Decomposition of triphenylbismuth dicrotonate in light in the presence of 2-methyl-2-nitrosopropane
Автор: Gushchin A.V., Kalistratova O.S., Maleeva A.I., Kuropatov V.A.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Химия элементоорганических соединений
Статья в выпуске: 1 т.8, 2016 года.
Бесплатный доступ
Triphenylbismuth dicrotonate Ph 3Bi(O 2CCH=CHCH 3) 2 in benzene solution in the presence of 2-methyl-2-nitrosopropane decomposes in light. The phenyl radicals formed as the result of Ph 3Bi(O 2CCH=CHCH 3) 2 decomposition are confirmed by the spin-trapping method; they can initiate the methylmethacrylate polymerization at room temperature. In the absence of light the decomposition has not been observed.
Electron paramagnetic resonance, triphenylbismuth dicrotonate, 2-methyl-2- nitrosopropane
Короткий адрес: https://sciup.org/147160346
IDR: 147160346 | УДК: 547.1’13 | DOI: 10.14529/chem160108
Текст научной статьи Decomposition of triphenylbismuth dicrotonate in light in the presence of 2-methyl-2-nitrosopropane
Previously, polymethylmethacrylate (PMMA) with addition of various Bi(V) organometallic compounds was obtained with the use of radical polymerization in the presence of initiators [1, 2]. It was established that Bi(V) acrylates accelerated the polymerization reaction. It was suggested that the acceleration was caused by decomposition of organometallic compounds on exposure to diffused light, as the polymerization slowed in the dark. The character of the process was not studied. When various diacyl derivatives of triphenylbismuth were used, no significant change in polymerization rate or molar mass was observed, indicating the predominant role of the Ph 3 Bi(V) fragment. Besides, the previous study of the photo-induced cation polymerization of oxiranes and vinyl monomers was carried out with triaryl(1-pyrenyl)bismuth salts as initiators [3, 4]. When such compounds were irradiated by visible light, the homolysis of Bi–C(pyrene) bond occured with the formation of pyrenyl radical and cation radical of triarylbismuth, which subsequently initiated polymerization. It is also known that organometallic compounds of bismuth(III) can cause the controlled living radical polymerization [5, 6].
In this connection it seems interesting to study decomposition of Bi(V) compounds by the example of triphenylbismuth dicrotonate (TPBDCr) in diffused light by the spin-trapping method, as well as formation of metal-containing PMMA, with the use of this compound in the role of initiator. The structure of TPBDCr and other acyl derivatives of triphenylbismuth has recently been described in the literature [7, 8].
Experimental
Purification of solvents and reagents. Benzene, Et 2 O and THF were dried over anhydrous calcium chloride, then distilled and kept over sodium wire. Chloroform was dried over anhydrous calcium chloride and distilled. Petroleum spirit was used without previous purification. Anhydrous BiCl 3 was purified by sublimation (350 °С, 0.5 torr).
Synthesis of Ph 3 Bi . Triphenylbismuth was synthesized according to conventional procedure [9] from BiCl 3 and PhMgBr with the use of the benzene and THF (1:2) mixture as the solvent.
Synthesis of Ph 3 Bi(O 2 CCH=CHCH 3 ) 2 . The synthesis of triphenylbismuth dicrotonate was carried out according to conventional procedure [10] by the oxidative addition reaction at room temperature in Et 2 O from triphenylbismuth, crotonic acid and tert -butylhydroperoxide (reagent ratio 1:2:1). The product was purified by recrystallization from the medium petroleum spirit – chloroform (4:1). The yield of the purified reaction product equaled 73 %, melting point 153 °С.
Химия элементоорганических соединений
Synthesis of 2-methyl-2-n it ros opropane (M N P ). MNP was synthesized by oxidation of tert butylamine by 20 % a que o us so lu tio n o f hy dr og e n p e r ox ide in the presence of sodium tungstate as a catalyst [11].
