI: 10.1128/JCM.02614-05
-
139. Kumar D, Dey T. Treatment delays in oncology patients during COVID-19 pandemic: A perspective . J Glob Health. 2020 ;10(1):010367. DOI: 10.7189/jogh.10.010367
-
140. Matsuo T, Kobayashi D, Taki F, et al. Prevalence of Health Care Worker Burnout During the Coronavirus Disease 2019 (COVID-19) Pandemic in Japan . JAMA Netw Open. 2020 ;3(8):e2017271. DOI: 10.1001/ jamanetworkopen.2020.17271
-
141. Rajkumar RP. COVID-19 and mental health: A review of the existing literature . Asian J Psychiatr. 2020 ;52:102066. DOI: 10.1016/j.ajp.2020.102066
-
142. Ochoa Arnedo C, Sánchez N, Sumalla EC, Casellas-Grau A. Stress and Growth in Cancer: Mechanisms and Psychotherapeutic Interventions to Facilitate a Constructive Balance . Front Psychol. 2019 ;10:177. DOI: 10.3389/fpsyg.2019.00177
-
143. Tsamakis K, Triantafyllis AS, Tsiptsios D, et al. COVID-19 related stress exacerbates common physical and mental pathologies and affects treatment (Review) . Exp Ther Med. 2020 ;20(1):159-162. DOI: 10.3892/ etm.2020.8671
-
144. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic
-
145. Richards M, Anderson M, Carter P, et al. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020 ;1(6):565-567. DOI: 10.1038/s43018-020-0074-y
-
146. Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium . Breast Cancer Res Treat. 2020 ;181(3):487-497. DOI: 10.1007/s10549-020-05644-z
-
147. Shinder BM, Patel HV, Sterling J, et al. Urologic oncology surgery during COVID-19: a rapid review of current triage guidance documents . Urol Oncol. 2020 ;38(7):609-614. DOI: 10.1016/j.urolonc.2020.05.017
-
148. Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016 ;18(1):10. DOI: 10.1186/s13058-015-0669-x
-
149. https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-treatment-supportive-care
-
150. Payne K. Ethical issues related to pandemic flu planning and response . AACN Adv Crit Care. 2007 ;18(4):356-360. DOI: 10.1097/01.AACN.0000298627.07535.76
-
151. Adams DP. Wartime bureaucracy and penicillin allocation: the Committee on Chemotherapeutic and Other Agents, 1942-44 . J Hist Med Allied Sci. 1989 ;44(2):196-217. DOI: 10.1093/jhmas/44.2.196
-
152. Rosenbaum L. Facing Covid-19 in Italy - Ethics, Logistics, and Therapeutics on the Epidemic's Front Line . N Engl J Med. 2020;382(20):1873-1875. DOI: 10.1056/NEJMp2005492
-
153. Xie J, Tong Z, Guan X, et al. Clinical Characteristics of Patients Who Died of Coronavirus Disease 2019 in China . JAMA Netw Open. 2020 ;3(4):e205619. DOI: 10.1001/jamanetworkopen.2020.5619
-
154. Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence . BMC Public Health. 2020 ;20(1):1193. DOI: 10.1186/s12889-020-09301-4
-
155. Lesslie M, Parikh JR. Implementing a Multidisciplinary Tumor Board in the Community Practice Setting . Diagnostics (Basel). 2017 ;7(4):55. DOI: 10.3390/diagnostics7040055
-
156. Wein W. Drug development: successes, problems and pitfalls-the industry perspective . ESMO Open. 2016 ;1(1):e000033. DOI: 10.1136/esmoopen-2016-000033
-
157. Yella JK, Yaddanapudi S, Wang Y, Jegga AG. Changing Trends in Computational Drug Repositioning . Pharmaceuticals (Basel). 2018 ;11(2):57. DOI: 10.3390/ph11020057
-
158. Kale VP, Habib H, Chitren R, et al. Old drugs, new uses: Drug repurposing in hematological malignancies . Semin Cancer Biol. 2021 ;68:242-248. DOI: 10.1016/j.semcancer.2020.03.005
-
159. Zhou Y, Hou Y, Shen J, et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2 . Cell Discov. 2020 ;6:14. DOI: 10.1038/s41421-020-0153-3
-
160. Fan HH, Wang LQ, Liu WL, et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model . Chin Med J (Engl). 2020 ;133(9):1051-1056. DOI: 10.1097/CM9.0000000000000797
-
161. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) . Nat Rev Drug Discov. 2020 ;19(3):149-150. DOI: 10.1038/d41573-020-00016-0
-
162. Liu X, Liu C, Liu G, et al. COVID-19: Progress in diagnostics, therapy and vaccination . Theranostics. 2020 ;10(17):7821-7835. DOI: 10.7150/thno.47987
-
163. Du Y, Tu L, Zhu P, et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study . Am J Respir Crit Care Med. 2020 ;201(11):1372-1379. DOI: 10.1164/rccm.202003-0543OC
-
164. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, et al. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease . Cytokine Growth Factor Rev. 2020 ;54:62-75. DOI: 10.1016/j.cytogfr.2020.06.001
-
165. Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020 ;11:1446. DOI: 10.3389/fimmu.2020.01446
-
166. Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis . Clin Cancer Res. 2012 ;18(11):3008-3014. DOI: 10.1158/1078-0432.CCR-11-3145
-
167. Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms . J Autoimmun. 2017 ;85:58-63.
-
168. La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation . Leukemia. 2020 ;34(7):1805-1815. DOI: 10.1038/s41375-020-0891-0
-
169. Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19 . Sci Immunol. 2020 ;5(48):eabd0110. DOI: 10.1126/sciimmunol.abd0110
-
170. https://www.cancer.gov/news-events/cancer-currents-blog/2017/acalabrutinib-fda-mantle-cell-lymphoma
-
171. Lee KG, Xu S, Kang ZH, et al. Bruton's tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response . Proc Natl Acad Sci U S A. 2012 ;109(15):5791-5796. DOI: 10.1073/pnas.1119238109
-
172. Weber ANR, Bittner Z, Liu X, et al. Bruton's Tyrosine Kinase: An Emerging Key Player in Innate Immunity . Front Immunol. 2017 ;8:1454. DOI: 10.3389/fimmu.2017.01454
-
173. Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b Treatment for COVID-19 . Front Immunol. 2020 ;11:1061.
