Development of heavy metal-based nanostructured complex technology for use in building mortar
Автор: Vladimir M. Ilin, Evgeni V. Boev, Aigul' A. Islamutdinova, El'mira K. Aminova
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Application of nanomaterials and nanotechnologies in construction
Статья в выпуске: 5 Vol.14, 2022 года.
Бесплатный доступ
Introduction. Heavy metals (copper, zinc, nickel, lead, chromium, cobalt, cadmium) get into constructional materials with natural and man-made raw materials. The chemical and mineralogical composition of large-tonnage wastes from the petrochemical industry is perfect for constructional materials production. Heavy metals in constructional compositions provide high strength and frost resistance. Currently, nanostructured metal-containing complexes are used in the production of mortars. Therefore, it is necessary to ensure the reliable binding of heavy metals into structurally stable compounds to avoid their emission and secondary environmental pollution. The steadily growing volumes of sludge reservoirs with high concentrations of heavy metals such as chromium (Cr +6), copper (+2), lead (+2), iron (+2), and Fe (+3) cause particular interest to researchers. Qualified extraction of the listed metals and binding them as nanocomponents in the composition of the complexing agent will ensure the creation of a nanostructural composition in the recipe for the preparation of mortar for various purposes. Methods and materials. Sorption methods are the main way to isolate heavy metals. The paper proposes a method for the production of alkyleneaminopolycarboxylic acids and studies its ability to form nanometallic complex compounds for the extraction of heavy metals. Results and discussions. In order to bind metal nanoparticles in oil sludge, the efficiency of the produced compounds, carboxymethyl derivatives of hexamine, was investigated. Optimum synthesis conditions were selected and the structure of the obtained complexing agents was proved by infrared and ultraviolet radiation methods as well as by the method of nuclear magnetic resonance. Conclusion. The resulting nanostructured additions have binding properties that provide high adhesion of the heavy metal to the organic substrate and mortar components, which makes it possible to provide a strong composition that maintains operational properties that meet technical requirements.
Nanostructured complexes, mortars, acrylic acid nitrile, monochloroacetic acid, piperazine, ethylenediamine, benzimidazole, heavy metals
Короткий адрес: https://sciup.org/142235386
IDR: 142235386 | DOI: 10.15828/2075-8545-2022-14-5-398-404
Текст научной статьи Development of heavy metal-based nanostructured complex technology for use in building mortar
Original article
One of the main tasks of modern organometallic chemistry is the development of new nanocatalytic systems and materials with predetermined practically useful properties. Varying the nature of the ligand in transition metal complexes by using compounds of various natures, including chelating ones, leads to a significant change in the properties of metal complexes, including their stability and reactivity. Among the numerous nitrogen-containing ligands obtained over the past decades, tweezer-type ligands are becoming increasingly important due to their special characteristics and the ability to “tune” the electronic properties of the complexes formed by them. Such an adjustment makes it possible to increase the thermodynamic stability of metal-complex systems, and in some cases it is possible to stabilize unstable degrees of oxidation of the metal center during the formation of chelated metal cycles [1–3]. The chemistry of such transition metal complexes is currently undergoing a period of intensive development due to their unique catalytic properties. The number of publications on this topic reaches several thousand and continues to increase rapidly. Due to the discovery of a powerful catalytic potential of transition metal complexes based on tweezertype ligands in various chemical transformations, there has been a clear tendency in the last few years to increase the total number of articles related to these ligands [5–7].
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
Organometallic complexes of this type have proven themselves to be highly effective catalysts for such processes as ketone hydrogenation [8], alkane dehydrogenation [8], oligo- and polymerization of unsaturated hydrocarbons [9], hydrosilylation and hydroborination [10] and a number of others.
The environmental situation around the world, including in the town of Sterlitamak (Republic of Bashkortostan, Russia), requires the development of effective solutions for the disposal of long-term burials of solid and liquid wastes from petrochemical industries, which have not yet found a qualified method of processing and disposal.
The main waste generating source is the large industrial associations, such as Joint-Stock Company “Bashkir Soda Company”, Management Company TAU “Neft-ekhim”, which includes JSC “Sterlitamak Petrochemical Plant” and JSC “Syntez-Kauchuk”, Federal State-owned Enterprise “Avangard” and other low-tonnage production.