Pol y me r iza t ion of M M A i n the pr e se n c e of T PB DCr. Solutions of TPBDCr (1–5 % m/m) were p r e par e d in me thy lme tha cry la te (MMA) without an initiator. The polymerizat io n wa s ca rr ied out i n degassed vacuumed a mpoule s b o th in diffused light and in the dark at temperatur e 19-24 °С.
Phot odec omposi ti on of T PBDC r in th e pr e senc e of M N P. The solutions for investigation of TPB DC r dec omposi t ion in diff us ed light were prepared as follows: in one elbow of H-ampoule for EPR a sa mple of MNP ( 0. 2 mol/L) was placed, while the other elbow was filled by t he benze ne solu ti on o f TPB DC r . T h e a mpoule was de g a ssed, sealed, and after mixing the EPR spectrum wa s r e g is ter e d.
EPR spectra wer e r e g is tere d on the Br uk e r ER 200D-SRC apparatus with the operating frequency 9.5 GHz in degassed tubes.
Results and Discussion
We h a v e inv e s tig a ted t he decomposition of triphenylbismuth dicrotonate Ph 3 Bi(O 2 CCH=CHCH 3 ) 2 in b e nze ne in th e pr ese nce of MNP by EPR method. The choice of the solvent is due to its low a ct ivi t y i n ra di c a l r ea ction s at t he c hose n conditions. The concentrations of TPBDCr and MNP ar e 0.2 mol/L , t he r e a ct ion has bee n c ar ried out in l ig ht i n a s e ale d an d degassed ampoule.
The r e ac t ion has r esul te d i n addition compounds of phenyl radicals forme d by de c omposit ion o f t r i phe ny lbi smuth dicr oto nat e , with th e spin t r a p P hN( O •) B u - t , as well as in di- tert -butylnitroxyl radicals t -BuN(O•)Bu- t for me d by r de c omposition of the spin trap itself, according to the following scheme:
Ph 3 Bi(O 2 CCH=CHCH 3 ) 2 → 2 Ph• + P hBi( O 2 CCHCH=CHCH 3 ) 2
Ph• + t-BuN=O → PhN(O•)Bu-t t-BuN=O → t-Bu• + NO t-Bu• + t-BuN=O → t-BuN(O•)Bu-t
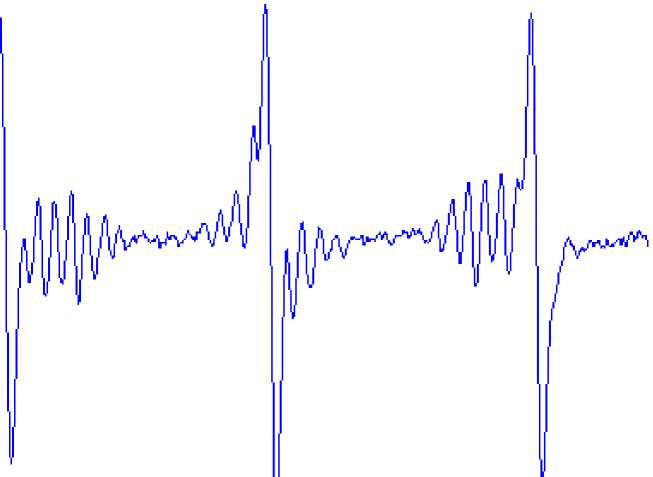
The EP R spe ctrum of t r i ph e ny lbismuth di c r o ton a te in be nze ne (with MNP as the spin trap) is shown in Fig. 1.
