Development of the basilar artery system in the human embryonic period
Автор: Gorbunov A.V., Kalugina M.G.
Журнал: Cardiometry @cardiometry
Рубрика: Original research
Статья в выпуске: 27, 2023 года.
Бесплатный доступ
The doctrine of the origin and development of cerebral arteries in the embryonic period of man in the XIX - XXI centuries was formed by I. Tandler (1899, 1902), H. Evans (1911), G.L. Streeter (1908, 1918), G.D. Aronovich (1939), D.N. Padget (1947 - 1972), N.V. Popova-Latkina (1954 - 1975), Y.N. Shapovalov (1963), A.G. Knorre (1967), I. Stanek (1977), B. Carlson (1983), A.V. Gorbunov (1998 - 2022), etc. To date, we have shown that basilar arteries are detected in embryos at 7 weeks of intrauterine development, posterior cerebral arteries with plexiform branching - at 8 weeks of intrauterine life, and persistent trigeminal arteries continue to be detected in prenatal embryos at 8-9 weeks of human prenatal ontogenesis. In prenatal embryos 7-8-9 weeks of prenatal ontogenesis, the basilar artery system provides the needs of the human intrauterine brain.
Basilar artery, embryo, pre-fetus
Короткий адрес: https://sciup.org/148326609
IDR: 148326609 | DOI: 10.18137/cardiometry.2023.27.142149
Текст научной статьи Development of the basilar artery system in the human embryonic period
Alexey V. Gorbunov, Maria G. Kalugina. Development of the basilar artery system in the human embryonic period. Cardiometry; Issue No. 27; May 2023; p. 142-149; DOI: 10.18137/cardiome-try.2023.27.142149; Available from: issues/no27-may-2023/development-basilar-artery-system
The ideas of H. Evans (1911), I. Tandler (1899, 1902), G.L. Streeter (1908, 1918), A.A. Zavarzin (1935) about the embryogenesis of the arterial bed of the brain are most successfully developed in the work of D.N. Padget (1947), where 8 stages of the development of the vascular system of the brain are postulated (correspond to the first 52 days of the prenatal period) 142 | Cardiometry | Issue 27. May 2023
[1 – 16, 25]. The author notes in stage 1 (24 days of intrauterine development (ID)) the plexiform structure of the primitive internal carotid arteries (ICA) in the oral sections and their recoil in the caudal direction of the primitive (persistent) trigeminal arteries (PTA); in stage 2 (28 days), paired longitudinal neural arteries arise-precursors of the basilar artery (BA), connected to the ICA by PTA; in stage 3 (29 days), a posterior connective artery (PCoA) forms from the caudal end of the ICA; in stage 4 (32 days), the main branches of the ICA are outlined and vertebral arteries (VA) are formed, BA is more often irregular; in stage 5 (35 days), further differentiation of cerebral vessels occurs, the posterior cerebral artery (PCA) begins to form from the PCoA , the upper cerebellar and lower anterior cerebellar arteries are outlined from the BA, and the posterior lower cerebellar artery from the PA; in stage 6 (40 days), the arterial circle of the large brain (ACLB) is visible, there are PA, anterior cerebral arteries (ACA) and middle cerebral arteries (MCA); in stage 7 (44 days), the formation of ACLB is completed, PTAs are reduced; in stage 8 (52 days), ACLB is already clearly visible, the trunks of all cerebral arteries are built according to the adult type. Thus, the formation of ACLB ends by the first 2 months of intrauterine development. In the following work, D.N. Padget (1948) reports that by the end of the 7th week of a person’s intrauterine life, ICA, BA, ACA, MCA, PCA and their branches of 1-2 orders are distinguished [17].