As a result, emissions contain 24 types of solid substances (6023.9 t/g), 51 gaseous substances (5844 t/g). The main environmental pollutants are ethylene, vinyl chloride, dichloroethane, mercury, ammonia and heavy metals. These wastes contain carcinogenic metals such as chromium Cr (VI), copper Cu (II), lead Pb (II), iron Fe (II) and Fe (III), the search for binding methods and the use of which is of interest to the scientific community. When using man-made raw materials in the production of constructional materials in accordance with methodical instructions (MU 2.1.674-97) “Sanitary and hygienic assessment of constructional materials with the addition of Industrial waste” [1], it is necessary that the content of water-soluble forms of heavy metals does not exceed the maximum permissible concentrations for surface waters (MPC) [2], since the impact of aggressive media, mechanical damage and other factors can lead to to violation of the integrity of the product, its design and contribute to the migration of hazardous components from the constructional material.
Therefore, the purpose of the research is a method for obtaining nanostructured complexing agents based on N,N/-bis(piperazinoethyl) ethylenediamine. Currently, one of the effective methods for removing heavy metals from oil sludge is the use of fibrous sorbents based on modified polyacrylonitrile or its copolymers. Sorption of metal nanoparticles in such sorbents occurs due to ion exchange reactions and complex formation of metal ions with the functional groups of the sorbent.
Heavy metals are always present in natural rocks, in addition to the main and secondary components.Their smallest amount is found in carbonate, the largest - in clayey rocks. Industrial waste is more enriched in heavy metals. Exceeding the maximum permissible concentra- tions (MPC) is observed in pyrite cinders, ash, phosphogypsum, mineral sludge, used foundry sands (UFS), etc.
According to the gross content of heavy metals in some industrial wastes of enterprises in the Republic of Bashkortostan, the MPC is exceeded: for lead from 1.3 to 45 times, for copper from 1.2 to 225 times, for zinc from 1.4 to 21 times, and for nickel by 5.7 times. The content of toxic metals, such as lead and chromium, belonging to hazard classes I and II, especially in an ionized state, is dangerous both for human health and the environment.
Complexing agents are also used in various areas of the chemical and petrochemical industries for the purification of various media from heavy metals. The reaction of N,N/-bis(piperazinoethyl)ethylenediamine with monochloroacetic acid yielded new alkyleneaminopolycarboxylic acids, which are of interest as highly effective complexing agents.
At present, compounds containing one or more nitrogen atoms are widely used in alkylation reactions in order to obtain industrially important corrosion inhibitors, complexing agents and surface-active compounds. The presence of metal leads to a greater destabilization of the products of secondary oil refining [3, 4]. Sorbents known in the prior art are characterized by low sorption properties, as well as instability and fragility of sorbent complexes with metal ions. Thus, it can lead to the destruction of the sorbent complexes and the desorption of toxic ions into purified water [5–8]. In addition, sorbents are characterized by low kinetic parameters, and most sorbents have an increased degree of swelling in water, which makes it difficult to use them in water treatment systems [9].
METHODS AND MATERIALS
Piperazine and its derivatives are not among the large-scale products of the organic synthesis industry, but many drugs and biologically active compounds have been created on their basis.
It should be obvious that the topic of discussion right now is the synthesis of new piperazine derivatives, which enables the production of innovative complexing agents with metal nanoparticles.
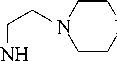
The interaction of N–(β aminoethyl) piperazine (AEP) with dichloroethane (DCE) at a temperature of 80–95оC in the presence of stabilizers (antioxidants) gave N,N/-bis(piperazinoethyl) ethylenediamine (hexamine) dihydrochloride, which was isolated as free base after treatment with an aqueous solution (44–46%) of caustic sodium or potassium with yields of 96.4–97.4% [10].
The reaction between AEP and DCE proceeds smoothly at a moderate temperature in the molar ratio of AEP: DCE: NaOH = 2: 1: 4 in an aqueous medium with the formation of hexamine 1. Screened phenols are used as stabilizers (antioxidants), which are taken in an
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION

^^ NH2
Cl Cl, NaOH
-2NaCl, -2H2O
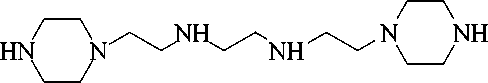
amount of 0.05–1% by weight of AEP and EDC. As a result, the yield of target products in the presence of stabilizers increases, and the process proceeds without technological difficulties.
