Current and future strategies for managing erectile dysfunction following nerve-sparing robot-assisted radical prostatectomy (literature review)
Автор: Gritskevich A.А., Baitman T.P., Borukaev A.U., Коstin A.А., Ishchenko R.V., Philimonov D.A., Golubitski K.О., Glebova A.E.
Журнал: Сибирский журнал клинической и экспериментальной медицины @cardiotomsk
Рубрика: Обзоры и лекции
Статья в выпуске: 2 т.40, 2025 года.
Бесплатный доступ
Erectile dysfunction (ED) is one of the most frequently developing and significantly reducing quality of life complications of radical prostatectomy (RPE). According to the number of studies, ED is diagnosed in 60-75% of patients after the RPE. The use of nerve-sparing surgical techniques has reduced the incidence of ED after RPE, but has not completely solved this problem. In addition, it is not always possible to preserve the vascular-nerve bundles for the reason of oncological radicalism. All of the above factors make the issue of restoring erectile function one of the priority areas of medical rehabilitation of patients after the radical surgery for prostate cancer (PC). The development of new strategies for the treatment of ED after NS RP, aimed at maintaining endothelial function in the cavernous arteries until the completion of remyelination and restoration of damaged cavernous nerves, is a relevant and actively developing area of modern andrology.
Prostate cancer, erectile dysfunction, nerve-sparing prostatectomy, signaling pathways, endothelial dysfunction
Короткий адрес: https://sciup.org/149148579
IDR: 149148579 | УДК: 616.65-089.87-78-036.86-035(048.8) | DOI: 10.29001/2073-8552-2025-2667
Текст научной статьи Current and future strategies for managing erectile dysfunction following nerve-sparing robot-assisted radical prostatectomy (literature review)
исследование выполнено за счет гранта Российского научного фонда № 24-75-00139,
Грицкевич А.А., Байтман Т.П., Борукаев А.Ю., Костин А.А., Ищенко Р.В., Филимонов Д.А., Голубицкий К.О., Глебова А.Э. Современные и будущие стратегии лечения эректильной дисфункции после нервосберегающей роботизированной радикальной простатэктомии: обзор литературы. Сибирский журнал клинической и экспериментальной медицины. 2025;40(2):21–31.
Prostate cancer ranks as the most frequently diagnosed cancer in men followed by lung cancer and is the second leading cause of death in men globally. [1]. A total of 1,466,680 new cases of prostate cancer were detected worldwide in 2022. [1] On average, one in eight men is diagnosed with prostate cancer during their lifetime. Prostate cancer is most frequently diagnosed in older patients; however there has been an aged shift towards young adults due to conventional and non-conventional risk factors. Moreover, advanced diagnosis and screening programs have contributed greatly to early cancer detection with over 90% of patients being diagnosed in local or regional stages when erectile function may be preserved [2].
The preservation of erectile function is of particular importance for young adults. The patient selection and surgical technique become the major determinants of postoperative erectile function. The treatment of choice depends on the risk group, present comorbidities, life expectancy, the estimated risk of regional lymph node involvement, prostate volume, and the presence of symptoms [3]. Radical prostatectomy is the standard and primary treatment for focal prostate cancer. It is indicated to patients assigned to the very low-risk group with the life expectancy exceeding 10 years, low and intermediate risk groups - with the life expectancy exceeding 10 years, and high and very high risk groups in the presence of symptoms or the life expectancy over 5 years [3].
The first description of the neurovascular bundles surrounding and innervating the penis in the context of sparing surgical treatment with negative risk reduction of erectile dysfunction (ED), which emerged at the end of the 20th century and became a breakthrough, which improved the quality of life of patients with prostate cancer with a low risk of progression [4, 5]. An improved understanding of the surgical anatomy of the prostate and its surrounding structures in the pelvis allowed surgeons to consider the rational plan for surgical dissection based on the balance of oncological radicalism and organ preservation. Maximal cavernous nerves structural preservation is indicated to patients with localized cancer, whereas in case of its greater spread radical surgery should be preferred [3]. The introduction of the nerve-sparing techniques resulted in the improved rate of postoperative recovery of erectile function sufficient for sexual intercourse without any compromise over oncological outcomes [6].
However, preserving erectile function after surgery is challenging, and the causes of erectile dysfunction following surgery requires further understanding [7]. The rate of erectile dysfunction after nerve-sparing prostatectomy varies widely from 20 to 80%. [8].
