Early and long-term outcomes of liver resections: a single specialized center experience
Автор: Chichevatov D.A., Kalentjev V.V., Glukhov A.E., Seliverstova O.M., Rodina G.A., Tsyganova M.V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Опыт работы онкологических учреждений
Статья в выпуске: 3 т.22, 2023 года.
Бесплатный доступ
The purpose of the study was to evaluate the outcomes of a series of liver resections performed in a single regional specialized cancer center. Material and Methods. Eighty-nine patients underwent liver and/ or extrahepatic bile duct resections in the Penza Regional Oncology Hospital over the 8-year study period. Malignancies were observed in 81 patients. Extended liver resections (4 segments or more) were performed in 58 (65.2 %) cases. Results. Postsurgical morbidity and mortality rates were 31.5 % (28 of 89) and 6.7 % (6 of 89), respectively. Six of 10 patients with primary liver carcinomas were alive without evidence of disease progression at a follow-up time ranged from 1.0 to 76.7 months. Adjuvant chemotherapy (ACT) was the only predictor (HR=0.40; 95 % CI 0.16-0.98) of overall survival in patients with metastatic colorectal cancer (mCRC). The median survival time after liver resections for mCRC with or without ACT was 54.5 (95 % CI: 14.5-94.5) vs 21.8 months (95 % CI: 14.2-29.4), respectively. In mCRC patients with ACT, the 5-year overall survival rate was 44.8 ± 12.9 %. Conclusion. Primary hepatobiliary carcinomas and colorectal cancer liver metastases are the most common reasons for liver resections. A series of liver resections in a low-volume hospital is feasible with the achievement of good outcomes.
Liver resection, malignant tumor, colorectal carcinoma, metastases
Короткий адрес: https://sciup.org/140300185
IDR: 140300185 | УДК: 616.36-089.87 | DOI: 10.21294/1814-4861-2023-22-3-90-98
Текст научной статьи Early and long-term outcomes of liver resections: a single specialized center experience
Up to date clinical indications for liver resections are multiple. However, colorectal cancer metastases, primary tumors of the liver and extrahepatic bile ducts are the most frequent reasons for liver resections [1, 2]. Whilst liver resection remains the most efficient curative procedure, it has not become an easily reproduced one. Predominantly it is employed in high-volume centers [3, 4].
The latter is explained by various circumstances relevant to liver resections. Such points as personal experience [5], the anesthesia protocol [6], and complexity of the future liver remnant (FLR) estimation taking account of possible comorbidity and chemotherapy application are critical [7–10]. All these requirements are considered to be more feasible in a high-volume center, which provides high-quality medical care associated with low postsurgical mortality [11]. However, this remains a controversial point [3, 11, 12]. Accumulation of specific patients in special centers is warranted while incidence of malignant pathology is extremely low. As per liver resection such an approach may be not appropriate, because the rate of liver surgical procedures is high and steadily increases.
The objective of the study is the assessment of outcomes after multiple liver resections in a single low-volume regional oncology center.
Material and Methods
Eighty-nine patients underwent liver and/or extrahepatic bile ducts (EBD) resection in the Penza Regional Oncology Center from 2014 to 2021. Patients with the liver surgical extent less than one segment (marginal resections) were excluded. There were 44 (49.4 %) men and 45 (50.6 %) women aged 28 to 81, the mean age being 61.8 ± 8.8.
Nosological forms are presented in Table 1. Benign liver pathologies were as diverse as the focal nodular hyperplasia (n=3), the hemangioma (n=2), the cystic lesion (n=1), the abscess (n=1), and the parasitic disease (n=1). Metastases of the pancreatic carcinomas (2), the pancreatic neuroendocrine tumor (NET) (n=1), the kidney cancer (n=3), retroperitoneal sarcomas (n=2), the ovarian cancer (n=1), the stomach gastrointestinal tumor (GIST) (n=1), the not otherwise specified (NOS) metastasis (n=1), and the liver fibrosarcoma (n=1) constitute the group of other tumors. Fifty-two (58.4 %) patients were operated for metastases of colorectal carcinomas.
