Curcumin as an anti-proliferative agent in breast cancer through RASSF1A, BAX, and CASPASE-3 protein
Автор: Rahmah N.A., Harliansyah H., Suyatna F.D., Kanoko M., Rustamadji P., Prihartono J., Bustami A., Haryono S.J., Hernowo B.S.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 6 т.21, 2022 года.
Бесплатный доступ
Background. Curcumin is a polyphenol that has pharmacological activity that can inhibit tumor cell growth and induce apoptosis through various mechanisms. However, the specific mechanism of curcumin cytotoxicity remains controversial because of many anti- and pro-apoptotic signaling pathways in various cell types. This study aims to examine the relationship among curcumin on RASSF1A, Bax protein levels, and caspase-3 activity in supporting the apoptotic mechanism in CSA03 breast cancer cells. Material and Methods. Curcumin administration to cancer cells is based on differences in dosage with 24-hour incubation. Cytotoxicity after curcumin administration was determined using MTS. RASSF1A and Bax protein levels were tested through ELiSA. Caspase-3 activity was used to determine apoptosis and was tested using flow cytometry. Results. The results indicated that curcumin had a cytotoxicity effect of 40.85 |jg/mL. At concentrations of 40 |jg/mL and 50 jg/mL, curcumin increases levels of protein RASSF1A (A = 26.53% and 47.35%, respectively), Bax (A = 48.79% and 386.15%), and caspase-3 (A = 1,678.51% and 1,871.889%) significantly. Conclusions. Curcumin exhibits anti-proliferative activity and apoptotic (Caspase-3) effects through activation of RASSF1A and Bax.
Bax, caspase-3, curcumin, csa03, rassf1a
Короткий адрес: https://sciup.org/140296698
IDR: 140296698 | УДК: 618.19-006.6:577.112 | DOI: 10.21294/1814-4861-2022-21-6-91-98
Текст научной статьи Curcumin as an anti-proliferative agent in breast cancer through RASSF1A, BAX, and CASPASE-3 protein
The frequency of breast cancer tends to increase. In 2020, there were 2.3 million women diagnosed with breast cancer and 685 000 deaths globally. As of the end of 2020, there were 7.8 million women alive diagnosed with breast cancer in the past 5 years, making it the world’s most prevalent cancer [1]. In Indonesia, breast cancer in 2010 ranked first in malignancy, accounting for 18.58 % of cancer cases, most often at the age of 35–54 years [2]. Recently, resistance to hormone therapy or chemotherapy has been reported since the beginning or during the treatment. Many strategies for selecting the therapy based on molecular mechanisms are currently being implemented to overcome the resistance in the breast cancer treatment [3].
One of the molecular mechanisms of breast cancer is epigenetic changes in the form of hypermethylation [4]. The tumor suppressor gene in breast cancer that often undergoes hypermethylation is the RASSF1A gene that plays a role in cell cycle processes, mitosis, and apoptosis [5]. RASSF1A can regulate the activity of the Bax protein to release cytochrome-c so that it activates caspase-3, which is the executor and is responsible for apoptosis [6]. Hypermethylation is reversible, and it is possible to re-express hypermethylated genes in cancer with demethylation drugs, so that the tumor suppressor genes that were previously inactive can be regulated and their function be improved. The administration of demethylation drugs in the tumor cell lines causes the reactivation of the tumor suppressor function, but demethylation drugs have side effects that limit the dose and duration of the treatment and have the potential to form mutagenic lesions [4, 7, 8]. Therefore, the use of new compounds from natural substances that can affect epigenetic changes and have low toxicity to inhibit the development of cancer cells is an important field of cancer treatment research.
One of the natural compounds that have an epigenome target is curcumin. Curcumin is the polyphenol that can efficiently induce apoptosis [9, 10]. However, the specific mechanism of curcumin cytotoxicity via the RASSF1A pathway is still controversial in various cell types [11]. One study demonstrated that curcumin increased Bax expression via the P53 pathway.