It should be noted that the interaction of amines with chlorohydrocarbons is accompanied by partial dehydrochlorination of the initial chlorohydrocarbon, in this case by DCE under the action of the AED itself.
Obviously, there is dehydrochlorination of chlorohydrocarbon under the catalytic action of amines, which leads to a decrease in the yield of target products.
ClCH2CH2Cl -HCl CH 2 CHCl
HN N NH 2 HCl --** HN N ^^ NH2*HCl
As a result of these side reactions, the raw materials used to obtain the target product are partially consumed. AEP hydrochloride A becomes inactive, but it also contributes to the dehydrochlorination of DCE. It has been established that screened phenols, including piperazino-phenols, taken as stabilizers (antioxidants) in the preparation of compound 1, suppress the process of DCE dehydrochlorination by 5–12%. 2, 6-di-tert-butylphenol, 2, 6-di-tert-butyl-4-methylphenol (ionol) were used as stabilizers in the reaction of the formation of hexamine.
We have obtained alkylene aminopolycarboxylic acids based on hexamine, which can be used in the technology of basic organic synthesis [11, 12].
Hexamine reacts with MCA (Chloroacetic acid) at a temperature of 80–85оC in a molar ratio of 1:2 for 6 hours with the formation of its hydrochloride carboxymethyl derivatives of hexamine. Treatment of the reaction mixture with an excess of alkali at 20–40оC for 4–6 hours led to its carboxymethyl derivatives 2, 3:
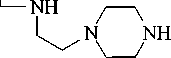
ClCH2COOH
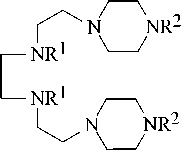
2, 3
R1 = H, R2= CH2COOH (2); R1= R2 = CH2COOH (3).
RESULTS
The effectiveness of compounds 2, 3 as complexing agents for extracting metals from sludge was studied. As an illustrative example, a 10 g sample of oil sludge was taken for testing, which was treated with 100 ml of a 3% solution of compounds 2, 3 for 30 min. The effectiveness of compounds 2, 3 as a complexing agent was determined from the residual content of the metal in the sample after treatment with a complexing agent. Table 1 shows the results of laboratory tests. The well-known complexone Trilon B, which is the disodium salt of ethylenediaminetetraacetic acid, was used as a comparison.
Table 1 shows that compounds 2, 3 show a high effect in the extraction of metals from activated carbon taken as a sample.
Table 2 shows the characteristics of alkyleneaminopolycarboxylic acids. Compounds 1–3 are viscous, high-boiling substances that decompose upon distillation in a vacuum.
The IR spectra of compounds 4–9 are complex. Their IR spectra contain absorption bands at 1590 and 1430 cm–1, typical for the ionized carboxyl group. There are also absorption bands at 1570 and 1550 cm–1, indicating the presence of a free NH group in compounds 1, 2, 4, 6, 10, 12.
IR spectra of di- and tetracyanoethyl derivatives of hexamine 2, 3 have absorption bands at 2230 cm–1char-acteristic of the nitrile (CN) group.
The UV spectra of benzimidazole compounds 10– 15 contain absorption bands with maxima at 247, 277, 284 nm, which are characteristic of the benzimidazole chromophore.
IR spectra were recorded on a UR-20 instrument in a thin layer or suspension in Nujol. UV spectra were taken on a Specord Uvvis instrument for alcohol solutions. 1Н and 13С NMR spectra (JMOD mode) were recorded on a Bruker AM 500 spectrometer (500.13 and 75.47 MHz, respectively) in CDCl3, internal standard Me4Si. The determination of primary, secondary, and tertiary amino groups is carried out by potentiometric titration. First, the mass fraction of total amine nitrogen is determined by potentiometrically titrating a 0.1 M solution of HBr in glacial acetic acid after protection of the amino group with cis-3, 6-endomethylene-1, 2, 3, 6-tetrahydrophthalic anhydride. The tertiary amino group is determined by patentiometric titration with 0.1 M HBr after treatment of the sample with a mixture of acetic acid and acetic anhydride (1:4).
