Development of control princi-ples for the desalination unit for closed life support systems of space purposes
Автор: Tikhomirov А.A., Trifonov S.V., Morozov Ye.A., Murygin A.V.
Журнал: Siberian Aerospace Journal @vestnik-sibsau-en
Рубрика: Technological processes and material science
Статья в выпуске: 3 vol.23, 2022 года.
Бесплатный доступ
Exploring the Solar system by humans implies the development of long-term habitable bases on several planets: the Moon, Mars, and others. Maintaining an environment favorable for a crew on such bases is possible due to life support systems (LSS), where a closed mass transfer of products and waste is imple-mented among the crew, the higher plants and other links. Closure increases the reliability and autonomy of the system, and reduces the cost of its supply.Controlling such mass transfer appears to be a difficult technical task requiring many man-hours, which is a valuable resource in the implementation of manned space missions. In the general case, this problem is solved by means of automation, however, it is neces-sary to take into account the features of the processes that support mass transfer, since this will allow find-ing ways to simplify the hardware and logical components, increase their versatility and reliability. This article presents an analysis of the technological processes of the experimental unit for extracting NaCl from solutions of mineralized human metabolites and proposes a simple control algorithm to be used for all processes of the unit without fundamental changes. Without developing a NaCl transformation cycle, it becomes almost impossible to develop a long-term functioning biological and technical life support sys-tem – the optimal LSS option for alien bases. In such systems, mass transfer occurs between the crew and the higher plants and there is a danger of NaCl accumulation in irrigation solutions and subsequent poi-soning of the plant link. Therefore, the problem of controlling the NaCl transformation cycle in mass trans-fer processes of a high degree of closure is relevant, and the universal principles of automated control can be used not only in space, but also in terrestrial applications: in closed agrotechnical cycles and scientific, and educational stands.
Process control, life support systems, utilization of organic waste, desalination, plant link
Короткий адрес: https://sciup.org/148329650
IDR: 148329650 | УДК: 544.623.032.52 | DOI: 10.31772/2712-8970-2022-23-3-551-560
Текст научной статьи Development of control princi-ples for the desalination unit for closed life support systems of space purposes
Closed artificial ecosystems, including humans and higher plants, are of scientific interest to study the cycling processes of natural and artificial ecosystems. The technologies developed to organize mass transfer between the crew and higher plants are planned to be used to develop biotechnical life support systems (BTLSS) of planetary bases, including the lunar and Martian ones [1 –3]. One of the key points in organizing mass transfer is the rapid and environmentally friendly processing of organic waste, including human metabolites, into fertilizers. There are a limited number of methods for such utilization [1–12], each of them has the disadvantage of a high relative content of NaCl in the processed products. Their constant use as fertilizers will lead to salinization of irrigation solutions and a decrease in the productivity of the space greenhouse during its long-term operation for many months. As a result, the crew will begin to experience a shortage of plant foods, and then water and oxygen. In this regard, developing desalination technologies seems relevant [13; 14], including the development of automated control of the relevant technological processes.
It is obvious that the development of NaCl extraction technology is most rationally performed in combination with waste processing technology, since human metabolites are the main source of NaCl in mass transfer. The Institute of Biophysics SB RAS is developing a complex for the physicochemical processing of wastes of plant and animal origin, including human metabolites, to involve the elements contained in these wastes in the cycle of mass transfer processes of BTLSS. This complex is based on the mineralization of waste by the method of "wet" incineration, the essence of which is the oxidation of organic waste in an aqueous solution of hydrogen peroxide under the action of an alternating electric current [12; 15; 16]. Such processing results in generating a mineralized solution, which can be used to prepare an irrigation solution for a link of higher plants [15; 16]. This method has a great advantage in the speed of utilization and environmental safety of the resulting products (solution and gas) [16].