Selecting the right cell line is vital to getting reliable research results, particularly for breast cancer cell lines. Breast cancer cells are heterogeneous, therefore, they are classified based on the expressed genes on the cancer cells luminal A breast cancer, luminal B-like breast cancer, luminal B breast cancer, HER2-enriched breast cancer, and triple-negative or basal-like breast cancer. Furthermore, the mutated gene controls the cell behaviour, including cell response to cancer drugs and treatment [12]. The previous study demonstrated that the administration of curcumin treatment to MCF-7 and MDA-MB-468 cells efficiently enhances the suppression of the growth of the breast cancer cells in vitro through reactivation of RASSF1A, upregulation of Bax, and induction of apoptosis [13]. Experimental research was carried out on the luminal A breast cancer cells from Indonesian patients, in particular, using CSA03 cells. The CSA03 cells were isolated from breast cancer tissue of a 56-year-old Indonesian woman with a clinical diagnosis of left invasive ductal mammary carcinoma T2N0M0. On immunohistochemical examination based on secondary data from the Department of Anatomic Pathology, Dr. Cipto Mangunkusumo Jakarta obtained ER-positive results (5 %, weak, Allred score 3), 83 PR-positive (20 %, moderate, Allred score 5), 83 HER2-negative, and Ki67 positive (20 %, weak). Based on the results of the IHC examination, CSA03 cells were included in the luminal A subtype of breast cancer.
The present study was aimed at investigating the cytotoxic effect and apoptosis-induced effects of curcumin via the RASSF1A pathway CSA03 cell line in vitro . The results of this study can be used as the basis of literature for further research.
Material and MethodsMaterial
The CSA03 breast cancer cells were obtained from the integrated laboratory of the Faculty of Medicine, University of Indonesia which was operated on at Dr. Cipto Mangunkusumo Jakarta. Dubelco’s minimum essential medium (DMEM) (Gibco, New York, USA) was supplemented with 10 % fetal bovine serum (Biowest, Riverside, USA), 5 % amphotericin, and 5 % penicillin-streptomycin (Biosciences, San Jose, USA). Curcumin was purchased from Sigma Chemical Co. (St. Louis, MO, USA). The MTS kit was obtained from Promega (Madison, USA). The RASSF1A kit was obtained from MyBioSource, and the Bax kit was obtained from Abcam. The caspase-3 level was determined using PE-conjugated the monoclonal active the caspase-3 antibody apoptosis kit (BD PharMingen, Heidelberg, Germany).
Morphology of Cells
The cell morphology examination was carried out on a paraffin block of the cancer patient using hematoxylin-eosin staining.
The Cell Culture
The CSA03 cells were cultured in a Tissue Flask containing complete culture media. After the cells were harvested, the cells were counted using a hemocytometer. There were 1 × 106 cells/well, and curcumin that had been dissolved in DMSO was added. After the harvesting of CSA03 cells, cell blocks were made, and hematoxylin-eosin staining was carried out to see morphological features of the cells.
The Cytotoxicity Examination
The cytotoxicity examination used the colorimetric principle with the MTS assay. As many as 5 × 103 cells of CSA03 per well were plated in 96-well plates and treated with curcumin at concentrations of 20, 30, 40, 60, 80 μg/mL. 0.1 % DMSO was used as a control. It was then incubated for 24 hours. After adding 20 mg/mL 3-(4, 5 dimethylthiazol-2-yl)-5-(3-carboximetoniphenol)-2-(4-sulphodenil)-2-H-tetrazo-lium) (MTS) to each well, it was incubated again for 2 hours at temperature 37 °C. Furthermore, the optical density was determined using a microplate reader that was set at 490 nm. IC50 was calculated based on
Δ Control uptake - Δ Sample uptake
% inhibition = --------------------------------------------× 100 %. Eq (1)
Δ Control uptake
Furthermore, the data from the cytotoxicity examination were used as the basis for determining the concentration of curcumin to be exposed to breast cell cultures. The determination of the curcumin concentration was carried out around the IC50 value.