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
Table 1
Extraction of metal salts contained in a sample of oil sludge after treatment with complexones
|
The designation of metal salts in oil sludge |
% metal salt content |
The content of metal salts after treatment with the obtained complexones |
||
|
Compounds, g |
Trilon B, ml |
|||
|
2 |
3 |
|||
|
HgCl2 |
5.40 |
0.0004 |
0.0002 |
0.35 |
|
CuSO4×5H2O |
3.62 |
0.0003 |
0.0001 |
0.6 |
|
ZnCl2 |
3.68 |
0.004 |
0.001 |
0.2 |
|
FeCl3×6 H2O |
2.88 |
0.03 |
0.007 |
0.2 |
|
NiCl2×6 H2O |
3.24 |
0.002 |
0.0006 |
0.4 |
|
6 MnCl2 |
4.28 |
0.003 |
0.0008 |
0.3 |
Table 2
Characteristics of the synthesized compounds
|
Compound |
Found, % |
Molecular-formular |
Computed, % |
IR spectra (ν, сm–1) |
Yield, % |
|
N |
N |
||||
|
1 |
29.23 |
C14H32N6 |
29,57 |
1570(NH) |
96.4 |
|
2 |
21.38 |
C18H36N6O4 |
21,00 |
1570 (NH) отс. (CN) |
80.7 |
|
3 |
15.84 |
C 22 H 40 N 6 O 8 |
16,28 |
отс. (NH) |
81.3 |
N, N1 bis[(piperazinoethyl)]ethylenediamine (1). A solution of 38.7 g (0.3 mol) of AEP in the form of a 65% aqueous solution, 0.48 g (1% weight of AEP and DCE) of ionol (compound 2), and 9.9 g of DCE is dosed at 90°C. The reaction mixture is heated at 90-95°C for 4 hours, then it is cooled and neutralized with a 46% aqueous sodium hydroxide solution. The molar ratio of AEP : DCE : NaOH = 3:1:4. The upper (amine) layer is separated and distilled in vacuum. As a result, there is 27.6 g (97.2%) of the product and composition, wt. %: compound (1) 98.8; DABO 0.9; EDA 0.05; DEET 0.1; unidentified compounds 0.15.
N,N1-[carboxymethyl (piperazinoethyl)ethylenedi-amine (2) . A mixture of 28.4 g (0.1 mol) of hexamine and 18.9 g (0.2 mol) of MCA at 85°C for 4-5 hours, the reaction mass is treated with an excess of 36% aqueous sodium hydroxide solution. Obtained compound С18H36N6O4 (6) yields 33.8 g (84.5%). Found, %: N 20.59. Computed, %: N 21.00.
1Н NMR spectrum (CDCl3, δ, ppm, J /Hz): 2.16-2.40 (m, 2H, NH + 16Н, piperazine), 2.45-2.52 (m, 4Н, С4Н2, С6Н2), 2.55-2.62 (m, 4Н, С11Н2, С14Н2), 2.65-2.70 (m, 4Н, С12Н2, С15Н2,), 4.24-4.40 (t, 4Н, С1Н2, С2Н2, 2 J 6.0, 3 J 11.8), 2.77-2.85 (m, 4Н, С3Н2, С5Н2), 8.33 (s, 2H, C13OOH, C16OOH).
13С NMR spectrum (CDCl3; δ, ppm): 18.45 (C12), 19.00 (C15),45.60 (C8, C8’, C10, C10’,), 48.10 (С1), 48.23 (С2), 49.05 (C11), 49.08 (C14), 52.26 (С4), 52.32 (С6), 53.00 (С3), 53.18 (С5), 45.75 (C7, C7’, C9, C9’), 174.45 174.50 (C13OOH), 174.52 (C16OOH).
N, N, N1, N1-tetracarboxymethyl-N, N1-bis[(pipera-zinoethyl)]ethylenediamine(3). A mixture of 28.4 g (0.1 mol) hexamine and 37.8 g (0.4 mol) MCA in a molar ratio of hexamine: MCA = 1:4 at 80-85 °C for 6 hours under the conditions for obtaining compound (4). As a result, the obtained compound С22H40N6O yields 43.2 g (83.8%). Found,%: N 15.84. Computed,%: 16.27.