In addition to the risk of salinization, the resulting reactor liquid cannot be directly introduced into the irrigation solution for plants, since the “wet” incineration method is practically unable to utilize urea, which increases the risk of development of opportunistic urobacteria in the system [16]. Therefore, the complex of physical and chemical processing of organic waste includes several processes [12]:
-
1) "wet" waste incineration;
-
2) urea decomposition;
-
3) NaCl extraction.
To establish a reliable mass-transfer cycle of BTLSS , it is necessary that all processes of the waste processing complex proceed within a technologically acceptable time (no more than 1–2 days). The ability to process the daily rate of human liquid secretions during the day would be more preferable.
The research demonstrates the development of principles for controlling the technological processes of urea decomposition and NaCl isolation from a solution of mineralized human metabolites obtained by the "wet" incineration method. Several physicochemical sequential and parallel processes are performed in the NaCl extraction unit being developed. Despite the differences in the chemistry of the processes, their dynamics are similar, and the flow conditions are provided by similar physicalchemical methods. This allows to build the hardware and software of the block elements in such a way that there is a possibility of mutual replacement of components. Due to this, the ability to survive and BTLSS reliability could be increased, and the risks of successfully completing the mission will be reduced.
Technological processes of the desalination unit
Figure 1 demonstrates a general scheme of the experimental complex for the physical and chemical processing of organic waste. According to this scheme, after processing in a “wet” incineration reactor, mineralized exometabolites enter the reactor for the enzymatic decomposition of urea using urease [16; 17]. In addition, this reactor is periodically supplied with HCl synthesized in the HCl synthesis reactor [18] to correct the pH level of the solution. The hydrolysis process of urea in solution is completed when its concentration level drops to a certain value.
Further, the solution enters the Cl 2 extractor, where, due to electrolysis, gaseous Cl 2 is formed, which enters the HCl synthesis reactor [18] necessary to obtain NaCl at the last stage of the technological process. The Cl 2 extraction occurs due to the electrolysis of the solution and ends when the concentration of Cl- in the solution drops to a certain value.
The HCl synthesis [12] proceeds until all the gaseous chlorine formed at the previous stage is converted into acid and fixed in water, that is, the process stops when a certain value of the HCl concentration in the aqueous solution is reached.
After the urea decomposition and the chlorine release, the solution enters the electrolyzer, where the allocation of the alkaline part happens from the mineralized metabolites, which contains the main share of metals. The acidic and neutral parts are returned to the irrigation solution. The process of extracting the alkaline part is aimed at lowering the Na+concentration in the solution, so the signal for its completion is the decrease in the level of this concentration to a certain value determined by the operator.
Further, in the reactor for the synthesis of sodium carbonate, Na 2 CO 3 is extracted from the alkaline solution, and the remaining components of the alkaline solution in the form of a carbonate solution are returned to the irrigation solution. Synthesis of Na 2 CO 3 is multistage:
-
1) bubbling air and heating the alkaline solution to a certain temperature until it is concentrated to a certain volume (level);
-
1) barbotage with air and heating the alkaline solution to a certain temperature until it is concentrated to a certain volume (level);
-
2) barbotage the solution of NaHCO 3 and KHCO 3 with CO 2 , until a certain gas pressure over the solution is reached;
-
3) cooling to a certain temperature both a solution of Na 2 CO 3 and K 2 CO 3 and maintaining a reduced pressure over the solution for a certain time.
During the final physical-chemical stage in the NaCl synthesis reactor, Na 2 CO 3 and HCl are mixed to form a NaCl solution. The process takes place in the liquid phase and ends when the required pH level is reached. Further, the resulting solution with a high content of NaCl can be filtered for the production of food salt or used for the cultivation of halophytes in the phototrophic link, providing humans with NaCl.
