Development of adaptive stereotactic radiotherapy method in treatment of primary malignant glial tumors in the brain
Автор: Vlasov S.G., Yengibaryan M.A., Shikhlyarova A.I., Sakun P.G., Voshedsky V.I., Rodionova O.G., Karnaukhova E.A., Solntseva A.A., Khatyushin V.E., Pandova O.V., Kuznetsova N.S., Kabanov S.N., Teplyakova M.A.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 22, 2022 года.
Бесплатный доступ
At present, there are some scattered evidence data pertaining to the use of an adaptive technique of radiotherapy in treatment of malignant glial tumors of the brain. Our evidence data obtained in MRI in the course of treatment show that the initial treatment plan may become irrelevant due to some changes observed in the tumor configuration. In its turn, it bears witness to the topicality of developing and introducing adaptive methods and techniques in the brain tumor treatment, which are capable to increase efficacy and tolerability in patients with primary malignant tumors of the brain. Aim. Our aim has been to develop an adaptive stereotactic method of radiotherapy in treatment of primary malignant brain tumors, which shall be capable to increase efficacy and tolerability of radiation therapy as well as reduce radiation dose to normal structures in the brain. Materials and methods. Our method has been elaborated with recruiting a group of 10 patients diagnosed with primary glioblastoma G IV, which have received specialized treatment at the National Medical Research Centre for Oncology at the Ministry of Health, the Russian Federation, in the period 2021-2022. The average age of the above patients is 43,4 years. All patients have undergone microsurgery covering the total resection of the tumor (3 patients) and subtotal removal of the malignant tumor (7 patients). The average time interval between the surgery and radiotherapy is 32,5 days. Before treatment, an intravenous contrast enhancement MRI of the brain with an axial pitch of 1 mm has been conducted employing the contrast T1, contrast-free T1 and T2 FLAIR modes. Planning of radiotherapy for this sort of patients has been carried out employing BrainLab Elements и Varian Medical Systems Eclipse. The CTV was defined as a 2,0 cm margin around GTV with an anatomical correction. The CTV-to-PTV margin was 0,1 cm. Doses have been administered as follows: a single dose of 2 Gy up to a total dose of 60 Gy in 30 fractions. The brain has been MRI-scanned in all patients with the use of intravenously introduced contrast agents with an axial pitch of 1 mm employing enhanced contrast/contrast-free T1 sequences and T2 FLAIR to redefine the radiotherapy targets (GTV, CTV, PTV). At fraction 30 we have completed MRI in order to estimate the treatment outcome. In the average, the radiotherapy course has taken 42-45 days, holidays included. For the purpose of the radiation therapy, used have been the Novalis Tx Varian Tx linear accelerator of kinetic energy of the beam of 6 MeV. Results We have developed our own adaptive stereotactic method of radiotherapy to treat the primary malignant glial tumors in the brain, which is capable of tracing the configuration of the post-surgery cavity, the residual tumor and the brain structures in the course of radiotherapy and adapting the therapy plan thereto that makes possible to reduce tissue volumes exposed to radiation due to a decrease in the tumoral and peritumoral volumes of the tumor and post-operative cavity. Conclusion. Our analysis has shown that in the course of radiotherapy some anatomical changes in the tumor configuration are found. An adaptive approach applied to radiation therapy allows monitoring the above changing volumes and correcting the treatment plan.