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
Table 3
Concentration of heavy metals in aqueous extracts
|
Additives, wt. % of the amount of UFS in concrete |
Concentration of heavy metals in water extracts, mg/l |
|
|
Cr6+ |
Pb2+ |
|
|
Sample 1 0.01% |
< 0.006 |
0.0235 |
|
Sample1 0.03% |
< 0.006 |
0.0188 |
|
Sample 2 0.01% |
< 0.006 |
0.0324 |
|
Sample 2 0.03% |
< 0.006 |
0.0311 |
|
MAC, mg/l |
0.05 |
0.03 |
Note: Sandy concrete composition 1:3, W/C = 0.45.
-
1Н NMR spectrum (CDCl3, δ, ppm, J /Hz): 2.16-2.36 (m,16Н, piperazine), 2.77-2.85 (m, 4Н, С3Н2, С5Н2), 2.50-2.65 (m, 4Н, С4Н2, С6Н2). 3.82-3.85 (d, 4Н, С15Н2, С17Н2, 2 J 15.4), 3.74-3.76 (d, 4Н, С11Н2, С13Н2, 2 J 15.7), 4.24-4.40 (t, 4Н, С1Н2, С2Н2, 2 J 6.0, 3 J 11.8), 9.42 (s, 2H, C12OOH, C14OOH), 9.45 (s. 2H, C16OOH, C18OOH).
13С NMR spectrum (CDCl3; δ, ppm): 16.42 (C12), 16.44 (C14), 18.45 (C18), 19.00 (C21), 45.60 (C8, C8’, C10, C10’,), 48.10 (С1), 48.23 (С2), 49.14 (C11), 49.20 (C13), 52.26 (С4), 52.32 (С6), 53.00 (С3), 53.18 (С5), 55.76 (C11), 55.78 (C13), 56.36 (C15), 56.40 (C17), 174.65 (C12OOH), 174.70 (C14OOH), 176.50 (C16OOH), 176.65 (C18OOH).
DISCUSSION
A novel developed technology that binds heavy carcinogenic metals such as chromium Cr (VI), copper Cu (II), lead Pb (II), iron Fe (II), and Fe (III) into a complex nanostructure capable of creating a stable composition in the mortar composition is N,N-bis(piperazinoethyl) ethylenediamine-based compounds. As a result, the study demonstrates that reacting N,N-bis(piperazinoethyl)eth-ylenediamine with monochloroacetic acid can result in new nanocomplexing agents called cyclic alkyleneaminopolycarboxylic acids.
In experiments, it was discovered that copper, zinc, nickel, cobalt, and iron are reliably blocked in the composition of concrete, and lead and hexavalent chromium are washed out with water; to bind the latter in a concrete solution, the possibility of retaining the washed-out metals with the help of hexamine derivatives was studied.
The study took into account that the proposed nanoadditive should not impair the properties of concrete, ensure the concentration of lead and chromium in aqueous extracts is below the MPC, while binding both lead and hexavalent chromium, be compatible, and also economically affordable.
The concentration of Cr6+ and Pb2+ in water extracts from sandy concrete on UFS after 10 days of exposure using nanostructured complex additives is given in Table 3.
Hexavalent chromium compounds are known not to be present in water extracts from concrete on UFS when sample 1 is added (0.01 weight percent of the UFS mass in concrete). Using sample 2 and values of 0.01 and 0.03 led to the determination of the maximum lead content.
Thus, the most effective method for reducing the concentration of free lead and chromium nanoparticles is sample 1-N, N1-bis[carboxymethyl (piperazinoethyl)] ethylenediamine.
CONCLUSION
The resulting nanostructured additions have binding capabilities that promote high adherence of the heavy metal to the organic substrate and mortar constituents, enabling the provision of a strong composition with operational properties that satisfy technical requirements.
APPLICATION OF NANOMATERIALS AND NANOTECHNOLOGIES IN CONSTRUCTION
Список литературы Development of heavy metal-based nanostructured complex technology for use in building mortar
- Parshall G.W. Intramolecular Aromatic Substitution in Transition Metal Complexes. Accounts of Chemical Research. 1970; 3 (4): 139–144.