The use of robotic technology revolutionized the management of prostate cancer providing the operator with advanced instrumentation, enhanced precision, vision, and the full 360 degree range of motion. Robot-assisted radical prostatectomy (RARP) is associated with minimal blood loss, less postoperative pain, shorter in-hospital stay, fast rehabilitation and superior quality of life. Detailed visualization of the prostate with cavernous nerves and its surrounding structures in the deep pelvis allow the surgeon to assess the oncologic process more accurately and preserve the cavernous nerves with complete excision of all tumor cells. However, it remains challenging to entirely prevent neuropraxia resulting from both direct and indirect injury to the neurovascular bundles during the procedure [9]. Mandel et al. found the recovery rate of erectile function less than 50% in patients suffered from neuropraxia 3 months following nerve-sparing robot-assisted radical prostatectomy (NS RARP). This group of patients may require over 24 months recovering erectile function [10]. Noldus et al. reported almost 50% of the patients who had recovery of erections sufficient for sexual intercourse without use of sexual aids [11]. Another study conducted by de Carvalho et al. had similar results one month after surgery with the further increase in the number of patients with recovered erectile function up to 86.7% [12]. Haese et al. reported 86.3% patients with recovered erections following NS RARP at 12 months after surgery among 10,790 patients [13]. Thus, 12-month potency rates after NS RARP varies from 66% to 88% at high-volume referral centers. Despite promising results, a large proportion of patients still require medical or surgical interventions to achieve erections after NS RARP [13-15].
There is currently no consensus on the most effective penile rehabilitation protocol and the optimal timing for interventions to achieve the best outcomes.
This review highlights the relevant molecular mechanisms provoking erectile dysfunction following NS RARP and is aimed at summarizing the current and future strategies for managing erectile dysfunction following NS RARP. The reported results are of particular importance for further studies on developing the optimal penile rehabilitation protocol for improving erectile function after NS RARP.
Research methodology
To achieve the objective of the review, a search and analysis of literature reviews and original articles published between 2020 and 2025 were conducted in the PubMed, Cochrane Library, and eLibrary databases. Several papers published before 2020 were also included in the review, as they contain valuable information relevant to the topic under review. The search was conducted using the following keywords: prostate cancer, erectile dysfunction, nervesparing prostatectomy, signaling pathways, endothelial dysfunction. A total of 109 sources were analyzed.
Molecular mechanisms of erection
Under physiological conditions, sexual stimulation of the cavernous nerve releases nitric oxide (NO), which stimulates an increase in the flow of oxygenated blood to the erectile tissue by relaxing arterial and arteriolar smooth muscle fibers. The classic mechanism for penile erection is activation of the NO/cGMP pathway. Under the action of neuronal nitric oxide synthase (nNO-synthase) and endothelial nitric oxide synthase (eNO-synthase), NO is synthesized from L-arginine in non-adrenergic non-cholinergic nerves and endothelial cells. The latter enters the smooth muscle cells of the corpora cavernosa, activates guanylate cyclase (GC), which converts guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP). The latter activates cGMP-dependent protein kinases, which affect ion channels and reduce the concentration of Ca2+ ions in the cytoplasm of smooth muscle cells, which ultimately leads to their relaxation and the onset of an erection [16, 17]. A similar mechanism of action is possessed by some peptides such as VIP and relaxation-inducing prostaglandins (R-PGs), which activate adenylate cyclase (AC), thereby increasing the level of cyclic adenosine monophosphate (cAMP), which in turn acts on protein kinase A (PKA), leading to relaxation of smooth muscle cells [17] (figure 1).
There are also a number of mechanisms that promote detumescence of the erect penis. The corpora cavernosa has a rich sympathetic adrenergic innervation that promotes smooth muscle contraction through the tonic release of norepinephrine. Norepinephrine causes contraction of the smooth muscles of the corpora cavernosa and constriction of the arterial vessels, which leads to a decrease in arterial inflow and, accordingly, collapse of the lacunar spaces, decompression of the subtunical venules and venous outflow from the corpora cavernosa [17, 18].
The main neurotransmitter of sympathetic fibers is norepinephrine. It binds to α-adrenergic receptors of smooth muscles, which triggers the inositol triphosphate signaling pathway. The binding of norepinephrine to the receptor through a cascade of reactions triggers phospholipase C, under the action of which the hydrolysis of the membrane lipid phosphatidylinositol-4,5-bisphosphate (PIF2) occurs with the formation of inositol-1,4,5-triphosphate (IPF3). IP3 binds to specific centers of the Ca2+ channel of the endoplasmic reticulum membrane and the cell membrane, which leads to an increase in the Ca2+ level through its release from intracellular stores (sarcoplasmic reticulum) and its entry from the extracellular environment (opening of L-type membrane calcium channels [17, 18].