Caspase-3 Examination
Caspase-3 examination was carried out using the immunofluorescence technique (apoptosis Kit Catalog No. 550914 from BD PharMingen). The cells that had been treated were then given the steps on the datasheet and put into the flow chamber. The electronic system converted the detected light signals into electronic signals that could be processed by a computer. Cells that did not indicate an autofluorescent pattern were grouped, compared against the whole cell, and their proportion was then determined as a percentage.
RASSF1A and the Bax Protein Assay
RASSF1A and Bax protein examination was conducted using the ELISA technique (RASSF1 ELISA Kit Catalog No. MBS2706790 from MyBioSource and Bax Catalog No. ab133048 from Abcam). The results of RASSF1A and Bax protein levels obtained from the ELISA reader were optical density (OD) values converted into RASSF1A protein levels (ng/mL) and Bax protein levels (pg/mL) using standards.
Data Analysis
Data were processed statistically using the Statistical Program for Social Science (SPSS) version 20.0 software for Windows. Hypothesis testing was performed using a non-parametric numerical comparative hypothesis test flow. To assess the difference between >2 groups of unpaired concentrations, the Kruskal– Wallis non-parametric test was used, and to assess the significant difference in the two groups, a Mann– Whitney posthoc analysis was made. The correlation test between the two variables was conducted using the Spearman non-parametric test. All experiments were performed in triplicate (n = 3, where n: replicate). The average growth rate (delta) was calculated using the following formula:
Data after exposure - Data without exposure
Growth = ------------------------------------------------------- × 100 % Eq (2)
rate (Delta) Data without exposure
Results
Cell Morphology
The morphological picture of CSA03 cells consists of cells with pleomorphic nuclei, fine or coarse granular chromatin, partially irregularly shaped nucleolus, a small vacuole, and eosinophilic cytoplasm (Fig. 1). Fig. 2 illustrates the CSA03 cells in Tissue Flask that are ready to be harvested. The results of hematoxylin-eosin staining on CSA03 cells that had been made block cells indicate the same nuclei image as the nu-
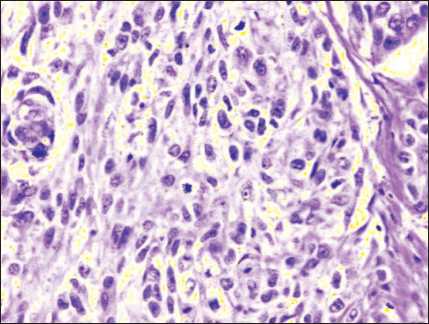
Fig. 1. The morphological feature of CSA03. Hematoxylin-eosin staining, × 400
Рис. 1. Морфологический признак CSA03. Окраска гематоксилином и эозином, ×400
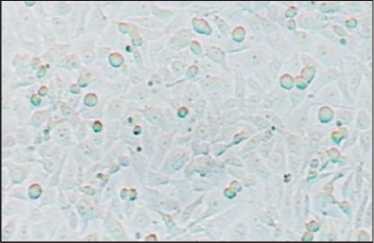
Fig. 2. Microscopic feature of CSA03 Cells in the Tissue Flask, ×400
Рис. 2. Микроскопия клеток CSA03 в колбе для тканей, ×400
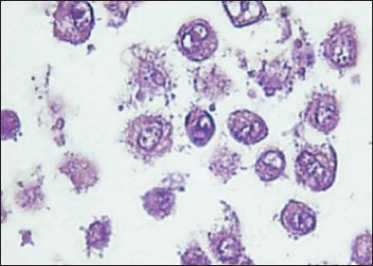
Fig. 3. Microscopic feature of CSA03 made of cell blocks. Hematoxylin-eosin staining, ×1,000
Рис. 3. Микроскопические характеристики CSA03, сделанного из клеточных блоков. Окраска гематоксилином и эозином, ×1000
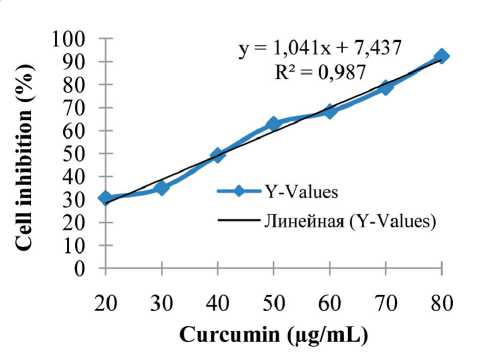
Fig. 4. Cell inhibition of curcumin on CSA03 cells (n=3) Рис. 4. Ингибирование куркумина клетками CSA03 (n=3)
clei image on the paraffin block; the cell morphology examination was carried out on the paraffin block of the cancer patient using hematoxylin-eosin staining (Fig. 3).