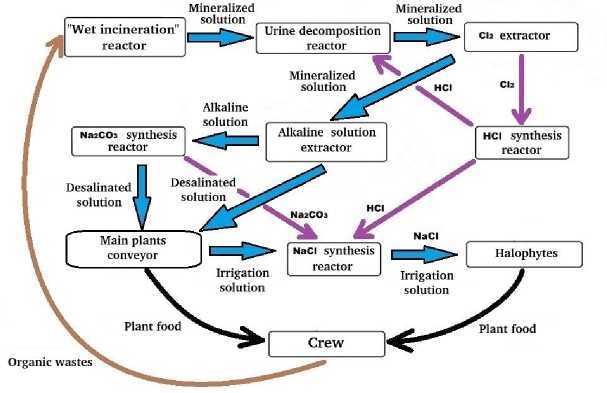
Рис. 1. Цикл NaCl в массообмене БТСЖО
Fig. 1. Cycle of NaCl in the mass transfer of BTLSS
The experiments have shown that the developed physicochemical technology for desalination of mineralized human metabolites in the future will make it possible to remove up to 80% of NaCl from the solution in less than a day, which can later be used to obtain food salt for the crew [19]. Identifying similar moments in the technological processes of the desalination unit will allow the use of universal, interchangeable elements of hardware and logical process control, which will help to significantly reduce the mass of the unit, increase its reliability, and it will be easier for the operator to maintain control, moving from one technological stage to another.
The principles to control the desalination unit
Each of the technological processes of the desalination unit can be characterized by the monotonous achievement of a target parameter of a certain value that is of interest to the operator. At the stage of urea decomposition, completing the process can be guided by a change in the concentration of urea, or the product of its hydrolysis, NH4+, which is technologically easier to implement with measuring electrodes [19]. The end of the processes of Cl2 release and HCl synthesis is determined by the readings of the Cl– concentration in the solution, the process of the allocation of the alkaline part is deter- mined by the readings of the Na+ concentration in the solution. Extracting sodium carbonate and synthesizing NaCl are completed when a certain time or pH value or solution level is reached. In all cases, the operator has to receive data, including graphical data, on the dynamics and current value, of only one target process parameter in order to reduce the likelihood of error and the risks of termination of the block functioning.
Appropriate conditions for the technological processes of the desalination unit should be ensured by maintaining a number of environmental parameters in the optimal range: temperature and pressure of the gas above the solution, temperature, pH and level of the solution. These parameters can be corrected by relay automation by turning on / off the actuator (heater, acid pump or air pump) depending on the readings of the sensors. It is also necessary to monitor critical deviations from the optimal parameters that can lead to destructing the production line.
Based on the described characteristics of the processes, controlling the desalination unit can be divided into two automated subsystems controlling the achievement of the target parameter and the parameters of the conditions, respectively (table). The first subsystem monitors the fact that the target parameter has reached a certain value (a signal on the completion of the technological stage), as well as the dynamics of this process to control the speed of its flow. The dynamics can be controlled by the operator, guided by the nature of the trend in the values of the target parameter, or by automation, by keeping the values of the derivatives of the target parameter within the specified ranges. The second subsystem maintains the environment parameters in the required range or urgently stops the process when they are beyond the critical value.
The controlled parameters of desalination stages Контролируемые параметры этапов обессоливания
|
Stages |
Controlled parameters |
|
|
Target parameter |
Condition parameters |
|
|
Urea decomposition |
NH 4 + Concentration |
tº solution, pH |
|
Cl 2 extraction |
Сl– Concentration |
tº solution, V solution |
|
HCl synthesis |
Сl– Concentration |
tº solution, V solution |
|
Alkaline solution extraction |
Na+ Concentration |
tº solution, V solution |
|
Na 2 CO 3 synthesis |
Solution V / gas P / Time |
tº solution |
|
NaCl synthesis |
pH |
tº solution |
Commentary . tº – temperature; V – volume; P – pressure.
The similarity of process control tasks makes it possible to use the same logic of the control algorithm (Fig. 2) at all stages of desalination. The algorithm operation should begin with a block of a predetermined process: checking the connection of sensors and actuators to assess the technical condition of the units. Further, a data block is required: the operator enters the values of the target parameters and the parameters of the process conditions - to be able to use a single control device at all stages of desalination. It will also allow to use the control system to conduct experiments with physical and chemical processes under adjustable experimental conditions.