-
174. Yeager CL, Ashmun RA, Williams RK, et al. Human aminopeptidase N is a receptor for human coronavirus 229E . Nature. 1992 ;357(6377):420-422. DOI: 10.1038/357420a0
-
175. Asmana Ningrum R. Human interferon alpha-2b: a therapeutic protein for cancer treatment . Scientifica (Cairo). 2014 ;2014:970315. DOI: 10.1155/2014/970315
-
176. Xu P, Huang J, Fan Z, et al. Arbidol/IFN-α2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study . Microbes Infect. 2020 ;22(4-5):200-205. DOI: 10.1016/j.micinf.2020.05.012
-
177. Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19 . N Engl J Med. 2020 ;382(24):2327-2336. DOI: 10.1056/NEJMoa2007016
-
178. Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized Mouse Xenograft Models: Narrowing the TumorMicroenvironment Gap . Cancer Res. 2016 ;76(21):6153-6158. DOI: 10.1158/0008-5472.CAN-16-1260
-
179. Jiang Y, Chen D, Cai D, et al. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: A network meta-analysis . J Med Virol. 2021 ;93(2):1171-1174. DOI: 10.1002/jmv.26443
-
180. Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2 . Nature. 2020 ;585(7824):273-276. DOI: 10.1038/s41586-020-2423-5
-
181. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report . N Engl J Med. 2020 ;383(19):1813-1826. DOI: 10.1056/NEJMoa2007764
-
182. Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets . Nat Rev Gastroenterol Hepatol. 2017 ;14(8):455-466. DOI: 10.1038/nrgastro.2017.71
-
183. Hashemian SM, Farhadi T, Velayati AA. A Review on Remdesivir: A Possible Promising Agent for the Treatment of COVID-19 . Drug Des Devel Ther. 2020 ;14:3215-3222. DOI: 10.2147/DDDT.S261154
-
184. Neogi U, Hill KJ, Ambikan AT, et al. Feasibility of Known RNA Polymerase Inhibitors as Anti-SARS-CoV-2 Drugs .
-
185. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
-
186. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200928-weekly-epi-update . pdf?sfvrsn=9e354665_6
-
187. Wu A, Peng Y, Huang B, et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China . Cell Host Microbe. 2020 ;27(3):325-328. DOI: 10.1016/j.chom.2020.02.001
-
188. Zhou H, Chen X, Hu T, et al. A Novel Bat Coronavirus Closely Related to SARS-CoV-2 Contains Natural Insertions at the S1/S2 Cleavage Site of the Spike Protein . Curr Biol. 2020 ;30(11):2196-2203.e3. DOI: 10.1016/j. cub.2020.05.023
-
189. Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City . Ann Oncol. 2020 ;31(8):1088-1089. DOI: 10.1016/j.annonc.2020.04.006
-
190. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study . BMJ. 2020 ;369:m1985. DOI: 10.1136/bmj.m1985
-
191. Meng Y, Lu W, Guo E, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis . J Hematol Oncol. 2020 ;13(1):75. DOI: 10.1186/s13045-020-00907-0
-
192. Rogado J, Obispo B, Pangua C, et al. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid . Clin Transl Oncol. 2020 ;22(12):2364-2368. DOI: 10.1007/s12094-020-02381-z
-
193. Gupta S, Hayek SS, Wang W, et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US . JAMAIntern Med. 2020 ;180(11):1436-1447. DOI: 10.1001/jamainternmed.2020.3596
-
194. Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study . Lancet Oncol. 2020 ;21(7):904-913. DOI: 10.1016/S1470-2045(20)30310-7
-
195. Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study . Lancet Oncol. 2020 ;21(10):1309-1316. DOI: 10.1016/S1470-2045(20)30442-3
-
196. Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study . Lancet. 2020 ;395(10241):1919-1926. DOI: 10.1016/ S0140-6736(20)31173-9
-
197. Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer . Nat Med. 2020 ;26(8):1218-1223. DOI: 10.1038/s41591-020-0979-0
-
198. Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience . Blood. 2020 ;136(10):1134-1143. DOI: 10.1182/blood.2020006965
-
199. Bisogno G, Provenzi M, Zama D, et al. Clinical Characteristics and Outcome of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Italian Pediatric Oncology Patients: A Study From the Infectious Diseases Working Group of the Associazione Italiana di Oncologia e Ematologia Pediatrica . J Pediatric Infect Dis Soc. 2020 ;9(5):530-534. DOI: 10.1093/jpids/piaa088
-
200. Pinato DJ, Lee AJX, Biello F, et al. Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe . Cancers (Basel). 2020 ;12(7):1841. DOI: 10.3390/cancers12071841
-
201. Patel VG, Zhong X, Liaw B, et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020 ;31(10):1419-1420. DOI: 10.1016/j.annonc.2020.06.023
-
202. Flinn IW, O'Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies . Blood. 2018 ;131(8):877-887. DOI: 10.1182/blood-2017-05-786566
-
203. Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes . J Invest Dermatol. 2006 ;126(10):2178-2189. DOI: 10.1038/sj.jid.5700289
-
204. Gunda V, Pathania AS, Chava S, et al. Amino Acids Regulate Cisplatin Insensitivity in Neuroblastoma . Cancers (Basel). 2020 ;12(9):2576. DOI: 10.3390/cancers12092576
-
205. Mahapatra S, Challagundla KB. Cancer, Neuroblastoma . StatPearls. Treasure Island (FL). 2020
-
206. Chava S, Reynolds CP, Pathania AS, et al. miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma . Mol Oncol. 2020 ;14(1):180-196. DOI: 10.1002/18780261.12588
-
207. Challagundla KB, Wise PM, Neviani P, et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy . J Natl Cancer Inst. 2015 ;107(7):djv135. DOI: 10.1093/jnci/djv135
-
208. Mudgapalli N, Shaw BP, Chava S, Challagundla KB. The Transcribed-Ultra Conserved Regions: Novel Non-Coding RNA Players in Neuroblastoma Progression . Noncoding RNA. 2019 ;5(2):39. DOI: 10.3390/ ncrna5020039
-
209. Yan P, Frankhouser D, Murphy M, et al. Genome-wide methylation profiling in decitabine-treated patients with acute myeloid leukemia . Blood. 2012 ;120(12):2466-2474. DOI: 10.1182/blood-2012-05-429175
-
210. Abraham SM, Lawrence T, Kleiman A, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1 . J Exp Med. 2006 ;203(8):1883-1889. DOI: 10.1084/ jem.20060336
-
211. Montecucco A, Zanetta F, Biamonti G. Molecular mechanisms of etoposide . EXCLI J. 2015 ;14:95-108. DOI: 10.17179/excli2015-561
-
212. Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer . Chemother Res Pract. 2014 ;2014:357027. DOI: 10.1155/2014/357027
-
213. Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy . Oncoimmunology. 2016 ;5(6):e1163462. DOI: 10.1080/2162402X.2016.1163462
-
214. Fink EC, Ebert BL. The novel mechanism of lenalidomide activity . Blood. 2015 ;126(21):2366-2369. DOI: 10.1182/blood-2015-07-567958
-
215. Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies . J Hematol Oncol. 2009 ;2:36. DOI: 10.1186/1756-8722-2-36
-
216. Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer . J Clin Invest. 2001 ;107(2):135-142. DOI: 10.1172/JCI11914
-
217. Fritsch M, Jordan VC. Long-term Tamoxifen Therapy for the Treatment of Breast Cancer . Cancer Control. 1994 ;1(4):356-366.