Ca2+ ions bind to the protein calmodulin, which functions as an intracellular Ca receptor, to form a calcium-calmodulin complex that activates regular myosin light chain (RLCM) kinase. When active, RLCM kinase phosphorylates myosin endotefial cell/ nerve
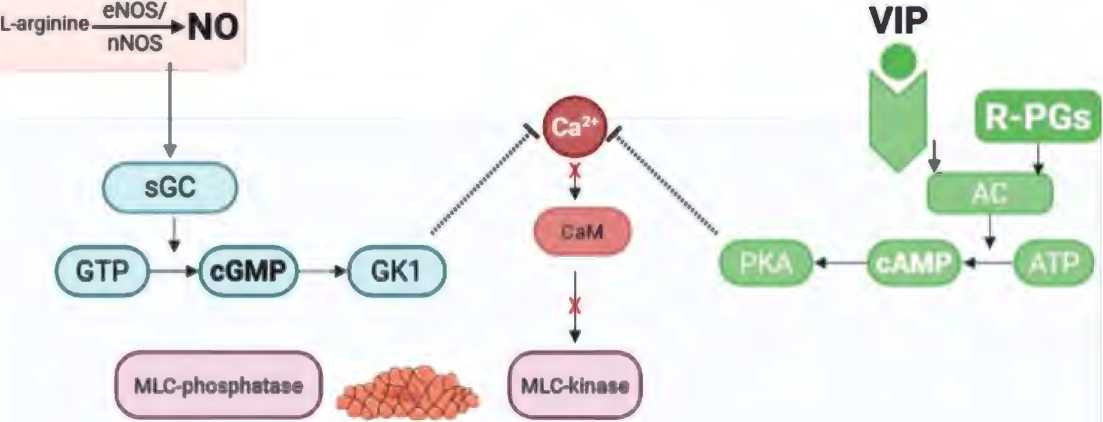
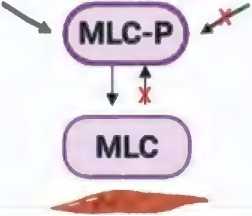
Figure 1. Signaling pathways that mediate relaxation of penile smooth muscle cells: NO/cGMP and VIP/R-PGs/ cAMP
Рисунок 1. Сигнальные пути, обеспечивающие расслабление гладкомышечных клеток полового члена: NO/ цГМФ и VIP/R-PGs/цАМФ
NE

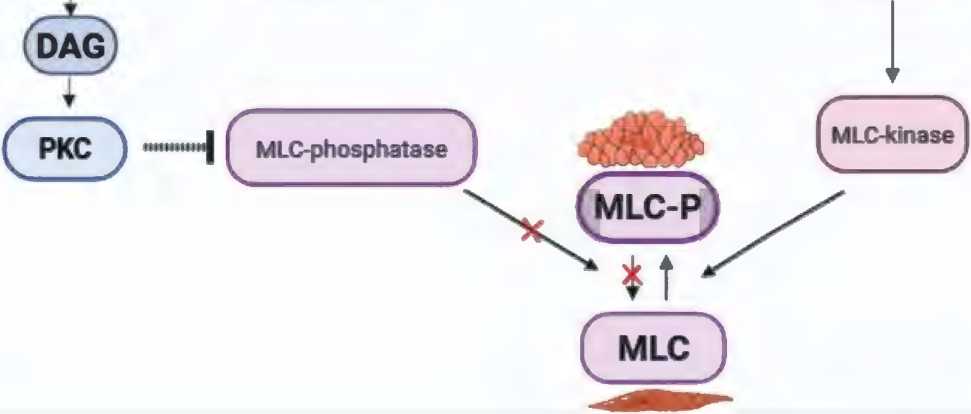
Figure 2. Signaling pathway that mediates contraction of penile smooth muscle cells: PLC/ IP3/ DAG (inositol triphosphate signaling pathway) Рисунок 2. Сигнальный путь, обеспечивающий сокращение гладкомышечных клеток полового члена: PLC/ IP3/ DAG (инозитолтрифосфатный сигнальный путь)
light chains, allowing myosin molecules to form crossbridges and bind to actin filaments, which promotes muscle contraction and vasoconstriction [17, 18] (figure 2).