Cytotoxicity Examination
In this study, the curcumin concentrations used were 0 µg/mL (without exposure), 40 µg/mL, and 50 µg/mL. The IC50 of curcumin on CSA03 cells was 40.85 µg/mL (Fig. 4). These results indicated that curcumin has a potent anti-proliferative effect on breast cancer cells.
Curcumin Upregulated the ProteinExpression of RASSF1A on CSA03 cells
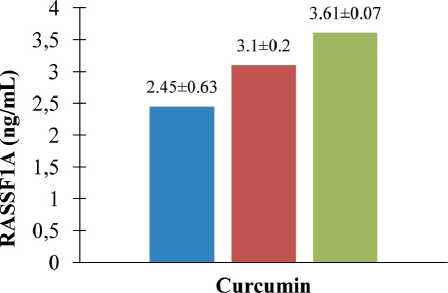
To investigate whether suppression of proliferation of breast cancer cells by curcumin is due to the downregulation of molecules involved in cell proliferation, we tested the protein levels of RASSF1A in cells. As illustrated in Figure 5, at the previously demonstrated pharmacological effective (anti-proliferation and apoptosis) concentrations, curcumin could significantly increase protein levels of RASSF1A on CSA03 cells. We found that curcumin treatment increased the protein expression of RASSF1A in a dose-dependent manner. At concentrations of 40 µg/mL and 50 µg/ mL, curcumin increased levels of protein RASSF1A (∆ = 26.53 % and 47.35 %, respectively).
Curcumin Upregulated the Protein of Bax on CSA03 cells
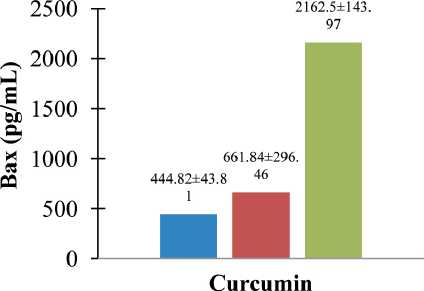
We further investigated mechanisms underlying curcumin-induced apoptosis of breast cancer cells. As illustrated in Fig. 6, Bax levels were significantly upregulated by curcumin in both cells in a dosedependent manner. Curcumin treatment increases the protein expression of Bax in a dose-dependent manner. At concentrations of 40 µg/mL and 50 µg/mL, curcumin increases levels of protein Bax (∆ = 48.79 % and 386.15 %, respectively).