-
218. Tam CS. Zanubrutinib: a novel BTK inhibitor in chronic lymphocytic leukemia and non-Hodgkin lymphoma . Clin Adv Hematol Oncol. 2019 ;17(1):32-34.
-
219. Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis . Bull NYU Hosp Jt Dis. 2007 ;65(3):168-173.
-
220. Dellis AE, Papatsoris AG. Perspectives on the current and emerging chemical androgen receptor antagonists for the treatment of prostate cancer . Expert Opin Pharmacother. 2019 ;20(2):163-172. DOI: 10.1080/1465 6566.2018.1548611
-
221. Miller J, Tarter TH. Combination therapy with dutasteride and tamsulosin for the treatment of symptomatic enlarged prostate . Clin Interv Aging. 2009 ;4:251-258. DOI: 10.2147/cia.s4102
-
222. Saha A, Sharma AR, Bhattacharya M, et al. Probable Molecular Mechanism of Remdesivir for the Treatment of COVID-19: Need to Know More . Arch Med Res. 2020 ;51(6):585-586. DOI: 10.1016/j.arcmed.2020.05.001
DOI: 10.1016/j.jaut.2017.06.010
DOI: 10.3389/fimmu.2020.01061
Pathogens. 2020 ;9(5):320. DOI: 10.3390/pathogens9050320
Перевод поступил в редакцию: 21.11.2021
Список литературы COVID-19 и сопутствующая онкологическая патология: терапевтические возможности и сложности
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. DOI: 10.1056/NEJMoa2001017
- Holshue ML, DeBolt C, Lindquist S, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929-936. DOI: 10.1056/NEJMoa2001191
- Cohen J, Normile D. New SARS-like virus in China triggers alarm. Science. 2020;367(6475):234-235. DOI: 10.1126/science.367.6475.234
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020; 109:102433. DOI: 10.1016/j.jaut.2020.102433
- Singu S, Acharya A, Challagundla K, Byrareddy SN. Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States. Front Public Health. 2020; 8:406. DOI: 10.3389/fpubh.2020.00406
- Li P, Kaslan M, Lee SH, et al. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789-804. DOI: 10.7150/thno.18133
- Liu J, Liao X, Qian S, et al. Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 2020;26(6):1320-1323. DOI: 10.3201/eid2606.200239
- Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523. DOI: 10.1016/S0140-6736(20)30154-9
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199-1207. DOI: 10.1056/NEJMoa2001316
- Santarpia JL, Rivera DN, Herrera VL, et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep. 2020;10(1):12732. DOI: 10.1038/s41598-020-69286-3
- Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984,-224(4653^1121-1124. DOI: 10.1126/science.6719137
- Cholankeril G, Podboy A, Aivaliotis VI, et al. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology. 2020;159(2):775-777. DOI: 10.1053/j.gastro.2020.04.008
- Liu F, Zhang Q, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics. 2020;10(12):5613-5622. DOI: 10.7150/ thno.45985
- Zhao W, Zhong Z, Xie X, et al. CT Scans of Patients with 2019 Novel Coronavirus (COVID-19) Pneumonia. Theranostics. 2020;10(10):4606-4613. DOI: 10.7150/thno.45016
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. DOI: 10.1016/S0140-6736(20)30183-5
- Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653-661. DOI: 10.1016/ S2352-4642(20)30177-2
- Cao J, Zheng Y, Luo Z, et al. Myocardial injury and COVID-19: Serum hs-cTnI level in risk stratification and the prediction of 30-day fatality in COVID-19 patients with no prior cardiovascular disease. Theranostics. 2020;10(21):9663-9673. DOI: 10.7150/thno.47980
- Tersalvi G, Veronese G, Winterton D. Emerging evidence of myocardial injury in COVID-19: A path through the smoke. Theranostics. 2020;10(21):9888-9889. DOI: 10.7150/thno.50788
- Dai M, Liu D, Liu M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020;10(6):783-791. DOI: 10.1158/2159-8290.CD-20-0422
- Li Y, Han X, Alwalid O, et al. Baseline characteristics and risk factors for short-term outcomes in 132 COVID-19 patients with diabetes in Wuhan China: A retrospective study. Diabetes Res Clin Pract. 2020; 166:108299. DOI: 10.1016/j.diabres.2020.108299
- Nishiga M, Wang DW, Han Y, et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543-558. DOI: 10.1038/s41569-020-0413-9
- What's New in the Guidelines. URL: https://www.covid19treatmentguidelines.nih.gov/whats-new.