Malignant glial tumors, adaptive stereotactic radiotherapy
Короткий адрес: https://sciup.org/148324643
IDR: 148324643 | DOI: 10.18137/cardiometry.2022.22.6976
Текст научной статьи Development of adaptive stereotactic radiotherapy method in treatment of primary malignant glial tumors in the brain
Stanislav G. Vlasov, Marina A. Yengibaryan, Alla I. Shikhlyarova, Pavel G. Sakun, Vitaly I. Voshedsky, Olga G. Rodionova, Elena A. Karnaukhova, Anna A. Solntseva, Vladislav E. Khatyushin, Olga V. Pandova, Natalia S. Kuznetsova, Sergey N. Kabanov, Maria A. Teplyakova.. Development of adaptive stereotactic radiotherapy method in treatment of primary malignant glial tumors in the brain. Cardiometry; Issue 22; May 2022; p. 6976; DOI: 10.18137/cardiometry.2022.22.6976; Available from:
The most severe malignant glial tumors of the brain are anaplastic gliomas Grade III and glioblastomas Grade IV, which form 14,3% of the total number of primary tumors in the brain [1]. The prognosis for this sort of patients is unfavorable, and, in general, a median survival of 10-15 months can be expected for them [2]. The present-day approach to treatment of malignant gliomas in the brain suggests the maximized removal of the tumor with minimized damage to the functional regions followed by radio- and chemotherapy [3]. Despite the progress in development of surgery technologies and medication modalities, radiation therapy remains one of the most effective methods to treat these diseases [4].
Currently a generally accepted technique of radiotherapy in treatment of malignant glial tumors is delivery of conformal radiation to the post-surgery region and the tumor subclinical regions with the use of medical linear accelerator (LINAC) [5]. When contouring in order to plan RT, the macroscopic volume of the tumor (Gross Tumor Volume referred to as GTV) shall include the postoperative tumor cavity and contrasted foci of the residual tumor tissue, which are visualized by the contrast enhanced T1 imaging, and the visualized hypertensive foci, which are detected in the T2 FLAIR sequence. At the stage of defining Clinical Target Volume (CTV), the region to be exposed to radiation shall be created from the gross tumor volume (GTV) by adding 1–3 cm margins with an obligatory exclusion of critical structures and healthy tissues of no interest. The Planning Target Volume (PTV) is defined as PTV=CTV+0,3-0,5 cm, since a margin is required to take into account possible errors due to the patient’s positioning, and it depends on immobilization devices used and visualization capabilities, when conducting RT. The purpose of radiotherapy is to deliver to the tumor an optimal total dose at which the desired therapy effect as much as possible can be achieved. Currently, radiation doses and radiated volumes are calculated based on recommendations issued by International Commission on Radiation Units and Measurements (ICRU) 83. As to the primary malignant gliomas, the most effective regime covers a single dose of 2 Gy up to a total dose of 60 Gy in 30 fractions [6].
Pathological angiogenesis is known to be typical for malignant glial tumors of the brain due to excess secretion of vascular endothelial growth factors 70 | Cardiometry | Issue 22. May 2022
(VEGF) that results in formation of a vascular bed, and in its turn it favors extensive growing and formation of the perifocal edema. An increase in the volume of perfusion of blood through the tumor and the vascular leakage lead to a greater permeability of hematoencephalic barrier (HEB) [7]. Considering these peculiarities, at present, the best and most informative standard method of diagnostics of the remaining part of the tumor and the post-surgery cavity of the latter is MRI with an intravenous contrast enhancement, with an axial pitch of 1 mm [8].
In case of classical remote source RT, a treatment plan shall be prepared on the basis of pre-treatment topometric spiral CT scanning, and subsequently a total dose of radiation shall be delivered according to the initially prepared plan. However some studies carried out by Cao Y. et. al. in 2021 confirm that the standard approach to the treatment may involve a lowering in efficacy and tolerability of radiotherapy due to involvement of healthy tissues in exposure and due to the presence of underexposed the pathology-affected tissues [9].
First the concept of the adaptive radiation therapy was offered by D. Yan et al. in 1998. They defined the above approach as a radiotherapy technique which implied a correction of the dosing plan considering changes in the configuration of the tumor or edema in the course of the radiation therapy [10]. Some scattered research works treating the adaptive approach in the glioma treatment can be found in the international reference literature. The accumulated data on investigations of the manner of the behavior of the post-operative cavity and the peritumoral edema in the course of radiotherapy bear witness to the relevance of the development of eligible adaptive methods of radiation therapy to treat the primary malignant glial tumors of the brain.