Eighty-one of 89 patients underwent surgery for malignant tumors. Morphological patterns are presented in Table 2. Seventy-eight epithelial tumors were staged in accordance with TNM (8thedition) classification (Table 3). There was the following stage distribution: I – 5 (6.4 %), II – 27 (34.6 %), III – 22 (28.2 %), and IV – 24 (30.8 %). Nineteen of 52 (36.5 %) mCRC patients were operated on synchronous distant metastases. Besides, one patient with HCC, one with hilar cholangiocarcinoma, two with pancreatic carcinomas, and one with carcinoma not otherwise specified have synchronous М1 as well. Three patients with nonstaged sarcomas underwent surgery for metachronous distant metastases.
We assessed FLR employing the CT Canon Aquil-ion One with the Liver Resection Planning package. Surgical extents are shown in Table 4. Anatomical portal liver resection was performed in 69 (77.5 %) of 89 patients. There were 30 (33.7 %) primary liver resections. In 59 (66.3 %) cases a liver surgical procedure was a subsequent step of treatment. None of the patients with mCRC were operated simultaneously.
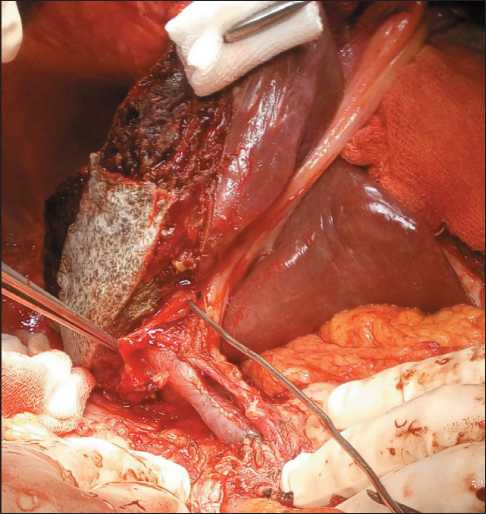
Fig. 1. Right-sided hemihepatectomy with resection of extrahepatic bile ducts. The probe is inserted into the left bile duct Рис. 1. Правосторонняя гемигепатэктомия с резекцией внепеченочных желчных путей. Зонд введен в левый желчный проток
surgical extentsОбъемы резекций печени
|
Surgical procedure/Операция |
Frequency/Частота |
Percentage/Процент |
|
Resection of EBD/Резекция внепеченочных желчных протоков (РВЖП) |
3 |
3.4 |
|
Mesohepatectomy/Мезогепатэктомия |
4 |
4.5 |
|
ALPPS* |
6 |
6.7 |
|
Fissural segmentectomy/Атипичная резекция |
7 |
7.9 |
|
Trisectionectomy/Трисекционэктомия |
12 |
13.5 |
|
Anatomical segmentectomy/Анатомическая резекция |
21 |
23.6 |
|
Hemihepatectomy/Гемигепатэктомия |
36 |
40.4 |
|
Total/Всего |
89 |
100.0 |
* – Associated Liver Partition with Portal Vein Ligation for Staged Hepatectomy table 5/Таблица 5
postsurgical complicationsПослеоперационные осложнения
individual lifetime in small groups of cases after liver resectionИндивидуальная продолжительность жизни после резекций печени в малых группах наблюдений
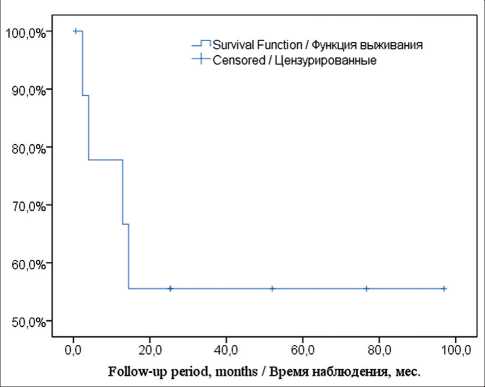
Fig. 2. The Kaplan–Meier curve of OS in the group of primary liver tumors
Рис. 2. Кривая общей выживаемости по методу Каплан– Мейера у больных с первичными опухолями печени
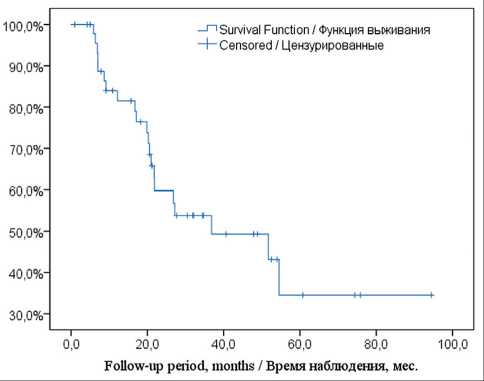
Fig. 3. The Kaplan–Meier curve of OS in the group of mCRC Рис. 3. Кривая общей выживаемости по методу Каплан– Мейера у больных метастатическим колоректальным раком
Statistics 23 software. Such notifications as X ± m must be regarded as a mean ± SE for survival and a mean ± SD for other descriptive statistics.