- Chan JF, Kok KH, Zhu Z, et al. Genomic characterization of the 2019 novel human-pathogenic Coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221-236. DOI: 10.1080/22221751.2020.1719902
- Wang H, Li X, Li T, et al. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 2020;39(9):1629-1635. DOI: 10.1007/s10096-020-03899-4
- Walls AC, Park YJ, Tortorici MA, et al. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281-292.e6. DOI: 10.1016/j.cell.2020.02.058
- Wan Y, Shang J, Graham R, et al. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7):e00127-20. DOI: 10.1128/ JVI.00127-20
- Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5(48):eabc8413. DOI: 10.1126/sciimmunol.abc8413
- Hoffmann M, Kleine-Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol Cell. 2020;78(4):779-784.e5. DOI: 10.1016/j. molcel.2020.04.022
- Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-1263. DOI: 10.1126/science.abb2507
- Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450-452. DOI: 10.1038/s41591-020-0820-9
- Burkard C, Verheije MH, Wicht O, et al. Coronavirus cell entry occurs through the endo-/lysosomal pathway in aproteolysis-dependentmanner. PLoS Pathog. 2014;10(11):e1004502. DOI: 10.1371/journal.ppat.1004502
- Milewska A, Nowak P, Owczarek K, et al. Entry of Human Coronavirus NL63 into the Cell. J Virol. 2018;92(3):e01933-17. DOI: 10.1128/JVI.01933-17
- Jaimes JA, Millet JK, Whittaker GR. Proteolytic Cleavage of the SARS-CoV-2 Spike Protein and the Role of the NovelS1/S2 Site. iScience. 2020;23(6):101212. DOI: 10.1016/j.isci.2020.101212
- Bagdonaite I, Wandall HH. Global aspects of viral glycosylation. Glycobiology. 2018;28(7):443-467. DOI: 10.1093/glycob/cwy021
- Cook J D, Lee J E. The secret life of viral entry glycoproteins: moonlighting in immune evasion. PLoS Pathog. 2013;9(5):e1003258. DOI: 10.1371/journal.ppat.1003258
- Tse LV, Hamilton AM, Friling T, Whittaker GR. A novel activation mechanism of avian influenza virus H9N2 by furin. J Virol. 2014;88(3):1673-1683. DOI: 10.1128/JVI.02648-13
- van Dorp L, Acman M, Richard D, et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020;83:104351. DOI: 10.1016/j.meegid.2020.104351
- Graham RL, Sparks JS, Eckerle LD, et al. SARS coronavirus replicase proteins in pathogenesis. Virus Res. 2008;133(1):88-100. DOI: 10.1016/j.virusres.2007.02.017
- Forni D, Cagliani R, Mozzi A, et al. Extensive Positive Selection Drives the Evolution of Nonstructural Proteins in Lineage C Betacoronaviruses. J Virol. 2016;90(7):3627-3639. DOI: 10.1128/JVI.02988-15
- Angeletti S, Benvenuto D, Bianchi M, et al. COVID-2019: The role of the nsp2 and nsp3 in its pathogenesis. J Med Virol. 2020;92(6):584-588. DOI: 10.1002/jmv.25719
- Pachetti M, Marini B, Benedetti F, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18(1):179. DOI: 10.1186/s12967-020-02344-6
- Wang C, Liu Z, Chen Z, et al. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol. 2020;92(6):667-674. DOI: 10.1002/jmv.25762
- Kang DH, Weaver MT, Park NJ, et al. Significant impairment in immune recovery after cancer treatment. Nurs Res. 2009;58(2):105-114. DOI: 10.1097/NNR.0b013e31818fcecd
- Wu MY, Li CJ, Yiang GT, et al. Molecular Regulation of Bone Metastasis Pathogenesis. Cell Physiol Biochem. 2018;46(4):1423-1438. DOI: 10.1159/000489184
- Rogado J, Pangua C, Serrano-Montero G, et al. Covid-19 and lung cancer: A greater fatality rate? Lung Cancer. 2020;146:19-22. DOI: 10.1016/j.lungcan.2020.05.034
- Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894-901. DOI: 10.1016/j. annonc.2020.03.296
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918. DOI: 10.1016/S0140-6736(20)31187-9
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337. DOI: 10.1016/S1470-2045(20)30096-6
- Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-1776. DOI: 10.1001/jama.2020.4683
- Trapani D, Marra A, Curigliano G. The experience on coronavirus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy Region. Eur J Cancer. 2020;132:199-206. DOI: 10.1016/j.ejca.2020.04.017
- Park JR, Bagatell R, London WB, et al. Children's Oncology Group's 2013 blueprintfor research: neuroblastoma. Pediatr Blood Cancer. 2013;60(6):985-993. DOI: 10.1002/pbc.24433
- Mehta V, Goel S, Kabarriti R, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935-941. DOI: 10.1158/2159-8290.CD-20-0516
- Lara Alvarez MÂ, Rogado Revuelta J, Obispo Portero B, et al. COVID-19 mortality in cancer patients in a Madrid hospital during the first 3 weeks of the epidemic. Med Clin (Engl Ed). 2020;155(5):202-204. DOI: 10.1016/j.medcle.2020.05.012
- Rugge M, Zorzi M, Guzzinati S. SARS-CoV-2 infection in the Italian Veneto region: adverse outcomes in patients with cancer. Nat Cancer. 2020;1(8):784-788. DOI: 10.1038/s43018-020-0104-9
- Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31(10):1386-1396. DOI: 10.1016/j.annonc.2020.06.007
- Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914-922. DOI: 10.1016/S1470-2045(20)30314-4
- Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737-e745. DOI: 10.1016/S2352-3026(20)30251-9
- Sanchez-Pina JM, Rodriguez Rodriguez M, Castro Quismondo N, et al. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol. 2020;105(5):597-607. DOI: 10.1111/ejh.13493
- Vuagnat P, Frelaut M, Ramtohul T, et al. COVID-19 in breast cancer patients: a cohort at the Institut Curie hospitals in the Paris area. Breast Cancer Res. 2020;22(1):55. DOI: 10.1186/s13058-020-01293-8
- Aries JA, Davies JK, Auer RL, et al. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol. 2020;190(2):e64-e67. DOI: 10.1111/bjh.16852
- Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215-220. DOI: 10.1038/s41586-020-2180-5