The next stage of studying the embryology of the cerebral arteries was to clarify the origin of arterial highways. It has been established that ICA originates either from the third pair of gill arches [1, 9], or from the third pair of gill arches and the anterior end of the dorsal aorta [5], or from the third pair of gill arteries connecting the ascending and descending aortic trunks [2, 7]. VA arise from the first six paired segmental arteries of the cervical region, originating from the dorsal aorta, and in embryos of 9-10 mm in length, basilar vessels penetrate the ventral fold of the cerebral vesicles together with the soft meninges [7, 9, 10]. The vessels of the arterial circle assume a normal position only in the 2nd half of the prenatal period [10]. Already at 2 months of ID, both ICA with ACA and MCA departing from them, as well as VA, BA, PCA and PCoA were noted [4]. At the early stages of embryogenesis, the nutrition of the entire brain comes from the ICA [8]. The anastomotic union of the arterial bed of the brain causes the emergence of new opportunities for the development of mechanisms of regulation of cerebral circulation and marks a qualitatively new stage in its organogenesis. Thus, the ACLB formed by the 9th week of intrauterine development coincides with the general formation of the cardiovascular system.
The arteries directly connecting the ICA with the BA are generally recognized in the human embryo – carotid-basilar anastomoses. The primitive trigeminal artery is more common than others (0.1–0.3%) – it connects the cavernous part of the ICA with the precursor arteries of the BA [6, 18, 19]. PTA was detected at an embryo length of 3 mm; in an embryo of 14 mm, it is obliterated [6].
The above ideas about the processes of formation of the arterial bed of the brain are summarized in the manuals “Human Embryology” [7], “Fundamentals of Embryology by Patten” [9], “Embryology” [19].
However, all the above studies indicate insufficient knowledge of the embryogenesis of the human arterial bed and the well-known schematicity of the fundamental guidelines on human embryology. There is also no systematic data on the development of apteria of the human brain in the light of the ever-increasing need to study the formation of clinically significant variants, anomalies and malformations of the arteries of the brain.
Material and Method
Taking into account the above, we conducted a study of the arteries of the human brain of 236 sections of 11 series of human embryos from 6 to 27 mm crownrump length (CRL) obtained as a result of artificial abortions from practically healthy women, obtained from the pathology bureau and the Bureau of Forensic Medical examination. The age of the embryos was determined taking into account the woman’s pregnancy history, the results of a clinical diagnostic examination and the results of comparisons of measurements of parietal-coccygeal, occipital-coccygeal and total length with tables and diagrams of manuals and summaries presented by F. Mall (1898), G. Streeter (1921, 1945), A. Schults (1923), L. Barth (1951), N.V. Popova–Lat-kina (1956), G.A. Schmidt (1957), B.M. Patten (1959), G. Oliver (1962), B.P. Khvatov (1969), A.G. Knorre (1971), Y.N. Shapovalov (1974), L.I. Falin (1976), O’Rahilly (1979), A.S. Leontyuk (1981) [1 – 3, 5, 8 – 15, 20].
Histological methods and techniques of staining sections made from embryos and pre-fetuses were used in the work. The embryos were fixed in a mixture of 50% alcohol with a 5% solution of formalin and Carnois liquid consisting of 6 parts of absolute ethanol, 3 parts of chloroform, 1 part of glacial acetic acid. The material was fixed in Carnois liquid for 4 hours at a temperature of 25° C, after which the material was transferred to absolute ethanol. For better circulation of ethanol, glass wool was placed on the bottom of the vessel. Next, dehydration was carried out through a series of alcohols of various concentrations. To fill the material, paraffin was used, in which 5% purified beeswax and a few drops of turpentine were added for plasticity. To get rid of gas impurities, paraffin was heated in a thermostat at a temperature of 70ºC, in open cups for several days. The material was filled with paraffin according to Levinson’s scheme (1957). The slices were obtained using a microtome. Further, the obtained sections were dewaxed in xylene and benzene, after which they were passed through alcohols in descending concentration and finished in distilled water. The preparations of the series of sections were stained with hematoxylin and eosin.
Thus, a total of 8 series of sagittal and 3 series of frontal sections of human embryos from 6 to 27 mm CRL were studied. On a series of sections, the rudiments of the departments of the nervous system were studied in connection with the embryonal status of the arteries feeding the brain. The diameters of the arteries, their length, thickness, and the area of branching in the embryonal status of the brain were measured. Morphometry was performed using an eyepiece micrometer.