I*№6
a.a
-0.2
-0.4
-0.6
I
-з i
3420 3425 3433 3435 3443 3445 3453 3455
-1.0

H_ [Oe]
Fig. 1. The EPR spectrum of triphenylbismuth dicrotonate in benzene, the spin trap is MNP
For the addition compound PhN(O•)Bu- t the values of hyperfine interaction constants have been determined: a N = 12.3 Oe, a Н1 = 1.8 Oe, а Н2 = 0.85 Oe, g = 2.0056, which are in agreement with the literature data for the addition compound generated in benzene by photolysis of Ph 3 Bi (a N = 12.0 Oe, a Н1 = 1.8 Oe, а Н2 = 1.0 Oe), Ph 3 Sb (a N = 11.9 Oe, a Н1 = 1.8 Oe, а Н2 = 0.9 Oe), Ph 3 As (a N = 11.8 Oe, a Н1 = 1.8 Oe, а Н2 = 0.9 Oe) [12], as well as with the data obtained for other sources of phenyl radical in benzene (a N = 12.3 Oe, a Н1 = 2.0 Oe, а Н2 = 0.9 Oe) [13]. For the addition compound t- BuN(O•)Bu- t the values a N = 15.4 Oe, g = 2.0061, which are also in agreement with the literature data for the corresponding addition compound generated in benzene by decomposition of α-isopropylazide (a N = 15.4 Oe, g = 2.0061) [14] and by photolysis of 2-methyl-2-nitrosopropane (a N = 15.4 Oe, g = 2.0057) [15].
Formation of phenyl radicals can also be confirmed by thermodynamic and X-ray diffraction data. It is known that the dissociation energy of the Bi–C(Ph) bond equals 46.2 kcal/mol for triphenylbismuth [16], while the dissociation energy of the Bi–O bond equals 80.6 kcal/mol for bismuth monoxide [17]. The X-ray diffraction analysis points at the bidentate character of acyl ligands binding with a bismuth atom; though the bond length of Bi–O is somewhat greater, the breaking occurs to the Bi–C bonds [5].
The fact of free radical formation was used by us to obtain PMMA. The polymerization of MMA, with various content of dissolved TPBDCr as an initiator, was carried out in diffused light at room temperature. Formation of the solid block was observed in less than 20 h. After storage of the ampoule for 30 days the obtained samples were yellow and opaque, practically insoluble in organic solvents, which indicated additional linking of macromolecules. Low solubility significantly hampered the subsequent analysis of such polymers. It is necessary to emphasize that storage of TPBDCr in methymethacrylate at similar conditions, but in the absence of light, did not lead to polymerization. Solidification of the solution was not observed even in 2 weeks. At present the study is on for production of transparent polymer samples, containing triphenylbismuth diacylates, at similar conditions.
Conclusions
-
1. Decomposition of triphenylbismuth dicrotonate in diffused light in benzene solution in the presence of 2-methyl-2-nitrosopropane leads to formation of phenyl radicals that are registered in the form of the addition compound PhN(O•)Bu- t.
-
2. Polymerization of methylmethacrylate with addition of triphenylbismuth dicrotonate (1–5 % m/m) as a radical initiator is carried out in diffused light at room temperature with formation of yellow-colored opaque polymethylmethacrylate blocks that are insoluble in organic solvents. In the absence of light the polymerization does not occur.
The research was supported by the Russian Foundation for Basic Research (Agreement No. 14-03-31625 mol_a).
Список литературы Decomposition of triphenylbismuth dicrotonate in light in the presence of 2-methyl-2-nitrosopropane
- Диакрилаты трифенилвисмута и трифенилсурьмы в синтезе металлсодержащего полиметилметакрилата/В.А. Додонов, А.В. Гущин, Ю.Л. Кузнецова и др.//Вестник Нижегородского университета им. Н.И. Лобачевского. Сер. «Химия». -2004. -Т. 1, № 4. -С. 86-94.
- Синтез и строение акрилата тетрафенилсурьмы и введение его в полиметилметакрилат/А.В. Гущин, Д.В. Шашкин, Т.С. Щербакова и др.//Вестник Нижегородского университета им. Н.И. Лобачевского. Сер. «Химия». -2010. -Т. 6. -С. 68-72.