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280.e8. DOI: 10.1016/j. cell.2020.02.052
- Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab. 2004;15(4):166-169. DOI: 10.1016/j.tem.2004.03.001
- Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637. DOI: 10.1002/ path.1570
- Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53(3):425-435. DOI: 10.1016/j.jmii.2020.04.015
- Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19(7):e13168. DOI: 10.1111/acel.13168
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273. DOI: 10.1038/s41586-020-2012-7
- Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117(21):11727-11734. DOI: 10.1073/pnas.2003138117
- Deshotels MR, Xia H, Sriramula S, et al. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64(6):1368-1375. DOI: 10.1161/HYPERTENSIONAHA.114.03743
- Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247-257. DOI: 10.1002/emmm.201000080
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383(4):334-346. DOI: 10.1056/NEJMoa2021680
- Zong H, Yin B, Zhou H, et al. Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumour Biol. 2015;36(7):5171-5177. DOI: 10.1007/s13277-015-3171-2
- Yu C, Tang W, Wang Y, et al. Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett. 2016;376(2):268-277. DOI: 10.1016/j. canlet.2016.04.006
- Zhou L, Zhang R, Yao W, et al. Decreased expression of angiotensin-converting enzyme 2 in pancreatic ductal adenocarcinoma is associated with tumor progression. Tohoku J Exp Med. 2009;217(2):123-131. DOI: 10.1620/tjem.217.123
- Xu J, Fan J, Wu F, et al. The ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Pleiotropic Roles in Cancer. Front Physiol. 2017;8:276. DOI: 10.3389/fphys.2017.00276
- Qian YR, Guo Y, Wan HY, et al. Angiotensin-converting enzyme 2 attenuates the metastasis of non-small cell lung cancer through inhibition of epithelial-mesenchymal transition. Oncol Rep. 2013;29(6):2408-2414. DOI: 10.3892/or. 2013.2370
- Stewart CA, Gay CM, Ramkumar K, et al. SARS-CoV-2 infection induces EMT-like molecular changes, including ZEBl-mediated repression of the viral receptor ACE2, in lung cancer models. bioRxiv. 2020,-Version 1: Preprint. 2020 May 29
- Yang J, Li H, Hu S, Zhou Y. ACE2 correlated with immune infiltration serves as a prognostic biomarker in endometrial carcinoma and renal papillary cell carcinoma: implication for COVID-19. Aging (Albany NY). 2020;12(8):6518-6535. DOI: 10.18632/aging.103100
- Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1(2):239-259. DOI: 10.2217/epi.09.33
- Lucas JM, Heinlein C, Kim T, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4(11):1310-1325. DOI: 10.1158/2159-8290.CD-13-1010
- Wang Q, Li W, Liu XS, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27(3):380-392. DOI: 10.1016/j.molcel.2007.05.041
- Shaw GL, Whitaker H, Corcoran M, et al. The Early Effects of Rapid Androgen Deprivation on Human Prostate Cancer. Eur Urol. 2016;70(2):214-218. DOI: 10.1016/j.eururo.2015.10.042
- Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040-1045. DOI: 10.1016/j.annonc.2020.04.479
- https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report
- Burgueno JF, Reich A, Hazime H, et al. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD. Inflamm Bowel Dis. 2020;26(6):797-808. DOI: 10.1093/ibd/izaa085
- Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5(47):eabc3582. DOI: 10.1126/sciimmunol.abc3582
- Wu ZS, Zhang ZQ, Wu S. Focus on the Crosstalk between COVID-19 and Urogenital Systems. J Urol. 2020;204(1):7-8. DOI: 10.1097/JU.0000000000001068
- Vaarala MH, Porvari K, Kyllönen A, et al. The TMPRSS2 gene encoding transmembrane serine protease is overexpressed in a majority of prostate cancer patients: detection of mutated TMPRSS2 form in a case of aggressive disease. Int J Cancer. 2001;94(5):705-710. DOI: 10.1002/ijc.1526
- Bahmad HF, Abou-Kheir W. Crosstalk between COVID-19 and prostate cancer. Prostate Cancer Prostatic Dis. 2020;23(4):561-563. DOI: 10.1038/s41391-020-0262-y
- https://covid19.who.int
- Spitz R, Hero B, Simon T, Berthold F. Loss in chromosome 11q identifies tumors with increased risk for metastatic relapses in localized and 4S neuroblastoma. Clin Cancer Res. 2006; 12(11 Pt 1):3368-3373. DOI: 10.1158/1078-0432.CCR-05-2495
- Xu PP, Tian RH, Luo S, et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10(14):6372-6383. DOI: 10.7150/thno.46833
- Spitz R, Hero B, Westermann F, et al. Fluorescence in situ hybridization analyses of chromosome band 1p36 in neuroblastoma detect two classes of alterations. Genes Chromosomes Cancer. 2002;34(3):299-305. DOI: 10.1002/gcc.10070
- https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview
- Datta PK, Liu F, Fischer T, et al. SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy. Theranostics. 2020;10(16):7448-7464. DOI: 10.7150/thno.48076
- Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681-687. DOI: 10.1038/s41591-020-0868-6
- Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol Sin. 2020;35(3):266-271. DOI: 10.1007/s12250-020-00207-4
- Tian S, Hu W, Niu L, et al. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020;15(5):700-704. DOI: 10.1016/j.jtho.2020.02.010
- Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893-903. DOI: 10.1016/S1470-2045(20)30309-0
- Diao B, Wang C, Tan Y, et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020; 11:827. DOI: 10.3389/fimmu.2020.00827
- McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2(5):662-673. DOI: 10.1002/cam4.106
- Vanni G, Materazzo M, Pellicciaro M, et al. Breast Cancer and COVID-19: The Effect of Fear on Patients' Decision-making Process. In Vivo. 2020;34(3 Suppl):1651-1659. DOI: 10.21873/invivo.11957