Aim. Our aim has been to develop an adaptive stereotactic method of radiotherapy designed to treat primary malignant tumors of the brain that is capable to increase efficacy and tolerability of radiation therapy and reduce radiation dose burden of normal structures in the brain.
Materials and methods
Our studies covered 10 patients diagnosed with primary glioblastoma G IV, who received specialized treatment at the National Medical Research Centre for Oncology at the Ministry of Health, Russia, in the period 2021-2022. Characteristics of the patients and histological structures of the tumors are given in Table 1 herein. The average age of the patients was recorded to be 43,4 years. All patients have undergone microsurgery implying the total resection of the malignant tumor (3 patients) and the subtotal resection of the malignant tumor (7 patients). An average interval between surgery and radiotherapy was 32,5 days. Before to start radiotherapy we have completed the intravenous contrast enhancement MRI of the brain with an axial pitch of 1 mm in the regimens as follows: contrast enhanced T1 imaging, contrast-free T1, T2 FLAIR imaging. Upon the completion thereof, we have provided the proper pre-radiation topometric preparation. We have fabricated an individualized immobilization device, namely, a three-coat thermoplastic mask for the purpose of stereotactic radiation therapy. All the patients have been examined with topometric tomography using the Siemens Somatom CT system. The delivered topometric data have been processed on virtual simulation workstation Singo Via. Upon completion of the simulation, the obtained multimodal images (spiral CT/MRI) have been matched with the BrainLab Elements software with identifying the critical structures. The produced data have been exported into the planning system Varian Medical Systems Eclipse. Utilizing this planning system, we have delineated the contours of volumes to be radiated: the GTV, CTV and PTV. When contouring the tumor, we have included into the GTV the foci visualized in the regimens contrast T1 + T2 FLAIR. The CTV was defined as a 2,0 cm margin around GTV with an anatomical correction. The CTV-to-PTV margin was 0,1 cm. Doses have been administered as follows: a single dose of 2 Gy up to a total dose of 60 Gyin in 30 fractions. Volumetric modulated arc therapy (VMAT) was employed to deliver the radiation dose continuously as the treatment machine rotated. The brain has been MRI-scanned in all patients at fraction 10 and 20 with the use of intravenous contrast agent with an axial pitch of 1 mm with the enhanced contrast/con-trast-free T1 and T2 FLAIR sequences in order to redefine the radiotherapy targets (GTV, CTV and PTV). At fraction 30 we completed MRI in order to estimate the treatment outcome. In the average, the radiotherapy course has taken 42-45 days, holidays included. For the purpose of the radiation therapy, used have been the Novalis Tx Varian Tx linear accelerator of kinetic energy of the beam of 6 MeV.
Table 1
Characteristics of patients
|
Characteristics |
Number |
|
Gender |
|
|
Male |
5 |
|
Female |
5 |
|
Histology |
|
|
Glioblastoma |
10 |
|
Surgery type |
|
|
Total resection |
3 |
|
Subtotal resection |
7 |
|
Radiotherapy start |
|
|
≥28-39 days |
10 |
|
Age |
|
|
>18 years |
10 |
|
Treatment |
|
|
Radiotherapy only |
10 |
Table 2
Mean values and standard deviations at the stages of re-planning for the GTV data at fraction 10, 20 and 30 and upon expiration of 1 month after treatment, with T1CU and FLAIR sequences, are represented herein; the CTV and PTV data refer to fraction 10 and 20
|
Critical structures |
Volume (cm3) |
|
GTV Fr10 T1CU |
-4,2±2,7 |
|
GTV Fr20 T1CU |
-3,2±2,4 |
|
GTV Fr30 T1CU |
-2,71±3,6 |
|
GTV 1 month T1CU |
-5,73±8,2 |
|
GTV Fr10 FLAIR |
-7,97±8,2 |
|
GTV Fr20 FLAIR |
-3,73±4,4 |
|
GTV Fr30 FLAIR |
-1,6±7,6 |
|
GTV 1 month FLAIR |
3,52±5,1 |
|
CTV 10 |
16,3±29,5 |
|
CTV 20 |
7,67±35,7 |
|
PTV 10 |
7,9±10,9 |
|
PTV 20 |
0,76±12,7 |
Results
The proposed method has been applied to treat 10 patients with primary glioblastomas of the brain. We have analyzed the GTV, CTV and PTV data at the different stages of the radiation therapy received, and the data in question are given in Table 2 herein. Our analysis has demonstrated that in the course of radiation therapy we observe anatomical changes in the tumor configuration. Our adaptive approach to the delivery of radiation has made it possible to control the changing volumes in question and adjust the initial treatment plan. Below we are presenting some clinical cases of adaptation of the treatment plan in the patients.