Activation of sympathetic nerves under conditions of alpha-adrenergic receptor blockade also has an antierectile effect, which suggests the presence of neurotransmitters other than norepinephrine. Thus, sympathetic fibers, with a particularly high density around the spiral arteries, contain neuropeptide Y (NPY). NPY receptors are associated with Gi proteins, and their activation primarily leads to a decrease in the formation of cAMP [19].
Endothelial cells synthesize and locally metabolize the vasoconstrictor factors endothelin-1, prostaglandin-F2a, and angiotensin II, which are involved in maintaining the penis in a flaccid state by stimulating the corresponding receptors and contracting the smooth muscles associated with the G protein located on the cavernous smooth muscle cells. The RhoA/Rho kinase system also plays an important role in maintaining corpora cavernosa smooth muscle tone and penile detumescence [17].
RhoA is a small protein that is a classic activator of Rho kinase (RhoK). It is expressed in cavernous smooth muscle 17 times more than in vascular smooth muscle. Binding of GTP results in activation of RhoA and its translocation to the outer membrane of the cell, where RhoA encounters and activates RhoK. RhoK inhibits RLCM phosphatase by phosphorylating its regulatory subunit MYPT1, and thus RLCMs remain phosphorylated and smooth muscle contraction is maintained with unchanged Ca2+ levels [17] (figure 3).
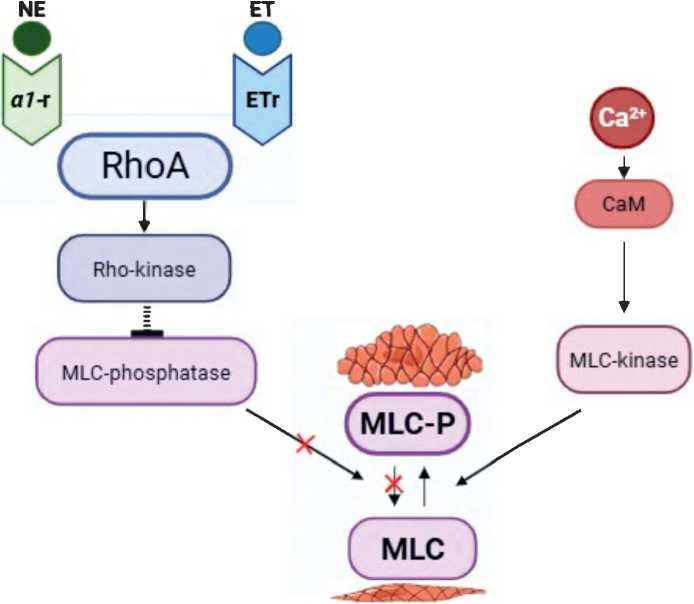
Figure 3. Signaling pathway that mediates contraction of penile smooth muscle cells: RhoA/Rho-kinase system Рисунок 3. Сигнальный путь, обеспечивающий сокращение гладкомышечных клеток полового члена: система RhoA/Rho-киназы
Mechanisms of erectile dysfunction after radical prostatectomy
Neurogenic mechanism
The intraoperative damage of the vascular-nerve bundles is considered the leading pathogenetic mechanism responsible for the development of erectile dysfunction following radical prostatectomy. This damage may be caused by the mechanical trauma during the prostate mobilization, thermal injury during coagulation or provoked by local postoperative inflammatory changes [16]. There are three main types of nerve fiber injury classified by Seddon in 1943 based on the presence of demyelination and the extent of damage to the axons and the connective tissues of the nerve: neuropraxia, axonotmesis, and neurotmesis [20].
Neuropraxia is the mildest type of nerve injury. It is a temporary disruption of conduction without the loss of axonal integrity and perineurium, with minimal degeneration occurring. The defect in the nerve fiber can be remyelinated and repaired by Schwann cells, and the nerve conduction will soon be restored. Nerve fibers are unable to conduct the potentials despite the continuity of the axons. When the axonal integrity is maintained, the ability to conduct nerve impulses recovers over varying periods depending on the severity of demyelination, ranging from several hours to months [20].
Axonotmesis involves the loss of continuity of the axons without the disruption of the structural integrity of the connective tissue fascicular elements. In axonotmesis, damage occurs to the axon and its myelin sheath while partial or complete integrity of the supporting connective tissue framework (endoneurium, perineurium, and epineurium) is preserved, leading to Wallerian degeneration distal to the site of injury. This type of injury is typically observed in cases of nerve compression [20].
