Effects of photoperiod on body mass, thermogenesis and body composition in Eothenomys miletus during cold exposure
Автор: Zhu Wan-Long, Yang Shengchang, Cai Jin-Hong, Meng Lihua, Wang Zheng-Kun
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.8, 2012 года.
Бесплатный доступ
Many small mammals respond to seasonal changes in photoperiod by altering body mass and adiposity. These animals may provide valuable models for understanding the regulation of energy balance. In present study, we examined the effect on body mass, rest metabolic rate, food intake and body composition in cold-acclimated (5 °C) in Eothenomys miletus by transferring them from a short (SD, 8h :16h L: D) to long day photoperiod (LD, 16h: 8h L:D). During the first 4 weeks of exposure to SD, E. miletus decreased body mass. After the next 4 weeks of exposure to LD, which the average difference between body masses of LD and SD voles was 4.76 g. This 14.74% increase in body mass reflected significant increases in absolute amounts of body components, including wet carcass mass, dry carcass mass and body fat mass. After correcting body composition and organ morphology data for the differences in body mass, only livers, kidney, and small intestine were enlarged due to photoperiod treatment during cold exposure. E. miletus increased RMR and energy intake exposure to LD, but maintained a stable level to SD after 28 days. Serum leptin levels were positively correlated with body mass, body fat mass, RMR as well as energy intake. All of the results indicated that E. miletus may provide an attractive novel animal model for investigation of the regulation of body mass and energy balance at organism levels. Leptin is potentially involved in the photoperiod induced body mass regulation and thermogenesis in E. miletus during cold exposure.
Eothenomys miletus, thermogenesis, body composition, serum leptin
Короткий адрес: https://sciup.org/14323612
IDR: 14323612
Текст научной статьи Effects of photoperiod on body mass, thermogenesis and body composition in Eothenomys miletus during cold exposure
Phenotypic flexibility is the ability of organisms to reversibly modulate traits in response to environmental challenge (Aneta et al., 2009). In recent years, the magnitude of this modulation has been extensively quantified at different levels of biological organization (Sabat and Bozinovic, 2000; Villarin et al., 2003; Kristan and Hammond, 2006).
Several studies have demonstrated that processes related to energy acquisition and expenditure is among the most sensitive to changes in environmental conditions (Naya et al., 2008). The responses to changing photoperiod can be readily induced in the laboratory by simulating the natural progression of day lengths or by acutely transferring animals between summer (long) and winter (short) day lengths (Krуl et al., 2005). There were many studies about the transfer of small mammals from long day photoperiod (LD, 16∙h:8∙h L:D) to short day photoperiod (SD, 8∙h:16∙h L:D), they showed the changes of body mass, thermogenesis and body composition (Ebling, 1994; Klingenspor et al., 2000; Mercer and Speakman, 2001; Hunter and Nagy, 2002). Leptin is a protein which is synthesized primarily by adipose tissue and is secreted into the bloodstream (Silva, 2006). Leptin is considered to be an adipostatic signal linking energy metabolism, regulating food intake, and providing prompt feedback to brain areas involved in regulation of energy balance (Barb and Kraeling, 2004). It is also hypothesized to contribute to the maintenance of body mass by altering food intake and energy expenditure (Friedman and Hallas, 1998).
The Hengduan Mountains region is located the boundary between the Palaearctic region and the Oriental region. It is alpine with high mountains and gorges; the diversity and abundance of mammals is high and it is considered to be “the harbor in fourth ice age”. Therefore small mammals may differ from those from other regions. Eothenomys were the proper genus in China, were a special group in Microtus, and were the typical animals in Hengduan Mountains region. E. miletus is the inherent species in Hengduan mountains region (Zhu et al., 2011a), has special status in Microtus. Now few studies about E. miletus were reported (Zhu et al., 2008; 2010a; 2010b; 2011b). In the present study, we examined the effect on body mass, thermogenesis and body composition in cold-acclimated E. miletus by transferring them from short to long day photoperiod. We predicted that E. miletus change their body mass, thermogenesis and body composition by transferring them from short to long day photoperiod, and leptin would be involved in the regulation of body mass and thermogenesis.
MATERIALS AND METHODS
Samples
E. miletus were captured in farmland (26°15'~26°45'N; 99°40'~99°55'E; altitude 2,590m) in Jianchuan County, Yunnan province, 2011. Average temperature is 9.1oC each year, minimum average temperature was -4.0oC in January, maximal average temperature was 24.1oC in July.