Case No.1 . Female patient, aged 62. The patient has undergone resection of glioblastoma in the right parietotemporal region in the brain. 30 days after the resection the patient has been hospitalized in the National Medical Research Centre for Oncology, at the Ministry of Health, RF, to receive radiation therapy.
We have fabricated a 3-coat stereotactic mask, performed spiral CT topometry investigation, with a pitch of 1 mm, and the intravenous contrast MRI examination with three-dimensional visualization. Utilizing the Brain Lab Elements, we have matched the produced multimodal scans (spiral CT/MRI) with identifying the critical structures. Upon processing, the produced data have been exported into the planning system Varian Medical Systems Eclipse. With the use of the planning system we have contoured the volumes to be exposed to radiation: the GTV, CTV and PTV at the stages of the intravenous contrast enhancement MRI of the brain. When contouring the tumor, the GTV has included the visualized foci detected in modes contrast T1 + T2 FLAIR. The margin around the Gross Tumor Volume (GTV) to create a Clinical Target Volume (CTV) was 2,0 cm with an anatomical correction. The CTV-to-PTV treatment margin was 0,1 cm. Radiation according to the applicable guidelines in radiotherapy of malignant glial tumors has been prescribed as follows: a single dose of 2 Gy to a total dose of 60 Gy in 30 fractions.
The initial radiated volume CTV=GTV+20mm has amounted to 176,5 cm3 with the largest diameter of 7,63 cm. At fraction 10 of radiotherapy, when conducting intravenous contrast MRI scanning and matching the obtained scans, in order to re-plan the treatment, we have found that the CTV to be radiated has been reported to be 134,3 cm3 with the largest diameter of 7,38 cm. When performing MRI of the brain with intravenous contrast enhancement at fraction 20 of the radiation therapy and matching the images with the fraction 10 plan, the CTV has been recorded to be 154,1 cm3 with the largest diameter of 7,48 cm. At fraction 30 of radiation therapy, according to the MRI scanning of the brain with contrast enhancement, the CTV value has been found to be 126,5 cm3 with the largest diameter of 7,33 cm.
Figures 1, 2 and 3. MRI scans of the brain in patients with primary malignant tumors at different treatment stages. Arrows indicate the respective CTV value. Fr0 indicates the CTV at the start of radiotherapy; Fr10 indicates the CTV at fraction session 10; Fr20 and Fr30 indicate the CTV values at fraction session 20 and fraction session 30, respectively.
Case No.2 Female patient P., aged 61. Cytoreductive resection of glioblastoma has been performed in the frontotemporal region in the brain. 29 days after the cytoreductive removal of the tumor the patient has been admitted to the National Medical Research Centre for Oncology at the Ministry of Health, RF, to receive radiation therapy.