- Namendys-Silva SA. Respiratory support for patients with COVID-19 infection. Lancet Respir Med. 2020;8(4):e18. DOI: 10.1016/S2213-2600(20)30110-7
- Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582(7813):469. DOI: 10.1038/d41586-020-01824-5
- Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396-403. DOI: 10.1016/j.ijid.2020.06.099
- Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica. 2020;44:e40. DOI: 10.26633/RPSP.2020.40
- Piechotta V, Chai KL, Valk SJ, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;7(7):CD013600. DOI: 10.1002/14651858.CD013600.pub2
- Michot JM, Albiges L, Chaput N, et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. 2020;31(7):961-964. DOI: 10.1016/j.annonc.2020.03.300
- Wan Y, Shang J, Graham R, et al. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7):e00127-20. DOI: 10.1128/ JVI.00127-20
- Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993,23(2 Suppl 1):82-91. DOI: 10.1016/s0049-0172(10)80012-5
- Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. 2020;12(8):e12476. DOI: 10.15252/emmm.202012476
- Piconi S, Parisotto S, Rizzardini G, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118(12):3263-3272. DOI: 10.1182/blood-2011-01-329060
- Yao TT, Qian JD, Zhu WY, et al. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J Med Virol. 2020;92(6):556-563. DOI: 10.1002/jmv. 25729
- Rivera DR, Peters S, Panagiotou OA, et al. Utilization of COVID-19 Treatments and Clinical Outcomes among Patients with Cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discov. 2020;10(10):1514-1527. DOI: 10.1158/2159-8290.CD-20-0941
- Geleris J, Sun Y, Platt J, et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;382(25):2411-2418. DOI: 10.1056/NEJMoa2012410
- Rosenberg ES, Dufort EM, Udo T, et al. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA. 2020;323(24):2493-2502. DOI: 10.1001/jama.2020.8630
- RECOVERY Collaborative Group, Horby P, Mafham M, et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383(21):2030-2040. DOI: 10.1056/NEJMoa2022926
- https://www.nih.gov/news-events/news-releases/nih-halts-clinical-trial-hydroxychloroquine
- https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine-with-or-without-azithromycin/
- Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355-362. DOI: 10.1038/s41577-020-0331-4
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970-10975. DOI: 10.1073/pnas.2005615117
- Liu J, Wan M, Lyon CJ, Hu TY. Nanomedicine therapies modulating Macrophage Dysfunction: a potential strategy to attenuate Cytokine Storms in severe infections. Theranostics. 2020;10(21):9591-9600. DOI: 10.7150/thno.47982
- Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474-e484. DOI: 10.1016/S2665-9913(20)30173-9
- Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43-49. DOI: 10.1016/j.ejim.2020.05.021
- Magee DJ, Jhanji S, Poulogiannis G, et al. Nonsteroidal anti-inflammatory drugs and pain in cancer patients: a systematic review and reappraisal of the evidence. Br J Anaesth. 2019;123(2):e412-e423. DOI: 10.1016/j. bja.2019.02.028
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860-867. DOI: 10.1038/ nature01322
- Renner A, Marth K, Patocka K, Pohl W. COVID-19 in a severe eosinophilic asthmatic receiving benralizumab -a case study. J Asthma. 2021;58(9):1270-1272. DOI: 10.1080/02770903.2020.1781165
- García-Moguel I, Díaz Campos R, Alonso Charterina S, et al. COVID-19, severe asthma, and biologics. Ann Allergy Asthma Immunol. 2020;125(3):357-359.e1. DOI: 10.1016/j.anai.2020.06.012
- Kaplan SS, Hicks CB. Lopinavir/ritonavir in the treatment of human immunodeficiency virus infection. Expert Opin Pharmacother. 2005;6(9):1573-1585. DOI: 10.1517/14656566.6.9.1573
- Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. DOI: 10.1056/NEJMoa2001282
- Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695-1704. DOI: 10.1016/S0140-6736(20)31042-4
- Group RC, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020: Published on July 17, 2020.
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. DOI: 10.1038/s41368-020-0074-x
- Cook AM, McDonnell AM, Lake RA, Nowak AK. Dexamethasone co-medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo-immunotherapy strategies. Oncoimmunology. 2015;5(3):e1066062. DOI: 10.1080/2162402X.2015.1066062
- Rubinstein SM, Steinharter JA, Warner J, et al. The COVID-19 and Cancer Consortium: A Collaborative Effort to Understand the Effects of COVID-19 on Patients with Cancer. Cancer Cell. 2020;37(6):738-741. DOI: 10.1016/j.ccell.2020.04.018
- Ceschi A, Noseda R, Palin K, Verhamme K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front Pharmacol. 2020; 11:557. DOI: 10.3389/ fphar.2020.00557
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-574. DOI: 10.1016/S0140-6736(20)30251-8
- Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44(6):2063-2071. DOI: 10.1128/JCM.02614-05
- Kumar D, Dey T. Treatment delays in oncology patients during COVID-19 pandemic: A perspective. J Glob Health. 2020;10(1):010367. DOI: 10.7189/jogh.10.010367
- Matsuo T, Kobayashi D, Taki F, et al. Prevalence of Health Care Worker Burnout During the Coronavirus Disease 2019 (COVID-19) Pandemic in Japan. JAMA Netw Open. 2020;3(8):e2017271. DOI: 10.1001/ jamanetworkopen.2020.17271
- Rajkumar RP. COVID-19 and mental health: A review of the existing literature. Asian J Psychiatr. 2020;52:102066. DOI: 10.1016/j.ajp.2020.102066
- Ochoa Arnedo C, Sánchez N, Sumalla EC, Casellas-Grau A. Stress and Growth in Cancer: Mechanisms and Psychotherapeutic Interventions to Facilitate a Constructive Balance. Front Psychol. 2019; 10:177. DOI: 10.3389/fpsyg.2019.00177
- Tsamakis K, Triantafyllis AS, Tsiptsios D, et al. COVID-19 related stress exacerbates common physical and mental pathologies and affects treatment (Review). Exp Ther Med. 2020;20(1):159-162. DOI: 10.3892/ etm.2020.8671