After entering the values of the parameters, the subsystem starts functioning, which controls the current parameters of the process conditions. The process block reads and writes condition parameter data. Next, the decision block compares the values of these parameters with the required ranges of values. If the parameter value is within the range, including its boundary values, then the process proceeds normally and control passes to the subsystem that monitors the achievement of the target value by the target parameter. If the value of the condition parameter is outside the required range, then the next decision block compares the current value of the condition parameter with the critical range of values. If the parameter value is within the critical range, including its boundary values, then the process block performs an act of adjusting the conditions by executive devices, after which the algorithm loop returns to the stage of the process block operation, which reads and writes data about the updated condition parameters. If the value of the conditions parameter is outside the critical range, then the predefined process block prepares the reactor for an emergency shutdown and the algorithm terminates by shutting down the reactor.
The target parameter control subsystem starts functioning if the values of the conditions parameters are in the specified range. The process block reads and writes data of the current value of the target parameter. Further, the decision block compares the current value with the required one. If the value of the parameter is less than required, then the process continues and the loop returns to the process block, which reads and writes data on the updated condition parameters. If the value of the target parameter is greater than or equal to the specified value, then the predefined process block prepares the reactor for a planned shutdown and the algorithm terminates by shutting down the reactor.
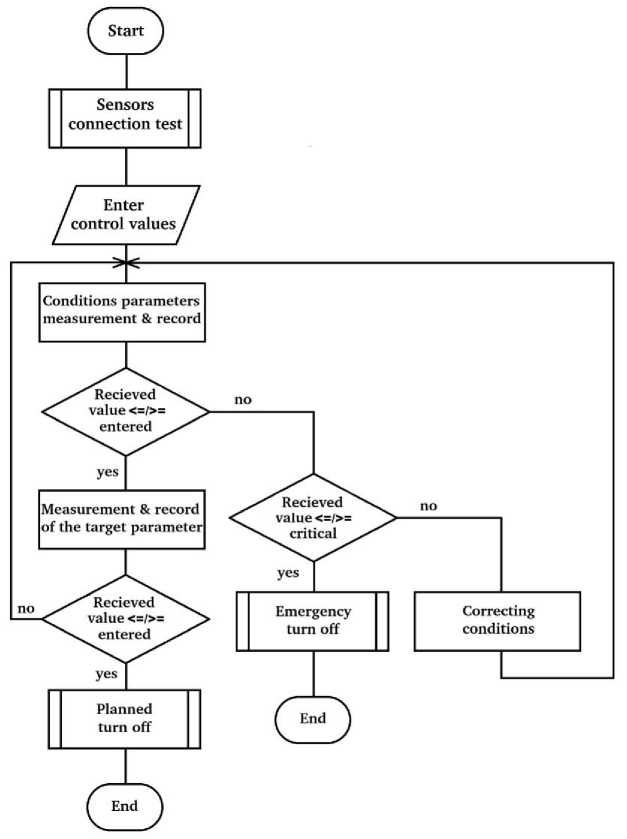
Рис. 2. Алгоритм работы программы автоматики
Fig. 2. Algorithm of functioning the automation program
Fig. 3 presents functional block diagram of automation. The block diagram elements are universal and can take part in several stages of operation of the NaCl extraction unit.