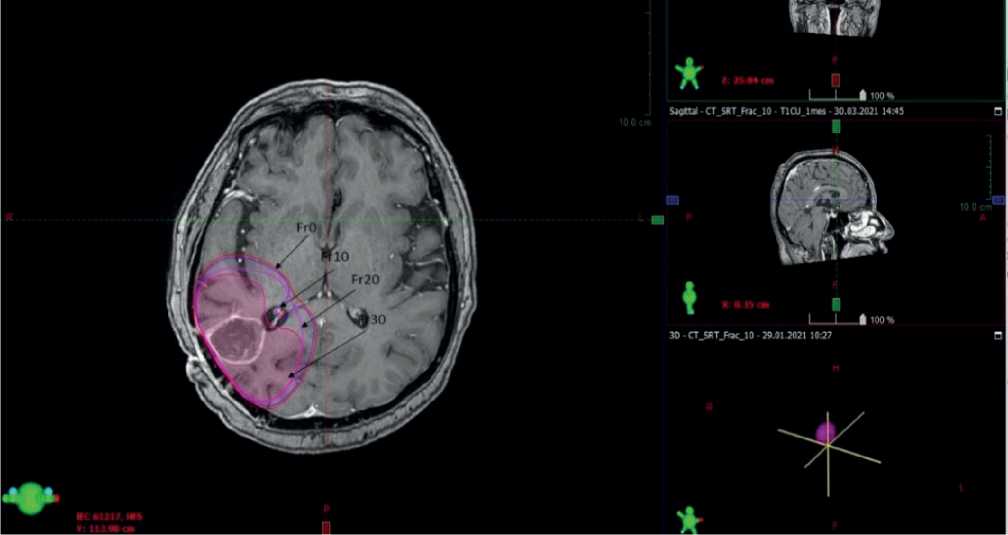
Figure 1
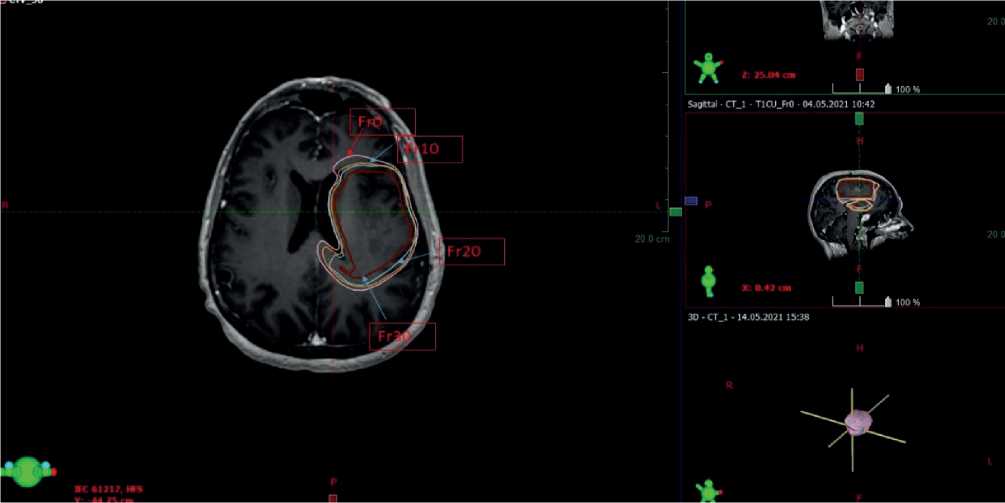
Figure 2
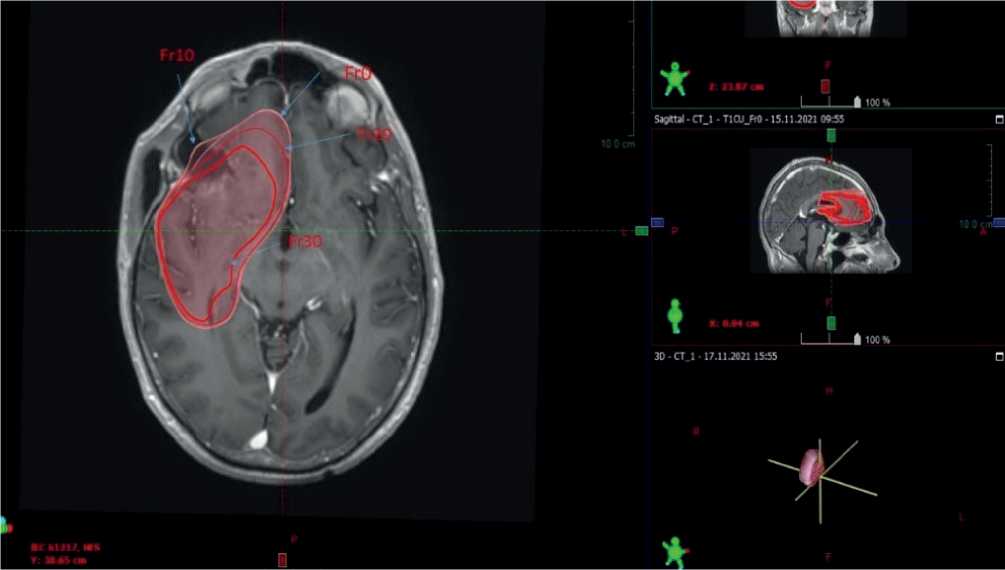
Figure 3
A stereotactic three-coat mask has been produced by us, a spiral CT topometry scanning with a pitch of 1 mm and the MRI examination with intravenous contrast enhancement in 3D visualization with a pitch of 1 mm have been conducted. Employing the BrainLab Elements software, we have matched the obtained multimodal scans (spiral CT/MRI) in order to detect the critical structures. The produced data have been exported into the planning system Varian Medical
Systems Eclipse. The latter has been applied to contour the respective radiated volumes: GTV, CTV and PTV at the stages of MRI scanning of the brain with intravenous contrast enhancement. When contouring the tumor, the GTV has included the visualized foci using the modes contrast T1 + T2 FLAIR. The CTV contained the GTV plus a margin of 2,0 cm with an anatomical correction. The CTV-to-PTV margin was 0,1 cm. Radiation according to the applicable guide- lines in radiotherapy of malignant glial tumors has been prescribed as follows: a single dose of 2 Gy to a total dose of 60 Gy in 30 fractions.
The initial volume to be exposed to radiation has been defined as CTV=GTV+20mm reaching 311,7 cm3 with the largest diameter of 7,35 cm. At fraction 10 of radiotherapy, when performing MRI of the brain with intravenous contrast enhancement and when matching the obtained scans in re-planning, the volume to be irradiated has been found to be 302 cm3 with the largest diameter of 7,24 cm. When applying MRI of the brain with intravenous contrast enhancement at fraction 20 