Results
From 10 to 17 (11 at the mean) liver resections were performed in our center annually. Post-surgical complications were registered in 28 (31.5 %) patients. These complications are presented in Table 5. Six (6.7 %) of 89 patients died. Necrosis of the hepatic-choledoch duct after standard hepatectomy and hepaticojejunostomy insufficiency after left bile duct resection that resulted in bile peritonitis were causes of death in two patients. Two patients died due to liver failure after the ALPPS procedure (n=1) and right-sided trisectionectomy for the long-lasting hilar CC complicated with obstructive jaundice and cholangitis (n=1). One case of peritonitis appeared as a result of stomach ulcer perforation. The patient underwent a series of second laparotomies, but the complication was not discharged. Mesenteric ischemia was a sequel of persistent atherosclerosis. Four died patients underwent surgery for mCRC, one for HCC, and one for hilar CC.
Long-term outcomes were assessed in the other 83 patients. Survival in small groups (less than 10) of patients is shown in Table 6. Kaplan-Meier survival was estimated in groups of primary liver carcinomas (n=10) and mCRC (n=48). The curve of OS in the group of primary carcinomas is presented in Fig. 2. Four of 10 patients with HCC/LCC died in the terms from 2.4 to 14.4 months. Six patients were alive without tumor recurrence at the follow-up time ranged from 1.0 to 76.7 months. The median follow-up time was 52.0 months.
The curve of OS in patients with mCRC is shown in Fig. 3. The median follow-up time was 34.7 momths. The median OS after liver resection was 36.8 months,
(95 % CI: 5.9 – 67.8). The 5-year survival rate was 34.5 ± 10.8 %. There was no tumor progression in 14 (29.2 %) of 48 patients. Secondary liver metastases appeared in 4 (8.3 %). In the other 30 (62.5 %) cases, tumor recurrence including distant metastases in other organs or multiple distant ones was revealed.
In the frame of this investigation the Cox regression analysis was performed. The following variables were regarded as potential predictors: age, sex (male, female), tumor localization (colon, rectum), ACT after colorectal resections (yes, no), neoadjuvant CT prior to liver resection (yes, no), ACT upon liver surgery (yes, no). The single factor affecting OS was ACT after liver resection (model χ2=4.26; p=0.04; HR=0.40; 95 % CI: 0.16–0.98). Curves of OS depending on ACT application are shown in Fig. 4.

Fig. 4. Kaplan–Meier curves of OS depended on ACT in the group оf mCRC
Рис. 4. Кривая общей выживаемости по методу Каплан–Мейера в зависимости от адъювантной химиотерапии у больных метастатическим колоректальным раком
The median survival time with/without ACT after liver resection was 54.5 (95 % CI: 14.5–94.5) vs 21.8 months (95 % CI: 14.2–29.4), respectively. The 5-year survival rate in the group of ACT was 44.8 ± 12.9 %. In the group of patients without ACT, the 5-year survival rate was 0 % . After liver resection ACT was not applied to 15 patients due to surgical complications (n=9) and previous preoperative CT (n=6).
Discussion
Performing liver surgery in high-volume centers only remains a controversial point [11]. As per some investigations [4], personal surgical experience, initial patient’s condition and a comorbidity level are more important influencing factors. Besides, some specific and quite satisfied results of low-volume centers contribution were published [12]. In our own opinion, a high level of surgical team rapport, affinity for single surgical modality, a stable patient stream, and performing up to 15 resections annually may provide proper skills among surgeons and anesthetists, on the assumption that the center deals with other high-tech operations in the same field.