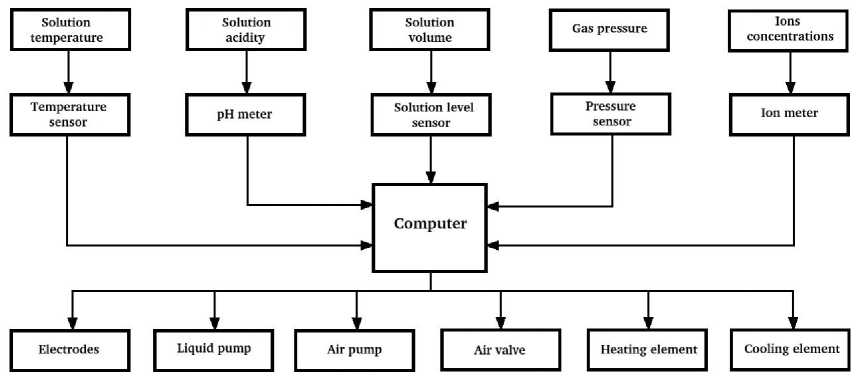
Рис. 3. Функциональная схема автоматики блока выделения NaCl
Fig. 3. Functional block diagram of the NaCl extraction block automation
Conclusion
The block for solution desalination being developed is a part of the subsystem for the physical and chemical utilization of BTLSS organic waste. This subsystem also includes a “wet” incineration reactor, the mineralization process that is multistage and has a chain character. The principles of its automation, described in [12; 19; 20], are based on multiple changes in the target parameter and require the introduction of temperature as an additional parameter while scaling the process. As a result, the application of the approach considered in this article is impossible when developing a control algorithm for a "wet" incineration reactor. However, it has got a number of advantages.
The presented algorithm can serve 6 out of 7 technological stages of the subsystem of physical and chemical utilization of organic waste. Using only one target parameter as a process completion indicator will help the operator maintain control as they move from one process step to the next. The ability to enter the control values of the parameters of the conditions and the target parameter allows you to apply automatic control in a scientific and educational application. For example, when developing experimental or educational stands to study the processes of fermentation, electrodialysis and crystallization.
In general, it can be concluded that the described principles of control of the desalination unit will increase the BTLSS reliability: first, by reducing the burden on the operator through automation; second, due to hardware duplication. A number of parameters controlled by the algorithm at different technological stages are measured in principle in the same way: by measuring electrodes, level, pressure and temperature sensors. This means that the same computing devices can be used from one technological step to another. The highlighted advantages increase the prospects to use BTLSS in future planetary bases, which will favorably affect the development of the solar system by a human being.
Список литературы Development of control princi-ples for the desalination unit for closed life support systems of space purposes
- Gitelson J. I., Lisovsky G. M., MacElroy R. D. Manmade Closed Ecological Systems. Taylor and Francis, New York, 2003, 402 p.
- Nelson M., Dempster W. F., Allen J. P. Integration of lessons from recent research for “Earth to Mars” life support systems. Advances in Space Research. 2008, Vol. 41. P. 675–683. Doi: https://doi.org/10.1016/j.asr.2007.02.075.
- Bamsey M., Graham T., Stasiak M., Berinstain A., Scott A., Rondeau Vuk T., Dixon M. Cana-dian advanced life support capacities and future directions. Advances in Space Research. 2009, Vol. 44. P. 151–161. Doi: https://doi.org/10.1016/j.asr.2009.03.024.
- Drysdale A. E., Ewert M. K., Hanford A. J. Life support approaches for Mars missions. Advanc-es in Space Research. 2003, Vol. 31 (1), P. 51–61. Available at: https://doi.org/10.1016/S0273-1177(02)00658-0.
- Farges B., Poughon L., Creuly C., Cornet J.-F., Dussap C.-G., Lasseur, C. Dynamic Aspects and Controllability of the MELiSSA Project: A Bioregenerative System to Provide Life Support in Space. Applied Biochemistry and Biotechnology. 2008, Vol. 151, P. 686–699. Doi: https://doi.org/10.1007/ s12010-008-8292-2.
- Guo S. S., Mao R. X., Zhang L. L., Tang Y. K., Li Y. H. Progress and prospect of research on controlled ecological life support technique. Reach. 2017, Vol. 6, P. 1–10. Doi: https://doi.org/ 10.1016/j.reach.2017.06.002.
- Walker J., Granjou C. MELiSSA the minimal biosphere: Human life, waste and refuge in deep space. Futures. 2017, Vol. 92, P. 59–69. Doi: https://doi.org/10.1016/j.futures.2016.12.001.