The required amount of material, as well as a sufficient number of measurements of morphometric parameters in the sample, was determined taking into account the recommendations set out in the works of G.S. Katinas [21, 22]. When planning this study, the following constants were taken: confidence interval (D) = 10% [23], significance level (P) ≤ 0.05, probability of error-free prediction ≥95% [24]. All the obtained quantitative parameters were subjected to variational and statistical processing with the calculation of their average value, its error, the mean square deviation, the coefficient of variation, the coefficient of confidence of the difference in average values, the probability of error according to the Student’s distribution, the correlation coefficients and its reliability [20].
Results
Below we present the original data of our own studies of the structural transformations of the arteries of the human brain in the embryonic period of human prenatal ontogenesis.
In embryos of 6 mm crown-rump length (CRL) (5 weeks of intrauterine development), posterior, middle and anterior cerebral bladders are detected. Single arterial deposits in the form of inclusions were found in the thin walls and cavities of the cerebral bladders. The area of these inclusions was 94.2 ± 7.54 mm2 (Fig. 1, Tab. 1).
In embryos of 9 mm CRL (6 weeks of intrauterine development (ID)), all brain bladders are clearly expressed, embryonal status of the terminal brain and the midbrain are revealed. Embryonal status of future vessels in the cavities and walls of brain bladders are revealed in the form of inclusions. Moreover, in the embryonal status of the posterior and middle cerebral bladders, as well as the embryonal status of the posterior brain, embryonal status of future arteries in the form of plexiform elements are determined. The area of inclusions and plexiform elements was 136.7 ± 10.93 mm2. (Tab. 1).
Table 1
Morphometry of the cerebral arteries of human embryos.
|
Parameter Age |
Area of embryonal arteries, μm2 |
Diameter of arteries, μm |
length of arteries, μm |
|
CRL 6 mm, 5 week |
94,2±7,54 |
– |
– |
|
CRL 9 mm, 6 week |
136,7±10,93 |
– |
– |
|
GR,% |
45,1 |
– |
– |
|
CRL 15 mm, 7 week |
241,8±21,76 |
– |
– |
|
GR,% |
76,9 |
– |
– |
|
CRL 21 mm, 7-8 week |
419,6±33,57 |
17,3±1,38 |
86,8±6,94 |
|
GR,% |
73,5 |
– |
– |
|
CRL 27 mm, 8 week |
1159,5±127,54 |
19,5±1,74 |
108,5±9,87 |
|
GR,% |
176,6 |
12,7 |
25,1 |
*CRL – crown-rump length
*GR – growth rate
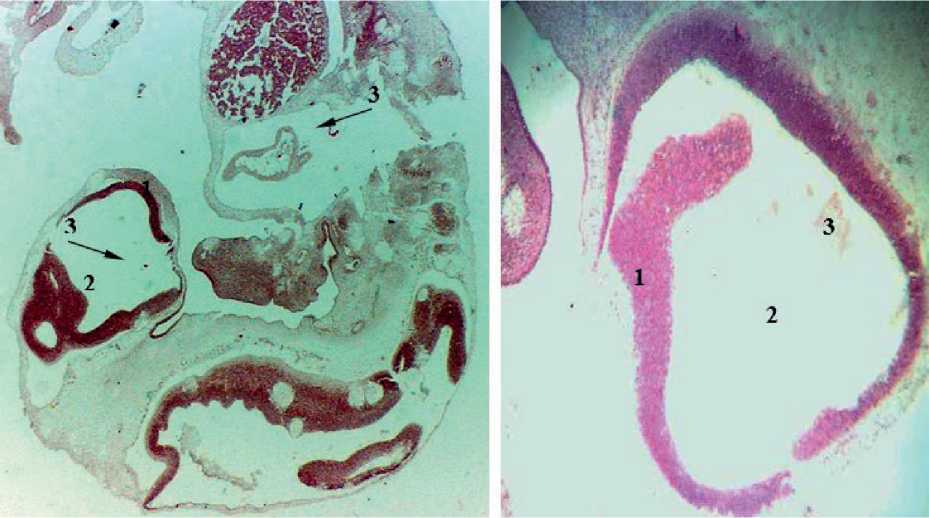
Figure. 1. Sagittal section of the embryo 5 weeks of intrauterine development: 1 – the wall of the cerebral bladder; 2 – the cavity of the cerebral bladder; 3 – arterial bookmarks in the form of inclusions.