Neurotmesis is the most severe type of injury, characterized by disruption of nerve fiber integrity and severe disorganization of the connective tissue framework of the nerve trunk. This type of injury results in persistent functional impairments of the nerve. In neurotmesis, the connective tissue framework is significantly deformed or even lost. The prognosis for spontaneous recovery without surgical intervention is unfavorable [20].
Damage to the cavernous nerve causes a decrease in nNOS density and in NO production, provoking tissue hypoxia, which leads to the development of structural changes in the penile tissue [16].
Vasculogenic mechanism
Endothelial dysfunction, smooth muscle changes, autonomic dysregulation, endocrine and metabolic disorders underlie vasculogenic erectile dysfunction [16].
The primary mechanism contributing to the onset of endothelial dysfunction includes a decrease in the production and bioavailability of NO, accompanied by a simultaneous increase in superoxide ion levels and the production of active vasoconstrictors. Endothelial dysfunction is characterized by imbalanced mediators that are crucial for the optimal functioning of all endothelial-dependent processes. These disturbances include the production, interaction, and degradation of endothelial vasoactive factors, as well as abnormal vascular reactivity and subsequent changes in the structure and growth of blood vessels. Endothelial dysfunction leads to a reduction in the synthesis of antifibrotic compounds (prostaglandin E1 (PGE-1), cyclic adenosine monophosphate (cAMP), vascular endothelial growth factor (VEGF)), and an increase in the activity of fibrogenic factors (transforming growth factor beta 1 (TGF-β1), endothelin-1 (ET-1)) [16, 21, 22].
Apoptosis and fibrosis of smooth muscle cells in the cavernous tissue play a crucial role in the development of erectile dysfunction following radical prostatectomy. These processes gradually lead to impairment of the veno-occlusive mechanism of erection. Musicki et al. have shown apoptosis to affect 15% of smooth muscle resulting in the disruption of the veno-occlusive mechanism of erection [23]. When comparing data from preoperative, early postoperative (<2 months), and late postoperative (>12 months) periods, a decrease in the proportion of elastic fibers and smooth muscle cells in cavernous tissue has been reported along with a significant increase in collagen fiber content [23].
Erectile function recovery: prehabilitation and rehabilitation approaches
Medical prehabilitation
Neuropraxia leads to a reduced oxygen supply in penile tissue, which can subsequently result in apoptosis and fibrosis of smooth muscle cells. Besides, neuropraxia contributes to the progressive fibrosis of the corpora cavernosa and degeneration of smooth muscle following RP. Enhanced oxygenation in the corpora cavernosa may help to mitigate oxidative stress, hypoxia, and fibrosis in smooth muscles. Long-acting phosphodiesterase type 5 inhibitors (PDE5-Is) activate the nitric oxide/cyclic guanosine monophosphate pathway, which may enhance the oxygenation in the corpora cavernosa in case of regular usage. The use of PDE5-Is is currently considered the most effective treatment for penile rehabilitation following NS RARP. Regular administration of PDE5-Is has shown significant efficacy and good tolerability, with minimal serious adverse events [24].
Noh et al. conducted a double-blind, prospective pilot study with a total of 41 patients who underwent NS RARP. They were randomly assigned into 2 groups according to the timing of rehabilitation initiation. Tadalafil was started 2 weeks before NS RARP in the preRARP group (n = 20), and 4 weeks after NS RARP in the postRARP group (n = 21). Twelve months after NS RARP, the IIEF-5 score was significantly higher in patients receiving PDE5-Is before the surgery (15.6 ± 2.1 vs. 12.8 ± 3.5, p < 0.001) [24]. A systematic review of 22 studies including a total of 2,711 patients demonstrated a higher rate of erectile function recovery in the prehabilitation group compared to the control group (56% vs. 24%; р = 0.007) [25]. Similar results were reported by Goh et al. suggesting the superiority of PDE5-I treatment in patients with erectile dysfunction after NSRP [26]. The efficacy of PDE5-Is treatment depends on the degree of preservation of the neurovascular bundles. Erectile function recovered in 75% of patients who underwent bilateral nerve-sparing surgery and received PDE5-Is treatment [27].
Therefore, penile prehabilitation using PDE5-I treatment should be considered as a promising treatment strategy enabling to achieve postoperative recovery of erectile function compared to postoperative rehabilitation.