Almost all types of standard anatomical liver resections are presented in our series, whilst 58 (65.2 %) procedures were large (including 4 or more segments). FLR calculated as 10 g of liver parenchyma per 1 kg of human body weight was considered to be absolutely acceptable. Unfortunately, such proportions could not be always obtained. Taking into account that majority of our patients who underwent large liver resections had received CT earlier, we regarded FLR as large as 40 % of the liver volume to be minimally valid [9]. Simple portal vein ligation (during staged colorectal surgery), embolization of the portal vein with subsequent chemoembolization of the hepatic artery, the ALPPS procedure were employed for induction of FLR hypertrophy [13, 14]. We believe that ALPPS is the most efficient inductor of FLR hypertrophy. Nevertheless, interest in ALPPS is declining nowadays due to a high risk of post-surgical complications and concurrent development of very safe non-inferior endovascular interventions [14]. We did not observe adverse effects after endovascular embolization or simple portal vein ligation. After 6 ALPPS procedures presented there were 3 bile leakages. The left bile duct injury required re-intervention in 1 case. The origin of bile leakage remained unknown in 2 patients as the complication was discharged by prolonged draining. Liver failure resulted in lethal outcome in 1 of 6 ALPPS’s. On the whole, we consider ALPPS to be acceptable especially in the case of bi-lobar liver damaging that requires trisectionectomy associated with 1–2 metastasectomies of FLR [8]. In one case we performed ALPPS after inefficient portal embolization.
Transplantation techniques such as resection and plasty of afferent and efferent vessels were applied to 4 patients [15]. All resections were performed under normothermia. We did not observe specific complications after such procedures.
Several authors reported the rate of post-surgical complications vastly ranging from 4.1 to 47.7 % [16]. In our series this item was as high as 31.5 %. As per Table 4, specific complications (bile leakage, bleeding, liver failure) constituted 64.3 % (18 of 28), whilst bile leakage predominated – 50.0 % (14 of 28). Re-intervention for bile leakage was performed in 4 patients; in two ones the complication was managed. The other 10 bile complications were cured by prolonged draining or US-navigated punctures. Parenchyma leakage was the most probable cause of these bile leakages. In general, the rate and set of post-surgical side events coincides with those reported by other authors [1, 16, 17].
Our colleagues announced the post-surgical mortality range from less than 1.0 % to 10.0 % [16]. We consider our own mortality 6.7 % to be high. However, 2 of 6 patients died due to comorbidity progression (stomach ulcer perforation and mesenteric ischemia). One death was caused by escalation of purulent cholangitis and liver failure developed before liver resection. This severe condition was a sequel of long-lasting non-drained obstructive jaundice and portal vein compression in the patient with hilar CC. Hence, this patient underwent surgery for life-threatening disease. Hence, only 3 patients died due to specific post-surgical complications. We believe the initial status of patients and the high rate of comorbidity undermined the level of mortality in our series. Besides, medical centers of the 3rd level in the Russian Federation cannot select patients most of the time, which adversely affects mortality. Other authors have analogous inference [4, 11].
In terms of long-term outcomes, all patients with benign pathology were expectedly cured successfully and stay alive without pathology progression.
Among 12 patients whom liver resection is not strongly prescribed to by clinical guidelines (neither mCRC nor hepato-biliar tumors), 8 demonstrated satisfied OS ranged from 31.0 to 88.0 mo under condition of additional drug therapy (Table 6). With the limited amount of such tumors we could not carry out a detailed controlled trial and draw strong conclusions. However, these results are believed to be a reason for individual planning of standard low-risk surgery if liver oligometastatic nature of the tumor is scrupulously proven.
Extrahepatic CC is regarded as a rare and very aggressive tumor associated with a poor prognosis. R0 resection is the only comprehensive curative option. Nevertheless, due to unfavorable site resectability of the tumor is low, post-surgical complications develop in 40–70 %, and surgical mortality is ranged within 5–15 % [18]. Even if R0 resection is accomplished, the incidence of tumor recurrence remains as high as 50–70 % and 5-year OS is as low as 10–40 % [18]. We presented the small series of extrahepatic CC consisting of 6 patients. Excepting Bismuth I tumors all patients had large liver resections with resection of extrahepatic bile ducts. One patient died after surgery due to progression of tumor complications (discussed above). Two died because of early tumor recurrence. Three patients are still alive without evident tumor whereas two have been alive for more than 5 years.