■ Without Treatment ■40pg/mL 50 ug/mL
Fig. 5. Curcumin upregulated RASSF1A of protein on CSA03 cells (statistical significance: p<0.05). ELISA assay of curcumin-induced apoptosis of cells for 24 h (RASSF1A protein level ± standard deviation, ng/mL Y-axis); untreated, 40 μg/mL, and 50 μg/mL (dose of curcumin, X-axis). Curcumin upregulated RASSF1A of protein on CSA03 cells in a dosedependent manner (n=3)
Рис. 5. Куркумин активировал белок RASSF1A на клетках CSA03 (статистическая значимость: p<0,05). ELISA-анализ индуцированного куркумином апоптоза клеток в течение 24 ч (уровень белка RASSF1A ± стандартное отклонение, нг/мл, ось Y); необработанный, 40 мкг/мл и 50 мкг/мл (доза куркуми-на, ось X). Куркумин активировал белок RASSF1A на клетках CSA03 дозозависимым образом (n=3)
■ Without Treatment ■ 40 ug/mL ■ 50 iig'm L
Fig. 6. Curcumin upregulated Bax of protein on CSA03 cells (statistical significance: p<0.05). ELISA assay of curcumin-induced apoptosis of cells for 24 h (Bax protein level, pg/mL, Y-axis); untreated, 40 μg/mL, and 50 μg/mL (dose of curcumin, X-axis) (n=3)
Рис. 6. Куркумин активировал белок Bax на клетках CSA03 (статистическая значимость: p<0,05). ELISA-анализ индуцированного куркумином апоптоза клеток в течение 24 ч (уровень белка Bax, пг/мл, ось Y); необработанные, 40 мкг/мл и 50 мкг/мл (доза куркумина, ось X) (n=3)
Curcumin Induces Apoptosis (Caspase-3)on CSA03 cells
In this study, the curcumin concentrations used were 0 µg/mL (without exposure), a low concentration below the IC50 (40 µg/mL), and a high concentration above the IC50 (50 µg/mL). The cells were treated of curcumin for 24 h. Our results indicated that curcumin in a dose-dependent manner induced apoptosis. As illustrated in Figure 7, the percentage of caspase-3 with a curcumin concentration of 40 μg/mL increased to 1678.51 % in CSA03 cells. Under the treatment with a curcumin concentration of 50 μg/mL, the level of caspase-3 increased to 1871.889 % in CSA03 cells.
Discussion
The effectiveness of curcumin as a chemopreventive agent has been investigated using cell culture. The types of breast cancer cells commonly used for research on anti-proliferative drugs are cancer cells that have similar molecular features to those of human breast cancer cells so that they are relevant for the development of anti-proliferative therapies for these types of subtypes. Therefore, experimental research on CSA03 cells is relevant to the situation in Indonesia, to determine the relationship between curcumin and levels of the RASSF1A protein, Bax protein, and caspase-3 level in supporting the apoptotic mechanism.
In this study, CSA03 cells originating from Indonesia were used. An MTS assay was performed to investigate the cytotoxicity effect of curcumin on CSA03 cells. The MTS assay in this study indicated that the IC50 of curcumin on CSA03 cells was 40.85 µg/mL. This result was lower than that on MCF-7 breast cancer cells (75.73 µg/mL), which had the same molecular picture as CSA03 cells [12]. This indicates that CSA03 cells are quite sensitive. At low concentrations, curcumin was able to inhibit growth by 50 % in CSA03 cells.
In some Southeast Asian countries, including Indonesia, turmeric is often used as a cooking spice, so that lower concentrations have shown a cytotoxicity level of 50 %. Frequent use of turmeric may interfere with the antitumor activity of curcumin. In the study of Hafner et al. on growth rates, it was reported that modification of genes or the microenvironment often results in changes in cell division [14, 15]. One of the cytotoxicity mechanisms of curcumin is the oxidative stress pathway. Curcumin, in high doses, can activate mitochondrial enzymes that lead to the production of ROS. The induction of ROS by curcumin can occur through its interaction with thioredoxin reductase, thereby altering the activity of NADPH oxidase, which in turn can lead to the production of ROS. Several studies have demonstrated that curcumin can induce ROS [16, 17].