In embryos of 15 mm CRL (7 weeks of ID), the embryonal status of the terminal brain, midbrain, posterior brain, future medulla oblongata and spinal cord are clearly revealed. In the embryonal status of the brain substance, the embryonal status of future arteries are more clearly identified. The arterial network is determined in the subarachnoid space tab. In the middle and underlying parts of the brain, the embryonal status of future arteries in the form of plexuses are more clearly distinguished. The area of arterial embryonal status was 241.8 ± 21.76 mm2. Caudally from the brain embryonal status, an accumulation of shaped elements in the lumen of the artery is revealed (Fig. 2, Tab. 1).
In embryos of 21 mm CRL (7-8 weeks of ID), the terminal brain, intermediate brain, midbrain, posterior brain and spinal cord are clearly identified. The embryonal status of the terminal brain contains a cavity of an irregular elongated shape, whereas in the embryonal status of the underlying parts of the brain, cavity formations have not been identified. In these parts of the brain, the embryonal status of future arteries and arterial plexuses are determined. Moreover, the embryonal status of the arterial plexuses in the terminal brain are less clear. The area of arterial formations was 419.6 ± 33.57 mm2. 1-2 large arteries with a length of 86.8 ± 6.94 microns and a diameter of 17.3 ± 1.38 microns are detected in the tabs of the posterior and spinal cord (Fig. 3, Tab. 1).
In embryos of 27 mm CRL (8 weeks of ID), the embryonal status of the terminal brain contains an irregularly elongated cavity. In the cavities and walls (there are 3 of them) of the embryonal status of the terminal brain, arterial embryonal status are found, sometimes an artery embryonal status with elements of plexiformity is clearly identified. The cavity of the terminal brain intimately adheres to the underlying departments and the arterial system is laid from them. Moreover, the embryonal status of this plexus is formed from the embryonal status of the arteries lying between the embryonal status of the terminal brain and the underlying parts of the brain, where
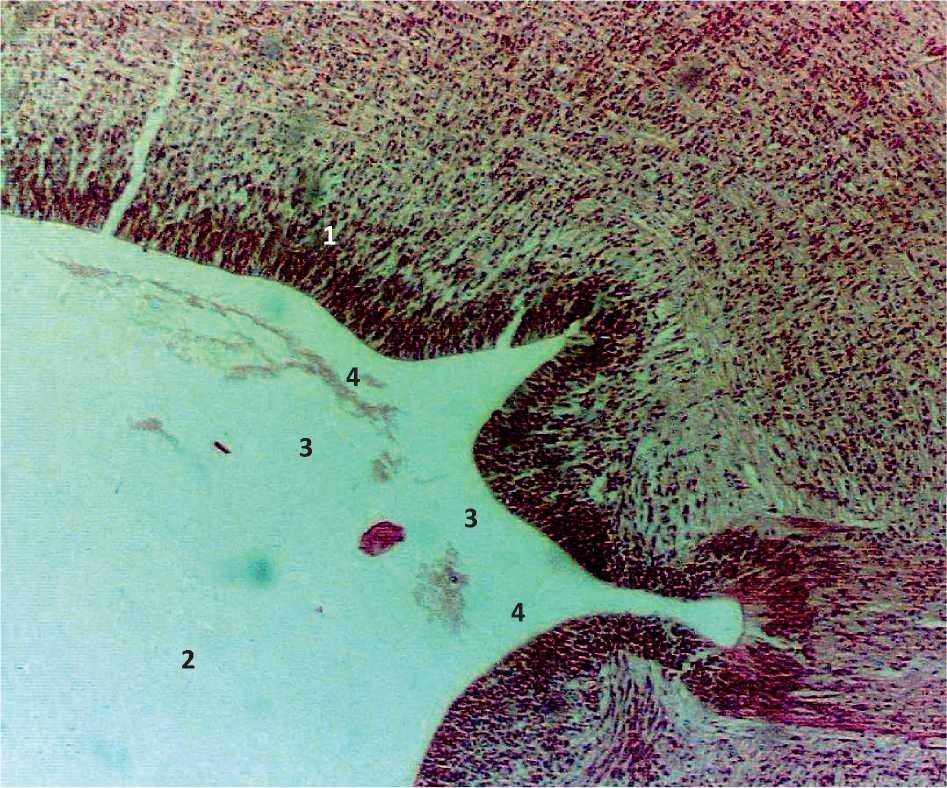
Figure. 2. Sagittal section of the embryo 7 weeks of intrauterine development: 1 – the wall of the cerebral bladder; 2 – the cavity of the cerebral bladder; 3 – artery bookmarks; 4 – the bookmark of the arterial plexus.