- https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic
- Richards M, Anderson M, Carter P, et al. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020;1(6):565-567. DOI: 10.1038/s43018-020-0074-y
- Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487-497. DOI: 10.1007/s10549-020-05644-z
- Shinder BM, Patel HV, Sterling J, et al. Urologic oncology surgery during COVID-19: a rapid review of current triage guidance documents. Urol Oncol. 2020;38(7):609-614. DOI: 10.1016/j.urolonc.2020.05.017
- Verma R, Foster RE, Horgan K, et al. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. 2016;18(1):10. DOI: 10.1186/s13058-015-0669-x
- https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-treatment-supportive-care
- Payne K. Ethical issues related to pandemic flu planning and response. AACN Adv Crit Care. 2007;18(4):356-360. DOI: 10.1097/01.AACN.0000298627.07535.76
- Adams DP. Wartime bureaucracy and penicillin allocation: the Committee on Chemotherapeutic and Other Agents, 1942-44. J Hist Med Allied Sci. 1989;44(2):196-217. DOI: 10.1093/jhmas/44.2.196
- Rosenbaum L. Facing Covid-19 in Italy - Ethics, Logistics, and Therapeutics on the Epidemic's Front Line. N Engl J Med. 2020;382(20):1873-1875. DOI: 10.1056/NEJMp2005492
- Xie J, Tong Z, Guan X, et al. Clinical Characteristics of Patients Who Died of Coronavirus Disease 2019 in China. JAMA Netw Open. 2020;3(4):e205619. DOI: 10.1001/jamanetworkopen.2020.5619
- Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20(1):1193. DOI: 10.1186/s12889-020-09301-4
- Lesslie M, Parikh JR. Implementing a Multidisciplinary Tumor Board in the Community Practice Setting. Diagnostics (Basel). 2017;7(4):55. DOI: 10.3390/diagnostics7040055
- Wein W. Drug development: successes, problems and pitfalls-the industry perspective. ESMO Open. 2016;1(1):e000033. DOI: 10.1136/esmoopen-2016-000033
- Yella JK, Yaddanapudi S, Wang Y, Jegga AG. Changing Trends in Computational Drug Repositioning. Pharmaceuticals (Basel). 2018;11(2):57. DOI: 10.3390/ph11020057
- Kale VP, Habib H, Chitren R, et al. Old drugs, new uses: Drug repurposing in hematological malignancies. Semin Cancer Biol. 2021;68:242-248. DOI: 10.1016/j.semcancer.2020.03.005
- Zhou Y, Hou Y, Shen J, et al. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. DOI: 10.1038/s41421-020-0153-3
- Fan HH, Wang LQ, Liu WL, et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin Med J (Engl). 2020;133(9):1051-1056. DOI: 10.1097/CM9.0000000000000797
- Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020;19(3):149-150. DOI: 10.1038/d41573-020-00016-0
- Liu X, Liu C, Liu G, et al. COVID-19: Progress in diagnostics, therapy and vaccination. Theranostics. 2020;10(17):7821-7835. DOI: 10.7150/thno.47987
- Du Y, Tu L, Zhu P, et al. Clinical Features of 85 Fatal Cases of COVID-19 from Wuhan. A Retrospective Observational Study. Am J Respir Crit Care Med. 2020;201(11):1372-1379. DOI: 10.1164/rccm.202003-0543OC
- Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, et al. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62-75. DOI: 10.1016/j.cytogfr.2020.06.001
- Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. DOI: 10.3389/fimmu.2020.01446
- Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18(11):3008-3014. DOI: 10.1158/1078-0432.CCR-11-3145
- Lussana F, Rambaldi A. Inflammation and myeloproliferative neoplasms. J Autoimmun. 2017;85:58-63. DOI: 10.1016/j.jaut.2017.06.010
- La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemichyperinflammation. Leukemia. 2020;34(7):1805-1815. DOI: 10.1038/s41375-020-0891-0
- Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5(48):eabd0110. DOI: 10.1126/sciimmunol.abd0110
- https://www.cancer.gov/news-events/cancer-currents-blog/2017/acalabrutinib-fda-mantle-cell-lymphoma
- Lee KG, Xu S, Kang ZH, et al. Bruton's tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc Natl Acad Sci U S A. 2012;109(15):5791-5796. DOI: 10.1073/pnas.1119238109
- Weber ANR, Bittner Z, Liu X, et al. Bruton's Tyrosine Kinase: An Emerging Key Player in Innate Immunity. Front Immunol. 2017;8:1454. DOI: 10.3389/fimmu.2017.01454
- Zhou Q, Chen V, Shannon CP, et al. Interferon-a2b Treatment for COVID-19. Front Immunol. 2020;11:1061. DOI: 10.3389/fimmu.2020.01061
- Yeager CL, Ashmun RA, Williams RK, et al. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357(6377):420-422. DOI: 10.1038/357420a0
- Asmana Ningrum R. Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica (Cairo). 2014;2014:970315. DOI: 10.1155/2014/970315
- Xu P, Huang J, Fan Z, et al. Arbidol/IFN-a2b therapy for patients with corona virus disease 2019: a retrospective multicenter cohort study. Microbes Infect. 2020;22(4-5):200-205. DOI: 10.1016/j.micinf.2020.05.012
- Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382(24):2327-2336. DOI: 10.1056/NEJMoa2007016
- Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized Mouse Xenograft Models: Narrowing the TumorMicroenvironment Gap. Cancer Res. 2016;76(21):6153-6158. DOI: 10.1158/0008-5472.CAN-16-1260
- Jiang Y, Chen D, Cai D, et al. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: A network meta-analysis. J Med Virol. 2021;93(2):1171-1174. DOI: 10.1002/jmv.26443
- Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273-276. DOI: 10.1038/s41586-020-2423-5
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813-1826. DOI: 10.1056/NEJMoa2007764
- Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14(8):455-466. DOI: 10.1038/nrgastro.2017.71
- Hashemian SM, Farhadi T, Velayati AA. A Review on Remdesivir: A Possible Promising Agent for the Treatment of COVID-19. Drug Des Devel Ther. 2020;14:3215-3222. DOI: 10.2147/DDDT.S261154