Intracavernosal injections of platelet-rich plasma
Currently the interest in the regenerative properties of platelets has significantly increased, and the areas of their application continue to expand each year. Platelet-rich plasma (PRP) is a concentrated source of growth factors and cytokines. Its high regenerative effects are attributed to the presence of highly active biological compounds in the alpha granules of platelets, such as platelet-derived growth factors (PDGF-AA, PDGF-AB, PDGF-BB), insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), hepatocyte growth factor (HGF), transforming growth factor beta (TGF-β1 and TGF-β2), vascular endothelial growth factor (VEGF), and others [27].
In vivo models with bilateral cavernous nerve injury have demonstrated that intracavernous injection of platelet-rich plasma led to improved erectile function, promoted cavernous nerve regeneration, and prevented structural changes in the corpus cavernosum due to its anti-inflammatory, reparative, neuroprotective, and neurotrophic effects [28].
Poulios et al. conducted a prospective double-blind randomized placebo-controlled clinical study involving 60 sexually active patients with mild to moderate erectile dysfunction. Patients were randomly assigned to two groups: the study group (n = 30) received intracavernous injections of 10 mL of PRP, while the control group (n = 30) received a placebo. The effect was assessed at 1, 3, and 6 months after completing the treatment protocol using the International Index of Erectile Function-5 (IIEF-5) scale and Sexual Encounter Profile (SEP) diaries. After 6 months, a minimally clinically significant difference was achieved in 20 patients (69%) in the PRP group compared to 7 patients (27%) in the placebo group [29].
Extracorporeal Shock Wave Therapy
Low-intensity shock wave therapy (LiSWT) is based on delivering acoustic wave energy to damaged tissues, stimulating reparative processes within them. This method has been proposed for treating patients with ED following radical prostatectomy [30].
The use of LiSWT in conjunction with PDE-5 inhibitors has proven effective and safe for this category of patients, with the therapeutic effect lasting for an extended period after completing the treatment course [31].
There is a lack of comprehensive data on the use of LiSWT for penile rehabilitation in the treatment of ED following prostate cancer therapy. Current Li-ESWT protocols are not standardized, and existing studies typically involve a small number of participants with short follow-up periods. Further research is necessary to establish optimal Li-ESWT protocols. Ideally, future studies should include longer followup durations to accurately assess the clinical significance of LiSWT in managing post-prostatectomy ED. Additionally, the effectiveness of LiSWT following radiotherapy remains unclear [32].
Combined Therapy: PRP + LiSWT
From 2016 to 2020, a prospective open-label placebo-controlled study was conducted involving 100 patients with ED. The patients were divided into three groups: group 1 (n = 20) received LiSWT on the penis and muscles involved in erection twice a week for six weeks; group 2 (n = 40) received both LiSWT and PRP injections into the penis and muscles involved in erection twice a week for six weeks; group 3 (n = 40) received LiSWT and PRP injections activated with a 10% CaCl2 solution into the penis and muscles involved in erection twice a week for six weeks. The study demonstrated high efficacy of PRP therapy, confirmed by improvements in IIEF-5 scores, SEP results, and pharmacodopplerography of penile vessels [33].
Regulatory peptides
Currently, there are no studies aimed at evaluating the effectiveness and safety of regulatory peptides for preventing erectile dysfunction following traumatic injury to cavernous nerves. The pioneers of peptide therapy are domestic scientists V.Kh. Khavinson and V.G. Morozov. In 1983, an article by V.G. Morozov and V.Kh. Khavinson titled “A New Class of Biological Regulators of Multicellular Systems – Cytomedians” appeared in the journal “Successes in Modern Biology”. The researchers found that low-molecular-weight peptide substances are formed within cells that transfer specific information between cells through amino acid sequences and conformational modifications, thereby regulating proliferation, differentiation, and intercellular interactions. These substances were isolated from various tissues and named peptide bioregulators or cytomedians [33].
Cytomedins belong to the mediatory link of the bioregulation system and participate in intergenic interactions at the level of specialized cell populations; they can exert normalizing effects on tissues from which they are derived as well as replace or complement biologically active compounds secreted by that morphological structure. It should be noted that similar to other peptide regulators, cytomedins penetrate inside cells, interact with the genome, and regulate its functional activity [34].