the embryonal status of the arteries are also revealed. The area of arterial formations was 1159.5 ± 127.54 microns, the length of the arteries was 108.5 ± 9.87 microns, the diameter was 19.5 ± 1.74 microns. In the caudo-ventral part of the posterior brain, a cavity with 2 walls is revealed, which has artery embryonal status in its lumen (Fig. 4, 5, Tab. 1).
Embryos of 7 weeks of ID have BA embryonal status. Embryos of 7-8 weeks of ID have PTA embryonal status. Embryos of 8 weeks of ID have BA embryonal status with plexiform branching and PCA embryonal status with plexiform branching. Morphometry revealed a linear increase in all parameter values in direct proportion to age. However, the area of the arterial bed in embryos from 7-8 to 8 weeks and the length of the arteries of embryos from 8-9 weeks in the body grow abruptly (Tab 1, Fig. 6 – 8).
Discussion and Conclusions
Thus, prior to our study, the representations were as follows. By the 4th week of intrauterine development, paired primary basilar arteries are formed, connected to the internal carotid arteries by persistent trigemi- nal arteries. At 4-5 weeks of intrauterine development, vertebral arteries are formed dorsally from the dorsal aorta from the cervical branches of the intersegmental arteries and participate in the formation of an integral basilar artery. At 5-8 weeks of intrauterine development, anastomoses