- Triaryl(1-pyrenyl)bismuthonium Salts: Efficient Photoinitiators for Cationic Polymerization of Oxiranes and a Vinyl Ether/Y. Matano, T. Shinokura, O. Yoshikava et al.//Org. Lett. -2008. -V. 10, № 11. -P. 2167-2170.
- Matano, Y. Pentavalent Organobismuth Reagents in Organic Synthesis: Alkylation, Alcohol Oxidation and Cationic Photopolymerization/Y. Matano//Top. Curr. Chem. -2012. -V. 311. -P. 19-44.
- Yamago, S. Development of Organotellurium-Mediated and Organostibine-Mediated Living Radical Polymerization Reactions/S. Yamago//J. Polym. Sci. Part A: Polym. Chem. -2006. -V. 44. -P. 1-12.
- Yamago, S. Precision Polymer Synthesis by Degenerative Transfer Controlled/Living Radical Polymerization Using Organotellurium, Organostibine, and Organobismuthine Chain-Transfer Agents/S. Yamago//Chem. Rev. -2009. -V. 109, № 11. -P. 5051-5068.
- Bis(but-2-enoato-кO)triphenylbismuth(V)/P.V. Andreev, N.V. Somov, O.S. Kalistratova et al.//Acta Crystal. Section E. -2013. -V. 69, № 6. -P. m333.
- Шарутин, В.В. Синтез и строение арильных соединений висмута/В.В. Шарутин, И.В. Егорова, О.К. Шарутина//Бутлеровские сообщения. -2004. -Т. 5, № 1. -С. 16-25.
- Кочешков, К.А. Методы элементоорганической химии. Сурьма, висмут/К.А. Кочешков, А.П. Сколдинов, Н.Н. Землянский. -М.: Наука, 1976. -483 с.
- Suzuki, H. Organobismuth Chemistry/H. Suzuki. -Amsterdam-London-New York-Oxford-Paris-Shannon-Tokyo, Elsevier, 2001. -619 p.
- Smith, R.J. Reaction of Di-tert-butylnitroxide with Methyl Trifluoromethanesulfonate. Unexpected Formation of N-Tert-butylhydroxylamine Radical Cation in Trifluoromethanesulfonic Acid/R.J. Smith, R.M. Ragni//J. Org. Chem. -1981. -V. 46, № 21. -P. 4307-4309 DOI: 10.1021/jo00334a048
- Xu, G. Application of Spin Trapping Technique in Photolysis of Compounds Ph3M (M = N, P, As, Sb, Bi)/G. Xu, J. Zhou, Y. Tang//Wuli Huaxue Xuebao. -1985. -V. 1, № 1. -P. 6-11 DOI: 10.3866/PKU.WHXB19850102
- Зубарев, В.Е. Применение спиновых ловушек для исследования механизма радикальных процессов/В.Е. Зубарев, В.Н. Белевский, Л.Т. Бугаенко//Успехи химии. -1979. -Т. XLVIII. -№ 8. -С. 1361-1392 DOI: 10.1070/RC1979v048n08ABEH002407
- Cook, M.D. Spin-trapping of Alpha-azidoalkyl Radicals/M.D. Cook, L.L. Ng, B.P. Roberts//Tetrahedron Lett. -1983. -№. 24. -P. 3761-3764 DOI: 10.1016/S0040-4039(00)94528-3
- Joshi, A. Spin Trapping of Radicals Generated in the UV Photolysis of Alkyl Disulfides/A. Joshi, G. Yang//J. Org. Chem. -1981. -V. 46, № 18. -P. 3736-3738 DOI: 10.1021/jo00331a035
- Freedman, L.D. Preparation, Reactions, and Physical Properties of Organobismuth Compounds/L.D. Freedman, G.O. Doak//Chem. Rev. -1982. -V. 82. -P. 15-57.
- Luo, Y. Comprehensive handbook of chemical bond energies/Y. Luo. -CRS Press, 2007. -1687 p.