E. miletus were breed for two generations in School of life Science of Yunnan Normal University, park in plastic box (260mmx160mmx150mm), one in a box without any bedding material, and were maintained at the room temperature of 25±1°C, under a photoperiod of 12L:12D (with lights on at 08:00). After 1 month stabilization, animas were randomly divided into two groups (n=7 each group). Following the acclimation period, all animals were kept in SD for a further 24days to obtain baseline measurements of body mass and food intake, after which 7 voles were exposed to a long day photoperiod (LD, 16h: 8h L: D, lights on 04:00h) at 5°C, whereas 7 control voles remained in original SD conditions. The voles were sacrificed between 09:00 and 11:00 by puncture of the posterior vena cava. Blood was centrifuged at 4000 rpm for 30 min, and serum was sampled and stored at -20°C for later measurement. All voles were dissected to evaluate organ morphology. All pregnant, lactating or young individuals were excluded.
Measurement of metabolic rates
Metabolic rates were measured by using AD ML870 open respirometer (AD Instruments, Australia) at 25oC within the TNZ (thermal neutral zone), gas analysis were using ML206 gas analysis instrument, the temperature was controlled by SPX-300 artificial climatic engine (±0.5oC), the metabolic chamber volume is 500ml, flow is 200 ml/min. The tree shrew were stabilized in the metabolic chamber for at least 60 min prior to the BMR measurement, oxygen consumption was recorded for more than 60∙min at 5∙min intervals. Two stable consecutive lowest readings were taken to calculate BMR (Li and Wang, 2005). Calculate method of metabolic rate is detailed by Hills (Hills, 1972).
Energy budget
Energy budget were measured by food equity (Rosenmann and Morrison, 1974), one animal in a metabolic cage ( 20×15×15cm3 ), had no nest materials. After adaptation one week, energy budget were measured. Animals were feeding fix quantify and time, next day weighted animal body mass, collected residual food, faeces. Residual food and faeces were dryness in vacuum airer until the mass were invariable, got sample (about 1g, precision: 0.0001g), the samples were measured by YX-ZR/Q automatism calorimeters instrument. The calorie of the diet fed to these animals were 18.0±0.8 kJ/g. Calculate the energy intake. The calculation was according to corresponding reports (Drozdz, 1975):
Energy intake (KJ/d) =food (g/d)* energy content (KJ/g)
Measurement of serum leptin level and thyroid hormones
Serum leptin levels were determined by radioimmunoassay (RIA) with the 125I Multispecies Kit (Cat. No. XL-85K, Linco Research Inc.).The lowest level of leptin that can be detected by this assay was 1.0 ng/ml when using a 100-μl sample size. And the inter- and intra-assay variability for leptin RIA were <3.6% and 8.7%, respectively.
Carcass and body fat content
Gastrointestinal tract (stomach, small intestine, cecum, large intestine) and the heart, lungs, pancreas, spleen, kidneys were extracted and weighed (±1 mg). The stomach and intestines were rinsed with saline to eliminate all the gut contents, before being dried and weighed. The remaining carcass and all the organs were dried in an oven at 60 °C to constant mass (at least 72 h), and then weighed again to obtain the dry mass. The remaining carcass and all the organs were dried in an oven at 60°C to constant mass (at least 72 h), and then weighed again to obtain the dry mass. The difference between the wet carcass mass and dry carcass mass was the water mass of carcass. Total body fat was extracted from the dried carcass by ether extraction in a Soxhlet apparatus (Zhang and Wang, 2007).
Statistical analysis
Data were analyzed using SPSS 15.0 software package. Prior to all statistical analyses, data were examined for assumptions of normality and homogeneity of variance, using Kolmogorov-Smirnov and Levene tests, respectively. Throughout the acclimation, changes in body mass, BMR and energy intake were analyzed by a twoway analysis of covariance (ANCOVA) with body mass as a covariate. Body composition between SD and LD were analyzed by independent t-test. Pearson's correlation was performed to detect possible correlations among serum leptin and body mass, body fat mass, RMR and energy intake. Results were presented as mean ± SEM, and P < 0.05 was considered to be statistically significant.
RESULTS
Body mass and serum leptin
No group differences were found between acclimation voles (F=0.972, P>0.05, Fig 1) before acclimation. During the acclimation, both acclimation voles decreased body mass in the first 4 weeks, SD voles showed relatively stable body mass after 4 weeks(F=0.073, P>0.05, Fig 1), whereas the LD voles showed a gradual increase over the next 4-week acclimation (F=2.449, P<0.05, Fig 1). Correlation analysis indicated that body mass was positively correlated with body fat mass (r = 0.836, P < 0.01; Fig 2A). There had positive correlation between serum leptin level and body mass (r = 0.960, P < 0.01; Fig 2B), between serum leptin level and body fat mass during the acclimation(r = 0.784, P < 0.01; Fig 2C).