are planned through the posterior connecting arteries with the primary branches of the internal carotid arteries, forming the arterial circle of the large brain. At 5-8 weeks of intrauterine development, the upper cerebellar and lower anterior cerebellar arteries are planned from the basilar arteries, and the posterior lower cerebellar arteries from the vertebral arteries. At the age of 6-7 weeks of intrauterine development, persistent trigeminal arteries are not detected.
Our research materials show that the basilar artery is detected in embryos at 7 weeks of intrauterine development, persistent trigeminal arteries are detected at 7-8 weeks of intrauterine development and posterior cerebral arteries with plexiform branching and basilar artery with plexiform branching are detected at 8 weeks of intrauterine development. In embryos of 7-8 weeks of prenatal human ontogenesis, arteries prob-
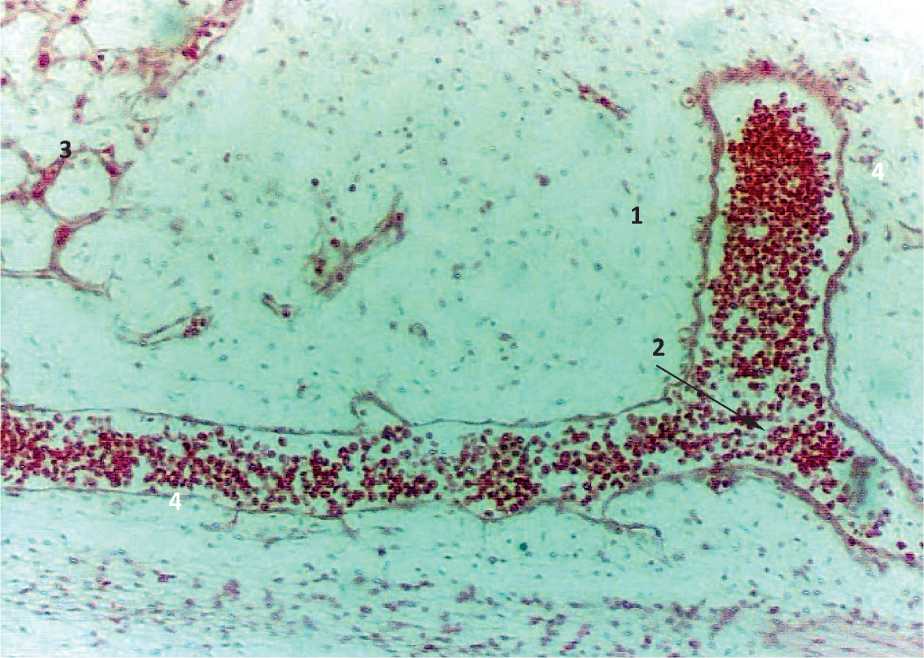
Figure. 3. Sagittal section of the embryo 7-8 weeks of intrauterine development: 1 – laying of the posterior brain; 2 – bifurcation of the bookmarks of large arteries; 3 – laying of the arterial plexus; 4 – accumulation of red blood cells.
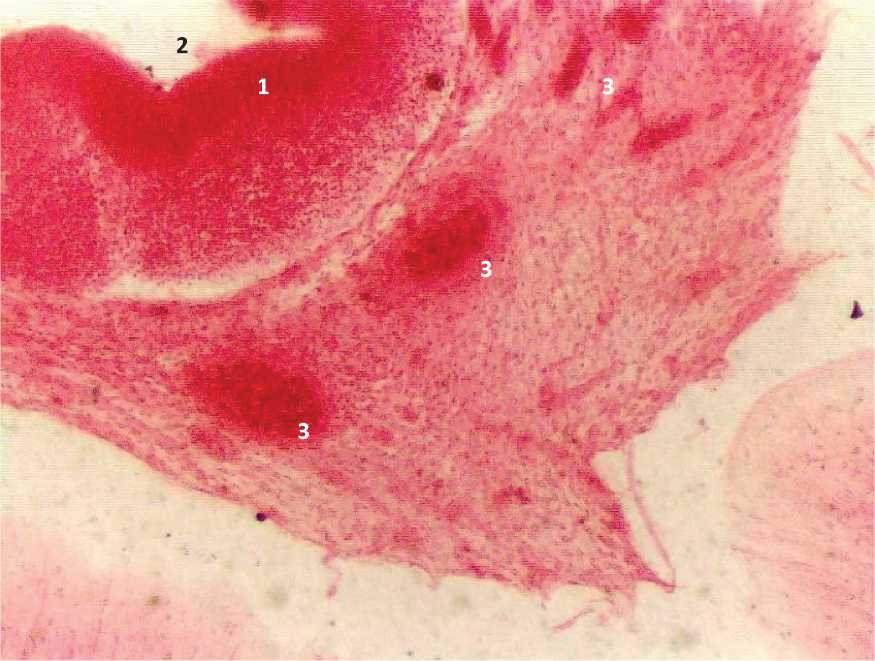
Figure. 4. Sagittal section of the embryo 8 weeks of intrauterine development: 1 – the wall of the terminal brain; 2 – the cavity of the terminal brain; 3 – artery bookmarks.