In the study of Lv et al. [18], it was reported that curcumin administration would increase Bax on MCF-7 cells in a concentration-dependent manner. In that study, curcumin was only given at one time, 48
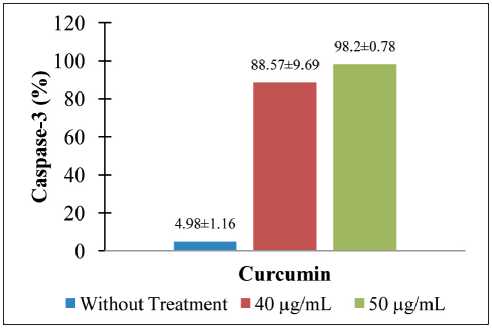
Fig. 7. Curcumin upregulated Caspase-3 on CSA03 cells (statistical significance: p<0.05) (n=3)
Рис. 7. Куркумин активировал Caspase-3 на клетках CSA03 (статистическая значимость: p<0,05) (n=3)
hours, so it is not known whether the effect of curcumin is time-course. However, the results of this study prove that the effect of curcumin as pro-apoptotic breast cancer cells is concentration-dependent. Furthermore, based on the results of the immunohistochemical examination, CSA03 cells have a molecular picture of luminal subtype A. Based on the secondary data results of the immunohistochemical examination, CSA03 cells have a molecular picture of luminal subtype A. CSA03 cells have low ER-positive, HER2-negative, low Ki67-positive so that the receptors on the cell surface for cancer growth and cell proliferation are also low, but cancer growth occurs with sufficiently large mass (>5 cm). In these circumstances, it should be suspected that a series of mechanisms occur and involve many genes in cells for cancer growth.
In cancer, there is a susceptibility to genetic variant loci that influence the regulation of gene expression, including variants in genes involved in DNA repair, cell cycle control, apoptosis, tumor suppressor ubiq-uitination, and mitotic kinases. The DNA methylation factor is also suspected to be one of causes of tumor growth. DNA methylation is one of the most common epigenetic modifications. These modifications do not alter the main sequence of DNA but are critical factors for normal development, gene expression patterns, and genomic stability. The RASSF1A gene is epigenetically inactivated in various solid tumors. In several studies reported, RASSF1A hypermethylation was detected in 48–70 % of breast cancer patients [19, 20].
Список литературы Curcumin as an anti-proliferative agent in breast cancer through RASSF1A, BAX, and CASPASE-3 protein
- World Health Organization. Breast cancer-WHO [Internet]. URL: https://www.who.int/news-room/fact-sheets/detail/breast-cancer [cited 2022 Jul 22].
- Kanker di Indonesia tahun 2010: Data Histopatologik. Badan Registrasi Kanker Perhimpunan Dokter Spesialis Patologi Indonesia dan Yayasan Kanker Indonesia.
- Abdulkareem I.H., Zurmi I.B. Review of hormonal treatment of breast cancer. Niger J Clin Pract. 2012; 15(1): 9-14. https://doi.org/10.4103/1119- 3077.94088.
- Thuy L.H.A., Thuan L.D., Phuong T.K. DNA hypermethylation in breast cancer. In: Pham PV, ed. (2017) Breast Cancer - From Biology to Medicine. London: InTech. 147-61. https://doi.org/10.5772/66900.
- Cao X., Tang Q., Holland-Letz T., Gündert M., Cuk K., Schott S., Heil J., Golatta M., Sohn C., Schneeweiss A., Burwinkel B. Evaluation of Promoter Methylation of RASSF1A and ATM in Peripheral Blood of Breast Cancer Patients and Healthy Control Individuals. Int J Mol Sci. 2018; 19(3): 900. https://doi.org/10.3390/ijms19030900.
- Law J., Salla M., Zare A., Wong Y., Luong L., Volodko N., Svystun O., Flood K., Lim J., Sung M., Dyck J.R., Tan C.T., Su Y.C., Yu V.C., Mackey J., Baksh S. Modulator of apoptosis 1 (MOAP-1) is a tumor suppressor protein linked to the RASSF1A protein. J Biol Chem. 2015; 290(40): 24100-18. https://doi.org/10.1074/jbc.M115.648345.
- Howell P.M., Liu Z., Khong H.T. Demethylating Agents in the Treatment of Cancer. Pharmaceuticals (Basel). 2010; 3(7): 2022-44. https://doi.org/10.3390/ph3072022.