Studies on laboratory animals have demonstrated that regulatory peptides improve lipid profile parameters in blood by normalizing the atherogenic index through increased HDL levels while reducing other cholesterol fractions; they prevent thrombosis and atherosclerosis development; lower blood pressure by inhibiting renin-angiotensin-aldosterone system activity; promote resorption of inflammatory infiltrates; and enhance tissue regeneration [35].
In an in vitro study on human and mouse endothelial cell lines, it was shown that regulatory peptides stimulate endothelial cell migration, increase cell activity in forming new vascular structures, exhibit pronounced anti-apoptotic and protective effects. Furthermore, regulatory peptides demonstrate regenerative activity that allows normalization of the cell cycle while reducing the proportion of cells in resting phase under cytotoxic factors' influence [36].
Given their biological effects, bioregulatory peptides targeting vascular walls may become a promising method for treating ED after radical prostatectomy by acting on key points in ED pathogenesis: restoring endothelial function while preventing smooth muscle apoptosis and fibrosis within cavernous tissue.
Neurotransmitters and neuropeptides
Several neurotransmitters and neuropeptides that promote penile erection have been identified in various regions of the brain. While there are drugs available that enhance the activity of these neurotransmitters and/or neuropeptides by targeting their receptors or through other synaptic mechanisms, very few of them effectively induce penile erection when administered systemically. Currently, the only drugs that target the central nervous system and have been used for treating erectile dysfunction (ED) in men include D2 receptor agonist apomorphine, α2-adrenergic receptor antagonists yohimbine and delequamine, serotonin reuptake inhibitor trazodone, α-melanocyte-stimulating hormone (α-MSH) receptor agonist melanotan II (MT-II) and its carboxylate derivative bremelanotide (PT-141) [17, 38, 39].
Despite the success of preclinical studies, apomorphine, yohimbine, delequamine and trazodone have not demonstrated an effect significantly greater than placebo in clinical trials [17, 38-40].
MT-II and PT-141 have successfully undergone both preclinical and clinical studies for the treatment of ED. These compounds exert their pro-erectile action by acting on central hypothalamic melanocortin (MC) receptors, demonstrating efficacy in both laboratory animals and in men after systemic administration [19]. Melanocortin receptors are associated with adenylate cyclase–cyclic adenosine monophosphate (cAMP) or phosphatidylinositol/Ca2+ signaling pathways [17]. Melanotan II and bremelanotide have been found to induce multiple episodes of penile erection in male volunteers and men with psychogenic impotence [40, 41]. However, further clinical studies of MT-II were cancelled due to the development of severe side effects, the main one being severe nausea, as well as a long waiting period for the onset of effect [42].
PT-141 is administered intranasally using a single-use dosing device. In a phase 1 study in 32 healthy men, PT-141 doses of 10 mg and 20 mg resulted in significantly longer baseline rigidity (≥80%). Doses up to 20 mg were tolerated by participants without adverse effects. In a Phase 2A study, administration of a 20 mg dose of PT-141 also resulted in a significantly longer duration of baseline rigidity (≥80%) compared to placebo, with a mean duration of approximately 24 minutes and a latency to onset of approximately 30 minutes. Most patients with ED tolerated single doses up to 20 mg well, with the most common side effects being hot flashes and nausea [43].
In a study of 342 patients with ED who had failed to respond to sildenafil, participants were randomly assigned to receive either PT-141 (10 mg) as an intranasal spray or placebo between 2 hours and 45 minutes before sexual activity. Efficacy was assessed using IIEF scores, mean intercourse satisfaction domain scores, and weekly coitus episodes. In the bremelanotide group, 51 subjects (34%) reported significantly better results compared with 9% in the placebo group, including improved ability to achieve and maintain an erection sufficient for intercourse and greater satisfaction. Single doses of bremelanotide were safe and well tolerated, with rare adverse effects including hot flushes, somnolence, nausea, vomiting, headache, and low back pain [44].
A separate study demonstrated the effects of coadministration of PT-141 with PDE5-I in 19 men with ED, with confirmed efficacy of sildenafil or vardenafil. The combination of PT-141 (7.5 mg) and sildenafil (25 mg) produced a significantly greater erectile response than sildenafil alone. The combination was safe and well tolerated by patients [45].
Further studies are needed to establish the long-term efficacy and safety of bremelanotide in the treatment of ED [42].