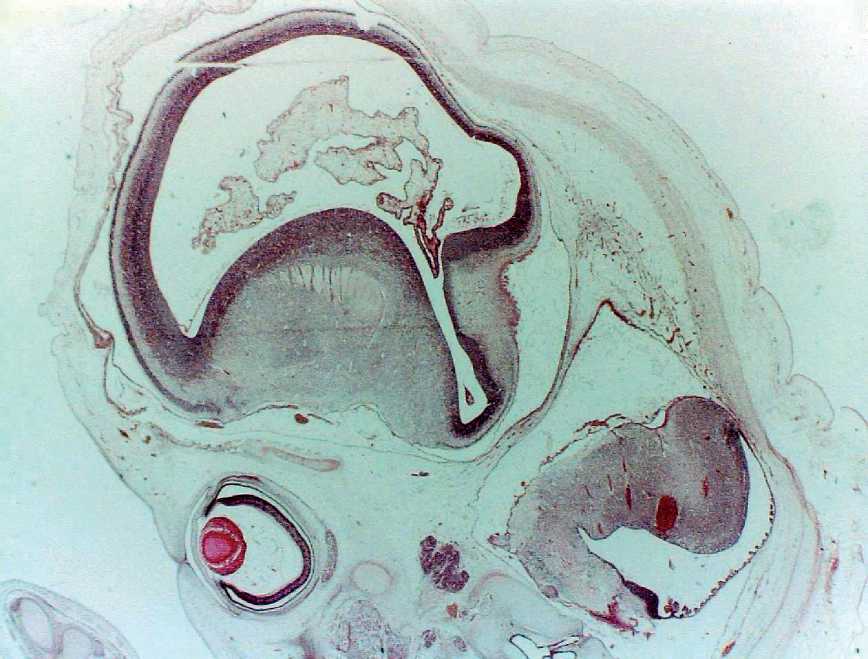
Figure. 5. Sagittal section of the embryo-pre-fetus 8-9 weeks of intrauterine development: 1 – the wall of the terminal brain; 2 – the cavity of the terminal brain; 3 – plexiform division of artery bookmarks; 4 – elements of fetal division of artery bookmarks; 5 – the bookmark of the eyeglass; 6 – the bookmark of the Sylvian aqueduct.
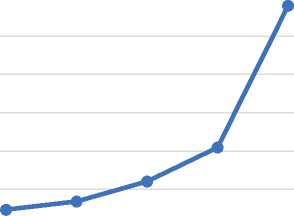
6 mm, 9 mm, 15 mm, 21 mm, 27 mm,
5 week 6 week 7 week 7-8 week 8 week
Age (crown-rump length, week of intrauterine development)
Figure 6. Changing the area of the arteries of the brain of human embryos.
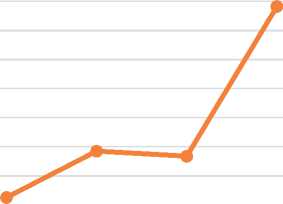
9 mm, 15 mm, 21 mm, 27 mm,
6 week 7 week 7-8 week 8 week
Figure 7. The rate of growth of the arteries of the brain of human embryos.

21 mm, 7-8 week
27 mm, 8 week diameter of arteries length of arteries
Figure 8. Increasing the length of the diameter of the arteries of the brain of human embryos.
ably vascularize the brain sufficiently for age-related needs, and in prenatal embryos of 8-9 weeks of prenatal human ontogenesis, the arterial bed of the brain probably provides the needs of the intrauterine brain, Persistent trigeminal arteries continue to be detected in prenatal embryos of 8-9 weeks of prenatal human ontogenesis.
Список литературы Development of the basilar artery system in the human embryonic period
- Paget DH. Willis Circle. This is embryology and anatomy. Intracranial artery aneurysms / Edited by W.E. Dandy. – Comstock Co. – Ithaca, New York, 1947. – Vol. 1. – pp. 207-259.
- Popova-Latkina N.V. Development of organs in the embryonic period in humans: Dis. ... Doctor of Medical Sciences. – Astrakhan, 1956. – 537 p.
- Popova-Latkina N.V. On the mechanisms of the occurrence of congenital anomalies and deformities. Dokl. USSR Academy of Sciences. 1965;161(2):493-6. [in Russian]
- Aronovich GD. Morphogenesis of cerebral arteries in human fetuses and newborns. – L.: Medgiz, 1939. – 40 p. [in Russian]
- Knorre AG. A brief outline of human embryology. – L.: Medicine, 1967. – 268 p. [in Russian]
- Luzha D. X-ray anatomy of the vascular system. – Budapest: Publishing House of the Hungarian Academy of Sciences, 1973. – 379 p.