of the treatment and when matching the obtained scan with the fraction 10 plan, we have found that the CTV has reached 300 cm3 with the largest diameter of 7,15 cm. At fraction 30 of the radiation therapy, MRI of the brain with intravenous contrast enhancement has shown that the CTV measures 279,5 cm3 with the largest diameter of 7,05 cm.
Case No.3. Female patient K., aged 52. At our Oncology Center the patient has undergone cytoreductive resection of glioblastoma in the left temporal lobe region in the brain. 36 days after the cytoreductive surgery the patient has been hospitalized to receive radiotherapy.
We have fabricated a stereotactic three-coat mask, conducted the spiral CT topometry investigation with a pitch of 1 mm and the MRI scanning with intravenous contrast enhancement in the 3D visualization mode with a pitch of 1 mm. Utilizing the BrainLab Elements software, we have matched the produced multimodal scans (spiral CT/MRI) with identifying the critical structures. The obtained data have been exported into the planning system Varian Medical Systems Eclipse. With the use of the planning system we have contoured the volumes to be exposed to radiation, namely GTV, CTV and PTV at the brain intravenous contrast enhancement MRI stages. When defining the contour of the tumor, the GTV has included the visualized foci from modes contrast T1 + T2 FLAIR. The CTV contained the GTV plus a margin of 2,0 cm with an anatomical correction. The CTV-to-PTV margin was 0,1 cm. Radiation according to the applicable guidelines in radiotherapy of malignant glial tumors has been prescribed as follows: a single dose of 2 Gy to a total dose of 60 Gy in 30 fractions.