- Foulks J.M., Parnell K.M., Nix R.N., Chau S., Swierczek K., Saunders M., Wright K., Hendrickson T.F., Ho K.K., McCullar M.V., Kanner S.B. Epigenetic drug discovery: targeting DNA methyltransferases. J Biomol Screen. 2012; 17(1): 2-17. https://doi.org/10.1177/1087057111421212.
- Goel A., Aggarwal B.B. Curcumin, the golden spice from Indian safron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010; 62(7): 919-30. https://doi.org/10.1080/01635581.2010.509835.
- Basnet P., Skalko-Basnet N. Curcumin: an anti-infammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011; 16(6): 4567-98. https://doi.org/10.3390/molecules16064567.
- Khazaei Koohpar Z., Entezari M., Movafagh A., Hashemi M. Anticancer Activity of Curcumin on Human Breast Adenocarcinoma: Role of Mcl-1 Gene. Iran J Cancer Prev. 2015; 8(3). https://doi.org/10.17795/ijcp2331.
- Dai X., Cheng H., Bai Z., Li J. Breast Cancer Cell Line Classifcation and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017; 8(16): 3131-41. https://doi.org/10.7150/jca.18457.
- Rahmah N.A., Harliansyah H., Suyatna F.D., Kanoko M., Rustamadji P., Prihartono J., Haryono S.J., Hernowo B.S. The role of curcumin on apoptosis through the RASSF1A and Bax pathways in breast cancer. Indonesian Journal of Cancer Chemoprevention. 2020; 11(2): 67-74. https://doi.org/10.14499/indonesianjcanchemoprev11iss2pp67-74.
- Hafner M., Niepel M., Chung M., Sorger P.K. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat Methods. 2016; 13(6): 521-7. https://doi.org/10.1038/nmeth.3853.
- He Y., Zhu Q., Chen M., Huang Q., Wang W., Li Q., Huang Y., Di W. The changing 50 % inhibitory concentration (IC50) of cisplatin: a pilot study on the artifacts of the MTT assay and the precise measurement of density-dependent chemoresistance in ovarian cancer. Oncotarget. 2016; 7(43): 70803-21. https://doi.org/10.18632/oncotarget.12223.
- Nicco C., Batteux F. ROS Modulator Molecules with Therapeutic Potential in Cancers Treatments. Molecules. 2017; 23(1): 84. https://doi.org/10.3390/molecules23010084.
- Skonieczna M., Hejmo T., Poterala-Hejmo A., Cieslar-Pobuda A., Buldak R.J. NADPH Oxidases: Insights into Selected Functions and Mechanisms of Action in Cancer and Stem Cells. Oxid Med Cell Longev. 2017. https://doi.org/10.1155/2017/9420539.
- Lv Z.D., Liu X.P., Zhao W.J., Dong Q., Li F.N., Wang H.B., Kong B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int J Clin Exp Pathol. 2014; 7(6): 2818-24.
- Zhang Y., Cao H., Yu Z., Peng H.Y., Zhang C.J. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iran J Reprod Med. 2013; 11(5): 415-22.
- Hagrass H.A., Pasha H.F., Shaheen M.A., Abdel Bary E.H., Kassem R. Methylation status and protein expression of RASSF1A in breast cancer patients. Mol Biol Rep. 2014; 41(1): 57-65. https://doi.org/10.1007/s11033-013-2837-3.
- Ung M., Ma X., Johnson K.C., Christensen B.C., Cheng C. Efect of estrogen receptor α binding on functional DNA methylation in breast cancer. Epigenetics. 2014; 9(4): 523-32. https://doi.org/10.4161/epi.27688.
- Cheng Y., Xie N., Jin P., Wang T. DNA methylation and hydroxymethylation in stem cells. Cell Biochem Funct. 2015; 33(4): 161-73. https://doi.org/10.1002/cbf.3101.
- Liu Z., Xie Z., Jones W., Pavlovicz R.E., Liu S., Yu J., Li P.K., Lin J., Fuchs J.R., Marcucci G., Li C., Chan K.K. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009; 19(3): 706-9. https://doi.org/10.1016/j.bmcl.2008.12.041.