- Neogi U, Hill KJ, Ambikan AT, et al. Feasibility of Known RNA Polymerase Inhibitors as Anti-SARS-CoV-2 Drugs. Pathogens. 2020;9(5):320. DOI: 10.3390/pathogens9050320
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200928-weekly-epi-update. pdf?sfvrsn=9e354665_6
- Wu A, Peng Y, Huang B, et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe. 2020;27(3):325-328. DOI: 10.1016/j.chom.2020.02.001
- Zhou H, Chen X, Hu T, et al. A Novel Bat Coronavirus Closely Related to SARS-CoV-2 Contains Natural Insertions at the S1/S2 Cleavage Site of the Spike Protein. Curr Biol. 2020;30(11):2196-2203.e3. DOI: 10.1016/j. cub.2020.05.023
- Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088-1089. DOI: 10.1016/j.annonc.2020.04.006
- Docherty AB, Harrison EM, Green CA, et al. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. DOI: 10.1136/bmj.m1985
- Meng Y, Lu W, Guo E, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol. 2020;13(1):75. DOI: 10.1186/s13045-020-00907-0
- Rogado J, Obispo B, Pangua C, et al. Covid-19 transmission, outcome and associated risk factors in cancer patients at the first month of the pandemic in a Spanish hospital in Madrid. Clin Transl Oncol. 2020;22(12):2364-2368. DOI: 10.1007/s12094-020-02381-z
- Gupta S, Hayek SS, Wang W, et al. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436-1447. DOI: 10.1001/jamainternmed.2020.3596
- Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904-913. DOI: 10.1016/S1470-2045(20)30310-7
- Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309-1316. DOI: 10.1016/S1470-2045(20)30442-3
- Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. DOI: 10.1016/ S0140-6736(20)31173-9
- Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020;26(8):1218-1223. DOI: 10.1038/s41591-020-0979-0
- Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134-1143. DOI: 10.1182/blood.2020006965
- Bisogno G, Provenzi M, Zama D, et al. Clinical Characteristics and Outcome of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Italian Pediatric Oncology Patients: A Study From the Infectious Diseases Working Group of the Associazione Italiana di Oncologia e Ematologia Pediatrica. J Pediatric Infect Dis Soc. 2020;9(5):530-534. DOI: 10.1093/jpids/piaa088
- Pinato DJ, Lee AJX, Biello F, et al. Presenting Features and Early Mortality from SARS-CoV-2 Infection in Cancer Patients during the Initial Stage of the COVID-19 Pandemic in Europe. Cancers (Basel). 2020;12(7):1841. DOI: 10.3390/cancers12071841
- Patel VG, Zhong X, Liaw B, et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020;31(10):1419-1420. DOI: 10.1016/j.annonc.2020.06.023
- Flinn IW, O'Brien S, Kahl B, et al. Duvelisib, a novel oral dual inhibitor of PI3K-5,y, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877-887. DOI: 10.1182/blood-2017-05-786566
- Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006;126(10):2178-2189. DOI: 10.1038/sj.jid.5700289
- Gunda V, Pathania AS, Chava S, et al. Amino Acids Regulate Cisplatin Insensitivity in Neuroblastoma. Cancers (Basel). 2020;12(9):2576. DOI: 10.3390/cancers12092576
- Mahapatra S, Challagundla KB. Cancer, Neuroblastoma. StatPearls. Treasure Island (FL). 2020
- Chava S, Reynolds CP, Pathania AS, et al. miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol. 2020;14(1):180-196. DOI: 10.1002/18780261.12588
- Challagundla KB, Wise PM, Neviani P, et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst. 2015;107(7):djv135. DOI: 10.1093/jnci/djv135
- Mudgapalli N, Shaw BP, Chava S, Challagundla KB. The Transcribed-Ultra Conserved Regions: Novel Non-Coding RNA Players in Neuroblastoma Progression. Noncoding RNA. 2019;5(2):39. DOI: 10.3390/ ncrna5020039
- Yan P, Frankhouser D, Murphy M, et al. Genome-wide methylation profiling in decitabine-treatedpatients with acute myeloid leukemia. Blood. 2012;120(12):2466-2474. DOI: 10.1182/blood-2012-05-429175
- Abraham SM, Lawrence T, Kleiman A, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203(8):1883-1889. DOI: 10.1084/ jem.20060336
- Montecucco A, Zanetta F, Biamonti G. Molecular mechanisms of etoposide. EXCLI J. 2015;14:95-108. DOI: 10.17179/excli2015-561
- Iqbal N, Iqbal N. Imatinib: a breakthrough of targeted therapy in cancer. Chemother Res Pract. 2014;2014:357027. DOI: 10.1155/2014/357027
- Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5(6):e1163462. DOI: 10.1080/2162402X.2016.1163462
- Fink EC, Ebert BL. The novel mechanism of lenalidomide activity. Blood. 2015;126(21):2366-2369. DOI: 10.1182/blood-2015-07-567958
- Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. DOI: 10.1186/1756-8722-2-36
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107(2):135-142. DOI: 10.1172/JCI11914
- Fritsch M, Jordan VC. Long-term Tamoxifen Therapy for the Treatment of Breast Cancer. Cancer Control. 1994;1(4):356-366.
- Tam CS. Zanubrutinib: a novel BTK inhibitor in chronic lymphocytic leukemia and non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 2019;17(1):32-34.
- Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(3):168-173.
- Dellis AE, Papatsoris AG. Perspectives on the current and emerging chemical androgen receptor antagonists for the treatment of prostate cancer. Expert Opin Pharmacother. 2019;20(2):163-172. DOI: 10.1080/1465 6566.2018.1548611
- Miller J, Tarter TH. Combination therapy with dutasteride and tamsulosin for the treatment of symptomatic enlarged prostate. Clin Interv Aging. 2009;4:251-258. DOI: 10.2147/cia.s4102
- Saha A, Sharma AR, Bhattacharya M, et al. Probable Molecular Mechanism of Remdesivir for the Treatment of COVID-19: Need to Know More. Arch Med Res. 2020;51(6):585-586. DOI: 10.1016/j.arcmed.2020.05.001