HCC and LCC were not multiple in our series either, that is why they were included in one common group of primary liver carcinomas. HCC expectedly prevailed over LCC (8 of 11 cases) [19, 20]. Only one patient had liver cirrhosis as the background of the cancer. The OS median was not obtained among 10 survived patients. We think the present results coincide with those reported by colleagues [19, 20].
Undoubtedly, long-term outcomes of mCRC surgical treatment are of great interest because this carcinoma constitutes the largest group of liver resections [3, 17]. All our patients underwent staged surgery whilst current meta-analyses do not substantiate significant OS differences after simultaneous or staged procedures (HR=0.97 (95 % CI: 0.88–1.08) with contemporary decreasing of hospital stay by one week (MD=-6.27
Список литературы Early and long-term outcomes of liver resections: a single specialized center experience
- Skipenko O.G., Zavenyan Z.S., Bagmet N.N., Tsar'kov P.V., Shatveryan G.A., Polishchuk L.O., Abdullaev A.G., Makarova V.V. Rezektsiya pecheni: blizhaishie rezul'taty 132 operatsii. Annaly khirurgicheskoi gepatologii. 2006; 11(4): 23-7.
- Vishnevskii V.A., Efanov M.G., Ikramov R.Z., Egorov V.I., Nazarenko N.A., Shevchenko T.V., Ionkin D.A., Kozyrin I.A., Kazakov I.V. Otdalennye rezul'taty rezektsii pecheni u bol'nykh s metastazami kolorektal'nogo raka i pervichnym rakom pecheni. Annaly khirurgicheskoi gepatologii. 2010; 15(1): 43-52.
- Marwah S., Khan M.M., Chaudhary A., Gupta S., Negi S.S., Soin A., Nundy S. Two hundred and forty-one consecutive liver resections: an experience from India. HPB (Oxford). 2007; 9(1): 29-36. https://doi.org/10.1080/13651820600985259.
- Lorenzo C.S., Limm W.M., Lurie F., Wong L.L. Factors affecting outcome in liver resection. HPB (Oxford). 2005; 7(3): 226-30. https://doi.org/10.1080/13651820510028864.
- Lukmonov S., Usmanov O., Ismailov M., Madatov K., Kurbankulov U., Allazarov U. Immediate and long-term results of liver resections with metastatic lesion. Ann Onc. 2018; 29(5): 71-2. https://doi.org/10.1093/annonc/mdy151.254.
- Bel'skii V.A., Mol'kov A.M., Utkin I.A., Rabosina I.A., Zagainov V.E. Protokol anestezii pri obshirnykh rezektsiyakh pecheni: smena paradigmy pod vliyaniem opyta transplantatsii pecheni (obzor literatury). Annaly khirurgicheskoi gepatologii. 2016; 21(2): 39-51. https://doi.org/10.16931/1995-5464.2016239-51.
- Grebenkin E.N., Borisova O.A., Fomin D.K., Akhaladze G.G. K voprosu o funktsional'nom rezerve pecheni. Annaly khirurgicheskoi gepatologii. 2017; 22(1): 25-31. https://doi.org/10.16931/1995-5464.2017125-31.
- Krasnodebski M., Bradford K.J., Wei S.H., Velasco J.D., Nishioka Y., Vauthey J.N. Chemotherapy in combination with resection for colorectal liver metastases - current evidence. Surgery in Practice and Science. 2020; 3. https://doi.org/10.1016/j.sipas.2020.100021.
- Zagainov E.M., Seregin A.A., Zaitsev A.I., Komarov D.V., Sharabrin E.G., Rykhtik P.I., Kukosh V.M., Zagainov V.E. Sovremennye metody stimulyatsii vikarnoi gipertrofii fragmenta pecheni pered obshirnoi rezektsiei: otsenka effektivnosti i puti uluchsheniya rezul'tatov. Annaly khirurgicheskoi gepatologii. 2016; 21(3): 25-33. https://doi.org/10.16931/1995-5464.2016325-33.