RMR and energy intake
Prior to acclimation, no group differences in RMR between acclimation voles (t=0.318, P>0.05). During the acclimation, both acclimation voles increased RMR in the first 4 weeks, SD voles showed relatively stable RMR after 4 weeks (F=0.125, P>0.05, Fig 3), whereas the LD voles showed a gradual increase over the next 4-week acclimation (F=3.245, P<0.05, Fig 3). Prior to acclimation, no group differences in energy intake between acclimation voles (t=0.364, P>0.05). During the acclimation, both acclimation voles increased energy intake in the first 4 weeks, SD voles showed relatively stable energy intake after 4 weeks(F=0.296, P>0.05, Fig 4), whereas the LD voles showed a gradual increase over the next 4-week acclimation (F=2.985, P<0.05, Fig 4). There had positive correlation between serum leptin level and RMR (r = 0.956, P < 0.01; Fig 5A), between serum leptin level and energy intake during the acclimation(r = 0.676, P < 0.01; Fig 5B).
Body composition
At the end of the acclimation, wet carcass mass, dry carcass mass and body fat mass showed a significant differences between SD voles and LD voles (t=2.242, P<0.01, Table 1), (t=2.717, P<0.05, Table 1), (t=2.035, P<0.05, Table 1), respectively. No differences were found in water of carcass between the two groups (t=1.603; P>0.05, Table 1). By contrast, the wet organ masses of liver, kidney and small intestine showed significant between the LD and SD voles (Table 2), the dry organ masses of liver and kidney showed significant between the LD and SD voles (Table 3).
Table 1 . Changes of body compositions exposed to short and long day photoperiods during cold exposure E. miletus
|
Parameters |
SD |
LD |
P value |
|
Body mass(g) |
22.52±0.51 |
26.12±0.98 |
0.042 |
|
Wet carcass mass(g) |
32.29±0.86 |
37.05±0.95 |
0.001 |
|
Dry carcass mass(g) |
11.27±0.34 |
13.44±0.72 |
0.019 |
|
Water of carcass(g) |
11.26±0.70 |
12.64±0.49 |
0.135 |
|
Body fat mass(g) |
4.62±0.14 |
5.50±0.29 |
0.025 |
|
Table 2. Changes of mean wet organ masses exposure E. miletus |
exposed to short and long day photoperiods during cold |
||
|
Parameters |
SD |
LD |
P value |
|
Heart(g) |
0.233±0.019 |
0.251±0.013 |
0.271 |
|
Lungs(g) |
0.308±0.015 |
0.324±0.008 |
0.401 |
|
Liver(g) |
1.525±0.071 |
1.848±0.078 |
0.012 |
|
Kidney(g) |
0.179±0.010 |
0.218±0.014 |
0.049 |
|
Spleen(g) |
0.020±0.002 |
0.020±0.003 |
0.896 |
|
Stomach(g) |
0.405±0.031 |
0.414±0.013 |
0.071 |
|
Small intestine(g) |
0.674±0.024 |
0.751±0.015 |
0.023 |