The initial volume to be radiated CTV=GT-V+20mm has been defined as 147,1 cm3 with the largest diameter of 5,35 cm. At fraction 10 of radiation therapy, when performing the brain intravenous con- 74 | Cardiometry | Issue 22. May 2022
trast enhancement MRI scanning and when matching the obtained image in re-planning, the CTV to be exposed to radiation has been found to be 132 cm3 with the largest diameter of 5,02 cm. At fraction 20, when scanning the brain with MRI with intravenous contrast enhancement and when matching the produced actual scan with the fraction 10 plan, the CTV has been measured 127,3 cm3with the largest diameter of 5 cm. At fraction 30 of radiation therapy, we have revealed with the brain MRI with intravenous contrast enhancement the CTV has measured 120 cm3 with the largest diameter of 4,9 cm.
Discussion
At present, there is no strong evidence that re-planning in treatment of brain tumors is of clinical efficacy. First it is connected with the fact that adaptive techniques of radiation therapy have been introduced quite recently for the above localizations, and they require further intense investigations. The latest studies on adaptive radiotherapy in advanced head and neck cancer demonstrate an improvement in the treatment outcomes due to local control of the tumor and a reduction of doses delivered to the critical structures [11].
The salient feature of malignant glial tumors is that they are fast-growing structures and that they trigger the surge in neurocognitive impairments that, in its turn, determines the peculiarities of the tumor aggressive progression [12]. Despite the fact that the malignant glial tumors are fast-progressing, the existing international guidelines governing radiation therapy, including RTOG and EORTC, overlook the required adaptation of the volumes to be exposed to radiation in the course of radiotherapy.
Studies conducted by Mehta S et al. analyzing daily MRI scans of the brain in three patients show that in the course of treatment in all patients observed has been a tendency to a regression in the tumor sizes [13]. In 2020 another paper has presented estimations of the radiotherapy plan adaptation in 43 patients with glioblastoma multiforme after cytoreductive surgery, based on CT/MRI scanning, at a single dose of 40 Gy followed by boost (treatment regimen 75 mg/m2 temozolomide on CRT days at a single dose of 2 Gy to a total dose of 60 Gy). It has been reported that “tumor shrinkage in 24 patients resulted in improved survival compared to 19 in whom tumor was unchanged or progressed (25.3 vs. 11.1 months, p=0.04). Adapted planning target volume allowed a reduction in irradi- ated volume, while increasing survival (12.06 vs. 28.98 months, p=0.026).” [14].
The method of the adaptation of the radiotherapy plan at the stage of treatment offered by us demonstrates a regression of the recorded tumor volumes during the treatment, and the re-planning measures taken are capable to reduce radiation exposure of normal tissues in the brain and increase efficacy and tolerability of the radiation therapy.
So, the offered method to implement the adaptive approach to RT to treat post-surgery malignant gliomas in order to optimize the target volume and minimize doses at risk organs is capable of improving the clinical outcome as compared with that achieved in the standard treatment cases. The evidence data on the changes in the configuration of the post-surgery cavity and the residual tumor, which have been collected by us in the course of radiotherapy, bear witness to the fact that we deal with the changes in the perifocal zone and in the redisual tumor in case of fractioned radiation therapy during the treatment. The application of the multimodal scans to optimize visualization in re-planning may increase the quality of radiotherapy in treatment of malignant glial tumors in the classical regimen of fractionation.
Conclusion
Our method of treatment has been properly patented in the RF under Patent RF No. 2759405 C1. METHOD FOR ADJUVANT ADAPTIVE STEREOTACTIC RADIATION THERAPY IN THE TREATMENT OF PRIMARY MALIGNANT GLIAL BRAIN TUMORS.
The developed method of the adaptive stereotactic radiation therapy designed to treat the primary malignant glial tumors of the brain allows tracing the changes in the configuration of the post-surgery cavity, the residual tumor and the brain structures in the course of radiation therapy and adapting the treatment plan that may make possible to increase efficacy and tolerability of radiation therapy as well as reduce radiation doses to normal structures in the brain that needs further investigation.
Statement on ethical issues
Research involving people and/or animals is in full compliance with current national and international ethical standards.
Conflict of interest
None declared.
Author contributions
The authors read the ICMJE criteria for authorship and approved the final manuscript.