- Hamady Z.Z., Kotru A., Nishio H., Lodge J.P. Current techniques and results of liver resection for colorectal liver metastases. Br Med Bull. 2004; 70: 87-104. https://doi.org/10.1093/bmb/ldh026.
- Eppsteiner R.W., Csikesz N.G., Simons J.P., Tseng J.F., Shah S.A. High volume and outcome after liver resection: surgeon or center? J Gastrointest Surg. 2008; 12(10): 1709-16; discussion 1716. https://doi.org/10.1007/s11605-008-0627-3.
- Cawich S.O., Maharaj R., Naraynsingh V., Pearce N., Francis W., Bonadie K.O., Thomas D.A. Clinical outcomes after major hepatectomy are acceptable in low-volume centers in the Caribbean. World J Hepatol. 2019; 11(2): 199-207. https://doi.org/10.4254/wjh.v11.i2.199.
- Voskanyan S.E., Artem'ev A.I., Naidenov E.V., Kolyshev I.Yu., Zabezhinskii D.A., Shabalin M.V., Bashkov A.N., Grigor'eva O.O., Shcherbin V.V., Zhurbin A.S. ALPPS v preodolenii malogo ostatochnogo ob"ema pecheni pri al'veokokkoze. Annaly khirurgicheskoi gepatologii. 2018; 23(4): 21-32. https://doi.org/10.16931/1995-5464.2018421-32.
- Schnitzbauer A.A., Lang S.A., Goessmann H., Nadalin S., Baumgart J., Farkas S.A., Fichtner-Feigl S., Lorf T., Goralcyk A., Horbelt R., Kroemer A., Loss M., Rilmmele P., Scherer M.N., Padberg W., KonigsrainerA., Lang H., Obed A., SchlittH.J. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012; 255(3): 405-14. https://doi.org/10.1097/SLA.0b013e31824856f5.
- Efanov M.G., Alikhanov R.B., Vishnevskii V.A. Transplantatsionnye tekhnologii v rezektsionnoi khirurgii mestnorasprostranennykh novoobrazovanii pecheni. Annaly khirurgicheskoi gepatologii. 2018; 23(4): 11-20. https://doi.org/10.16931/19955464.2018411-20.
- Shabunin A.V., Parfenov I.P., Bedin V.V., Tavobilov M.M., Grekov D.N., Drozdov P.A., Karpov A.A. Rezektsiya pecheni. Spetsificheskie oslozhneniya i ikh profilaktika. Khirurgiya. Zhurnal imeni N.I. Pirogova. 2020; (3): 5-12. https://doi.org/10.17116/hirurgia20200315.
- Polishchuk L.O., Kozmin L.D., Stroyakovskii D.L., Dorovskoi E.S., Skipenko O.G. Neposredstvennye rezul'taty rezektsii pecheni posle neoad"yuvantnoi khimioterapii kolorektal'nogo raka. Khirurgiya. Zhurnal im. N.I. Pirogova. 2010; (1): 30-40.
- Soares K.C., Kamel I., Cosgrove D.P., Herman J.M., Paw-lik T.M. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014; 3(1): 18-34. https://doi.org/10.3978/j.issn.2304-3881.2014.02.05.
- Balogh J., Victor D., Asham E.H., Burroughs S.G., Bok-tour M., Saharia A., Li X., Ghobrial R.M., Monsour H.P. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016; 3: 41-53. https://doi.org/10.2147/JHC.S61146.
- Bednarsch J., Czigany Z., Heij L.R., Liu D., den Dulk M., Wiltberger G., Bruners P., Ulmer T.F., Neumann U.P., Lang S.A. Compelling Long-Term Results for Liver Resection in Early Cho-langiocarcinoma. J Clin Med. 2021; 10(13): 2959. https://doi.org/10.3390/jcm10132959.
- Gavriilidis P., Sutcliffe R.P., Hodson J., Marudanayagam R., Isaac J., Azoulay D., Roberts K.J. Simultaneous versus delayed hepatectomy for synchronous colorectal liver metastases: a systematic review and meta-analysis. HPB (Oxford). 2018; 20(1): 11-9. https://doi.org/10.1016/j.hpb.2017.08.008.