- Stanek I. Human embryology. – Bratislava: Publishing House of the Slovak Academy of Sciences, 1977. – 474 p.
- Belenkaya R.M. Stroke and variants of brain arteries. – M.: Medicine, 1979. – 176 p. [in Russian]
- Carlson B. Fundamentals of embryology by Patten. – M.: Mir, 1983. – 224 p. [in Russian]
- Popova-Latkina NV. Analysis of topographic and anatomical correlations between the brain and spinal cord, skull and spine, their interaction and mutual influence on each other in human embryogenesis. Functional and structural foundations of systemic activity and mechanisms of brain plasticity: Collection of scientific tr. – M.: USSR Academy of Medical Sciences. 1975; IV:278-282.
- Gorbunov AV. Formation and variants of the topography of the internal carotid artery at the stages of prenatal and postnatal human ontogenesis: Dis. ... Candidate of Medical Sciences / Astrakhan State Medical Academy. – Astrakhan, 1998. – 173 p.
- Gorbunov A.V. Morphogenesis of cerebral arteries and its experimental and clinical significance: Dis. ... Doctor of Medical Sciences. Astrakhan State Medical Academy. Astrakhan, 2007. 287 p.
- Gorbunov AV, Moldavskaya AA. Morphogenesis and variants of the development of the arteries of the human brain. Moscow-Astrakhan-Tambov, Center-press, 2008. 216 p.
- Gorbunov, Alexey Viktorovich. Variants of the development of the arteries of the human brain and cerebrovascular disorders [Text] / A.V. Gorbunov ; M-in internal affairs of the Russian Federation. Federation, GOU VPO Moscow. un-t, Tambov. phil. – Tambov : [Publishing house -Vopershina R. V.], 2009. – 309 p. : ill.- Bibliogr.: pp. 259-306 (760 titles). – 300 copies. – ISBN 978-5-91253-242-9.
- Evolution of the arteries of the human brain [Electronic resource] : monograph / A.V. Gorbunov. – Tambov: Publishing Center FGBOU VO “TSTU”, 2022. – 1 electron. opt. disk (CD). – System requirements : PC not lower than Pentium II class; CD-ROM drive; 00.0 MB ; RAM ; Windows 95/98/XP ; mouse. [in Russian]
- Paget D.H. Development of cranial arteries in the human embryo // Continuation. The embryo. Carn. In-t, 1948. – Vol. 32. – publ. 212. – pp. 205-261.
- Wismer G.L. Variant of the Willis circle, analogous to the primitive trigeminal artery of the fetal type // Neuroradiology. – 1989. – Volume 31(4). – pp. 366-368.
- Salas E., Zial I.M., Sekhar L.N., Wright D.K. Persistent trigeminal artery: anatomical study // Neurosurgery. – 1998 September. – Volume 43(3). – pp. 557-561.
- Lippincott Williams and Wilkins H.yu. Embryology. 2001.
- Shapovalov Yu.N. Development of the human embryo during the first two months: Dis. ... Doctor of Medical Sciences / Crimean GMI. – Simferopol, 1963. – 502 p.
- Katinas G.S., Bulgak V.I., Nikiforova E.N., Svetikova K.M. On finding the standard error of the mean taking into account the variability of the trait within the organism. Arch. anat., histol. and embryol.1969. – No. 9. – pp. 97-104.
- Katinas G.S., Bykov V.L. Method of natural periodization of processes. Arch. anat. – 1976. – Vol. 71, issue 9. – pp. 98-103. [in Russian]
- Avtandilov G.G. Medical morphometry. – M.: Medicine, 1990. – 393 p. [in Russian]
- Lakin G.F. Biometrics. – M.: Higher School, 1968. – 284 p. [in Russian]
- A.R. Tsantili, V. Karampelias, A. Samolis et al., Anatomical variations of human vertebral and basilar arteries: current literature review, Morphology, https://doi.org/10.1016/j.morpho.2022.07.001