Список литературы Development of adaptive stereotactic radiotherapy method in treatment of primary malignant glial tumors in the brain
- Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014-2018. Neuro Oncol. 2021 Oct 5; 23(12 Suppl 2):iii1-iii105. doi: 10.1093/neuonc/noab200. PMID: 34608945; PMCID: PMC8491279.
- Davis ME. Epidemiology and Overview of Gliomas. Semin Oncol Nurs. 2018 Dec; 34(5): 420-429. doi: 10.1016/j.soncn.2018.10.001. Epub 2018 Nov 2. PMID: 30392758.
- Ma R, Taphoorn MJB, Plaha P. Advances in the management of glioblastoma. J Neurol Neurosurg Psychiatry. 2021 Oct; 92(10): 1103-1111. doi: 10.1136/jnnp-2020-325334. Epub 2021 Jun 23. PMID: 34162730.
- Frosina G. Radiotherapy of High-Grade Gliomas: First Half of 2021 Update with Special Reference to Radiosensitization Studies. Int J Mol Sci. 2021 Aug 19; 22(16): 8942. doi: 10.3390/ijms22168942. PMID: 34445646; PMCID: PMC8396323.
- Weller M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021 Mar; 18(3): 170-186. doi: 10.1038/s41571-020-00447-z. Epub 2020 Dec 8. PMID: 33293629; PMCID: PMC7904519.
- Hodapp N. Der ICRU-Report 83: Verordnung, Dokumentation und Kommunikation der fluenzmodulierten Photonenstrahlentherapie (IMRT) [The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT)]. Strahlenther Onkol. 2012 Jan; 188(1): 97-9. German. doi: 10.1007/s00066-011-0015-x. PMID: 22234506.
- Ahir BK, Engelhard HH, Lakka SS. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma. Mol Neurobiol. 2020 May;57(5):2461-2478. doi: 10.1007/s12035-020-01892-8. Epub 2020 Mar 9. PMID: 32152825; PMCID: PMC7170819.
- Verburg N, de Witt Hamer PC. State-of-the-art imaging for glioma surgery. Neurosurg Rev. 2021 Jun; 44(3): 1331-1343. doi: 10.1007/s10143-020-013379. Epub 2020 Jun 30. PMID: 32607869; PMCID: PMC8121714.
- Cao Y, et al. Study on the Appropriate Timing of Postoperative Adaptive Radiotherapy for High-Grade Glioma. Cancer Manag Res. 2021 Apr 28; 13: 3561-3572. doi: 10.2147/CMAR.S300094. PMID: 33953610; PMCID: PMC8089024.
- Yan D, et al. The use of adaptive radiation therapy to reduce setup error: a prospective clinical study. Int J Radiat Oncol Biol Phys. 1998 Jun 1; 41(3): 715-20. doi: 10.1016/s0360-3016(97)00567-1. PMID: 9635724.
- Sonke JJ, Aznar M, Rasch C. Adaptive Radiotherapy for Anatomical Changes. Semin Radiat Oncol. 2019 Jul; 29(3): 245-257. doi: 10.1016/j.semradonc.2019.02.007. PMID: 31027642.
- Zhao B, Wang X, Zheng J, Wang H, Liu J. Effects of metformin treatment on glioma-induced brain edema. Am J Transl Res. 2016 Aug 15;8(8):3351-63. PMID: 27648126; PMCID: PMC5009388.
- Mehta S, et al. Daily Tracking of Glioblastoma Resection Cavity, Cerebral Edema, and Tumor Volume with MRI-Guided Radiation Therapy. Cureus. 2018 Mar 19; 10(3): e2346. doi: 10.7759/cureus.2346. PMID: 29796358; PMCID: PMC5959724.
- Végváry Z, et al. Adaptive Radiotherapy for Glioblastoma Multiforme - The Impact on Disease Outcome. Anticancer Res. 2020 Aug; 40(8): 4237-4244. doi: 10.21873/anticanres.14425. PMID: 32727750.