Molecules that act on the smooth muscles
Sonic hedgehog protein
Sonic hedgehog (SHH) protein, a crucial regulator of penile smooth muscle (SM) and its apoptosis, plays a vital role in maintaining normal erectile function. The penile cavernosa is composed primarily of smooth muscle, elastin fibers, and a small amount of collagen. After peripheral nerve injury, there is a reduction in smooth muscle and elastic fibers in the penis, accompanied by an increase in collagen. This irreversible process contributes to the development of erectile dysfunction. In the adult penis, inhibition of SHH signaling leads to a significant 12-fold increase in smooth muscle apoptosis [46].
Research has shown that erectile dysfunction resulting from cavernosal nerve damage can be effectively treated by using a hydrogel combined with SHH protein. To successfully inhibit the apoptosis caused by cavernosal nerve injury, SHH proteins can be delivered to the smooth muscle of the penile corpora cavernosa through peptide amphiphilic (PA) based nanofibers, which have the ability to self-assemble into hydrogels [47].
In bilateral cavernosal nerve crush models using Sprague-Dawley (SD) rats, treatment with SHH peptide amphiphilic (PA) for 6 weeks showed significant regeneration. In the SHH PA group, both myelinated and non-myelinated fibers remained intact compared to the control group, where nonmyelinated fibers exhibited degeneration [47]. Quantification of penile smooth muscle using trichrome staining revealed that SHH-treated rats had 127% more smooth muscle at 4 days post-injury compared to controls (p = 0.0004) [47].
Quantification of the proliferative index in corpora cavernosa tissues using Ki67/DAPI indicated that SHH PA treatment increased the proliferative index by 50% at two days post-injury (p = 0.003), by 38% at four days (p = 0.042), and by 31% at seven days (p = 0.022). Dual immunohistochemical analysis for Ki67/CD31 and Ki67/α-ACTIN demonstrated that proliferation occurred in both endothelial cells and smooth muscle. These findings suggest that SHH PA treatment effectively inhibits apoptosis, preserves smooth muscle in the penis, and promotes its proliferation. Additionally, under regenerative conditions following prostatectomy, other studies have shown that SHH PA treatment can reduce collagen levels, enhance neurite formation, and facilitate regeneration of the cavernosal nerve [48]. Moreover, SHH PA inhibits both caspase 9 and caspase 8 apoptotic pathways, and the suppression of caspase 9 does not lead to a compensatory activation of caspase 8. This suggests that SHH PA holds considerable clinical potential as a preventative therapy for erectile dysfunction by effectively managing both intrinsic and extrinsic apoptotic mechanisms [48].
Myosin phosphatase target subunit 1 (MYPT1)
The primary regulatory unit of myosin light chain phosphatase, MYPT1, plays a role in regulating erectile dysfunction through the G-protein coupled receptor pathway. Additionally, lotusine has been shown to restore the levels of MYPT1 and enhance the function of injured penile smooth muscles. These findings present new therapeutic targets for the future treatment of ED [49].
In the report by C.M. Wang et al., the reduction of MYPT1 not only hindered the relaxation associated with NO/pKG signaling but also increased calcium-sensitized contraction in penile smooth muscle. This indicates that up-regulating MYPT1 could potentially restore the abnormal relaxation and contraction properties, thereby exhibiting therapeutic efficacy. Future studies may also explore the combination of MYPT1 up-regulation therapy with other treatment strategies, including those aimed at reducing calcium levels [50].
Conclusion
At present, a significant number of methods have been proposed for restoring erectile function lost after RP. Reflecting the lack of unified approaches to managing such patients, the dissatisfaction with treatment outcomes among both physicians and their patients, and the ongoing search for an optimal therapeutic strategy. The leading pathogenetic mechanism for the development of ED after RP is considered to be intraoperative damage to the neurovascular bundles due to mechanical trauma during prostate mobilization, thermal effects from coagulation, and the development of local postoperative inflammatory changes. Vascular changes associated with RP contribute to the creation of a hypoxic environment, leading to cavernous fibrosis. Thus, in the formation of a hypoxic environment, progressive endothelial dysfunction of the cavernous arteries develops. Concurrently, remyelination and repair of damaged nerves occur through Schwann cells. The process of nerve recovery after traumatic injuries can take a long time, while endothelial dysfunction of the cavernous arteries continues to progress, causing irreversible changes. The development of new treatment strategies for ED following nerve-sparing prostatectomy aimed at maintaining endothelial function in the cavernous arteries until the completion of remyelination and repair of damaged cavernous nerves is a relevant and actively evolving area in modern urology.