- Dehand J., Pfeffer M. Cyclometallated compounds. Coordination Chemistry Reviews. 1976: 18 (3): 327–352.
- Shilov A.E., Shul’pin G.B. Activation of C–H bonds by metal complexes.Chemical Reviews. 1997; 97 (8): 2879–2932.
- Peris, Crabtree R.H. Key factors in pincer ligand design. Chemical Society Reviews. 2018; 47 (6): 1959–1968.
- Selander N., Szabó K.J. Catalysis by palladium pincer complexes. Chemical Reviews. 2011; 111 (3): 2048–2076.
- González-Sebastián L., Morales-Morales D. Cross-coupling reactions catalysed by palladium pincer complexes. A review of recent advances. Journal of Organometallic Chemistry. 2019; 893: 39–51.
- Kumar L.M., Bhat B.R. Cobalt pincer complex catalyzed Suzuki-Miyaura cross coupling-A green approach. Journal of Organometallic Chemistry. 2017; 827: 41–48.
- Albrecht M., Van Koten G. Platinum group organometallics based on “pincer” complexes: Sensors, switches, and catalysts. Angewandte Chemie. International Edition. 2001; 40 (20): 3750–3781.
- Motolko K.S., Price J.S., Emslie D.J., Jenkins H.A., Britten J.F. Zirconium complexes of a rigid, dianionic pincer ligand: Alkyl cations, arene coordination, and ethylene polymerization. Organometallics.2017; 36 (16): 3084–3093.
- Obligacion J.V., Chirik P.J. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nature Reviews Chemistry. 2018; Vol. 2 (5): 15–34.
- MU 2.1.674–97. Sanitarno-gigienicheskaya ocenka strojmaterialov s dobavleniem promotkhodoV. M.: Minzdrav Rossii; 1997.
- SaNPIN 2.1.3684-21. Sanitarno-ehpidemiologicheskie trebovaniya k soderzhaniyu territorij gorodskikh i sel’skikh poselenij, k vodnym ob”ektam, pit’evoj vode i pit’evomu vodosnabzheniyu, atmosfernomu vozdukhu, pochvam, zhilym pomeshcheniyam, ehkspluatacii proizvodstvennykh, obshchestvennykh pomeshchenij, organizacii i provedeniyu sanitarno-protivoehpidemicheskikh (profilakticheskikh) meropriyatij. Moscow: As amended on February 14; 2022.
- Shadrunova I.V., Mineeva I.A., Shadrunov V.A. Rol’ i mekhanizm dejstviya organicheskikh kompleksoobrazovatelej pri sernokislotnom vyshchelachivanii mednykh i medno-cinkovykh rud: Tezisy dokladov Mezhdunarodnoj nauchnotekhnicheskoj konferencii. Magnitogorsk; 2001.
- Beka M., Nad’pal I. Issledovanie kompleksoobrazovaniya novejshimi metodami. M.: Mir; 1989.
- B’errum Y.A. Obrazovanie amminov metallov v vodnom rastvore. M.: Izd. inost. Literatury; 1961.
- Koordinacionnaya khimiya redkozemel’nykh ehlementov. Metod. posobie pod red. Spicyna V.I., Martynenko J.I.I. M.: Izd. MGU; 1974.
- Fidelis I., Siekierski S. On the regularities or tetrad effect in complex formation by f-electron elements. A doubledouble effect. J. Inorg. Nucl. Chem. 1971; V33: 3191–3194.
- Nugent L.J. Theory of the tetrad effect in the lanthanide (III) and actinide (III) series. J. Inorg. Nucl. Chem. 1970; V32: 3485-3490.
- Peppard D.F., Mason G.W., Lewis S. A tetrad effect in the liquid-liquid extraction ordering of lanthanide (III). J. Inorg. Nucl. Chem. 1969; V31: 2271–2272.
- B’errum Y.A. Obrazovanie amminov metallov v vodnom rastvore. M.: Izd. inost. Literatury; 1961.
- Koordinacionnaya khimiya redkozemel’nykh ehlementov. Metod. posobie. red. Spicyna V.I., Martynenko J.I.I. M.: Izd. MGU; 1974.