- Escobar C., Nabity J. Past, present, and future of closed human life support ecosystems – a review. 47th International Conference on Environmental Systems. 2017, P. 18. Doi: http://hdl.handle.net/2346/73083.
- Putnam D. F. Composition and Concentrative Properties of Human Urine. NASA contract report, Washington, 1971, 107 p.
- Kudenko Yu. A., Gribovskaya I. A., Zolotukchin I. G. Physical-chemical treatment of wastes: A way to close turnover of elements in LSS. Acta Astronautica. 2000, Vol. 46, P. 585–589. Doi: https://doi.org/10.1016/S0094-5765(00)00007-2.
- Kudenko Yu. A., Gribovskaya I. A., Pavlenko R. A. Mineralization of wastes of human vital activity and plants to be used in a life support system. Acta Astronautica. 1997, Vol. 41, P. 193–196. Doi: https://doi.org/10.1016/S0094-5765(97)00215-4.
- Morozov Ye. A., Trifonov S. V., Saltykov M. Yu, Murygin A. V., Tikhomirov A. A. [Physico-chemical waste mineralization reactors subsystem for closed bio technical life support systems for space application]. Siberian Journal of Science and Technology. 2017, No. 3, P. 585–591 (In Russ.).
- Cherif M., Mkacher I., Dammak L. Water desalination by neutralization dialysis with ion-exchange membranes: Flow rate and acid/alkali concentration effects. Desalination. 2015, Vol. 361, P. 13–24. Doi: https://doi.org/10.1016/j.desal.2015.01.024.
- Ushakova S. A., Kovaleva N. P., Gribovskaya I. V., Dolgushev V. A., Tikhomirova N. A. Ef-fect of NaCl concentration on productivity and mineral composition of Salicornia europaea as a poten-tial crop for utilization NaCl in LSS. Advances in Space Research. 2005, Vol. 36, P. 1349–1353. Doi: https://doi.org/10.1016/j.asr.2004.09.017.
- Ushakova S. A., Tikhomirov A. A., Tikhomirova N. A. Kudenko Yu. A., Litovka Yu. A., An-ishchenko O. V. A biological method of including mineralized human liquid and solid wastes into the mass exchange of bio-technical life support systems. Advances in Space Research. 2012, Vol. 50, P. 932–940. Doi: https://doi.org/10.1016/j.asr.2012.05.023.
- Tikhomirov A., Kudenko Yu., Trifonov S., Ushakova S. Assessing the feasibility of involving gaseous products resulting from physicochemical oxidation of human liquid and solid wastes in the cycling of a bio-technical life support system. Advances in Space Research. 2012, Vol. 49, P. 249–253. Doi: https://doi.org/10.1016/j.asr.2011.10.003.
- Sutormina E. F., Trifonov S. V., Kudenko Yu. A., Ivanova Yu. A., Pinaeva L. G., Tikhomirov A. A., Isupova L. A. Physicochemical processing of human exometabolites for closed life support systems. Chemistry for Sustainable Development. 2011, Vol. 19, P. 375–382.
- Tikhomirov A. A., Kudenko Yu. A., Trifonov S. V. Ustrojstvo kataliticheskogo sinteza HCl iz gazoobraznogo H2 i Cl2 [Apparatus of HCl catalytic synthesis from gaseous Cl2 and H2]. Patent RF, no. 157597, 2015.
- Trifonov S. V., Murygin A. V., Tikhomirov A. A. Physical-chemical method for desalting or-ganic waste for agricultural cycles. IOP Conference Series: Earth and Environmental Science. 2021, Vol. 839, No. 4, P. 042062. Doi: https://doi.org/10.1088/1755-1315/839/4/042062.
- Saltykov M. Yu., Morozov Ye. A., Trifonov S. V., Murygin A. V., Tikhomirov A. A. [Com-puter automation of organic wastes “wet incineration” installation for closed ecosystems]. Vestnik SibGAU. 2016, Vol. 17, No. 2, P. 438–443 (In Russ.).