|
Cecum(g) |
0.420±0.015 |
0.438±0.014 |
0.443 |
|
Large intestine(g) |
0.325±0.013 |
0.335±0.013 |
0.608 |
Table 3. Changes of mean dry organ masses exposed to short and long day photoperiods during cold exposure E. miletus
|
Parameters |
SD |
LD |
P value |
|
Heart(g) |
0.043±0.002 |
0.045±0.002 |
0.610 |
|
Lungs(g) |
0.066±0.002 |
0.070±0.001 |
0.131 |
|
Liver(g) |
0.384±0.015 |
0.497±0.022 |
0.001 |
|
Kidney(g) |
0.040±0.001 |
0.043±0.001 |
0.020 |
|
Spleen(g) |
0.004±0.001 |
0.004±0.001 |
0.763 |
|
Stomach(g) |
0.093±0.008 |
0.093±0.009 |
0.324 |
|
Small intestine(g) |
0.029±0.001 |
0.027±0.001 |
0.676 |
|
Cecum(g) |
0.041±0.003 |
0.041±0.002 |
0.981 |
|
Large intestine(g) |
0.044±0.003 |
0.044±0.004 |
0.906 |
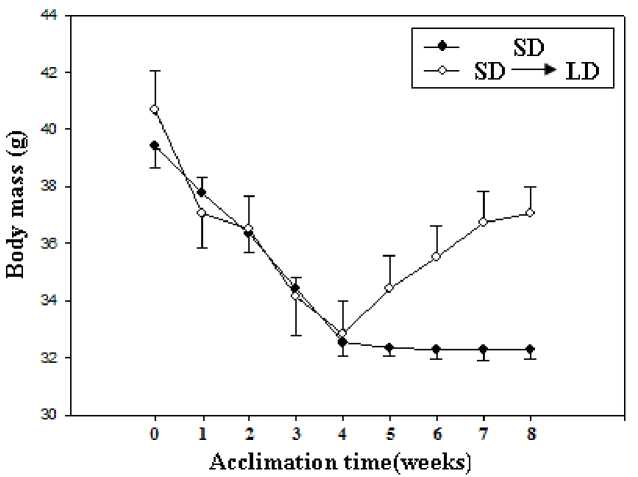
Figure∙1 Effect of body mass exposed to short and long day photoperiods during cold exposure in E miletus
DISCUSSION
Many small mammals respond to winter-associated environmental cues by reducing body or enhancing thermogenesis (Heldmaier et al., 1982; 1989), such as Microus brandti (Li and Wang, 2005), Djungarian hamster (Steinlechner et al., 1983). Moreover, photoperiod is one of the environmental cues triggering seasonal responses in small mammals. Some small mammals reduced body mass while increased thermogenesis in SD conditions (Geiser et al., 2007; Arnold et al., 2011), such as Siberian hamsters (Demas et al., 2002), Brandt's voles (Zhao and Wang, 2005). In the present study, it was clear that E. miletus showed decreasing of body mass in short day photoperiod during cold exposure in the first 4 weeks. But when E. miletus transferred from a short (SD, 8h: 16h L: D) to long day photoperiod (LD, 16h: 8h L: D). Body mass of E. miletus increased exposure to long day photoperiod. After the next 4 weeks of exposure to LD, which the average difference between body masses of LD and SD voles was 4.76 g. This 14.74% increase in body mass reflected significant increases in absolute amounts of body components, including wet carcass mass, dry carcass mass and body fat mass. At the end of the acclimation, our present study showed that SD voles had lower body mass and body fat mass compared with LD voles. As dry while no differences were found in water of carcass carcass mass in SD voles was also lower than in LD mass between the two groups.
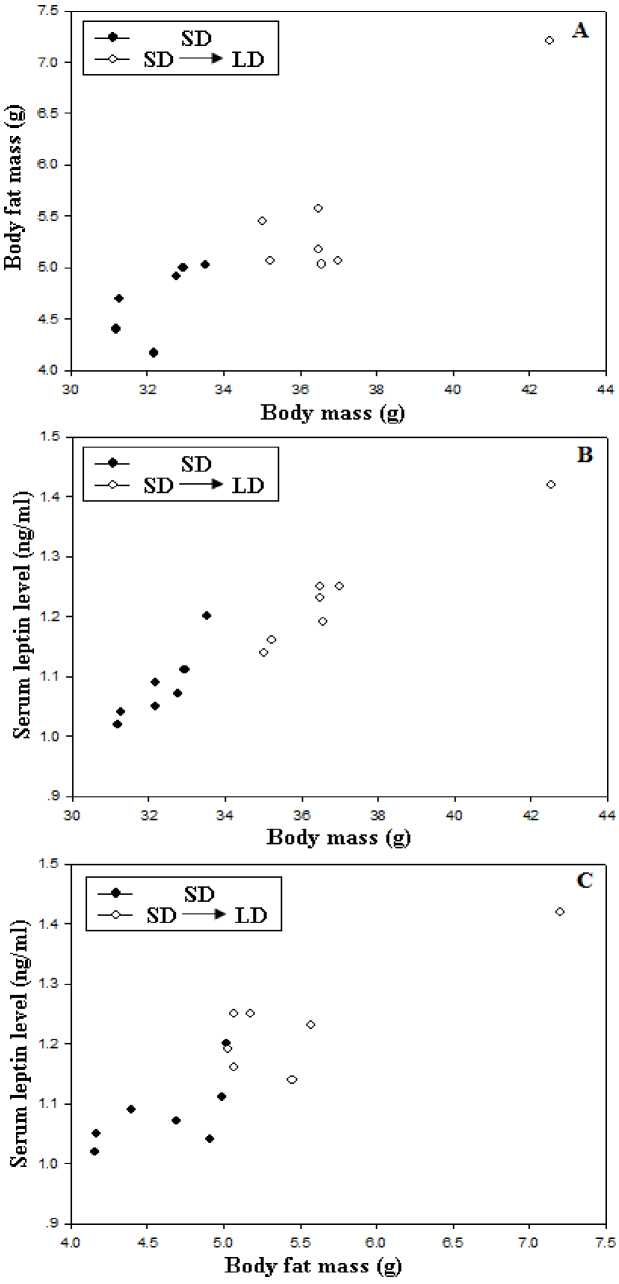
Figure 2 Correlation of body mass with body fat mass (A), serum leptin levels with body mass (B), body fat mass (C) in E. miletus
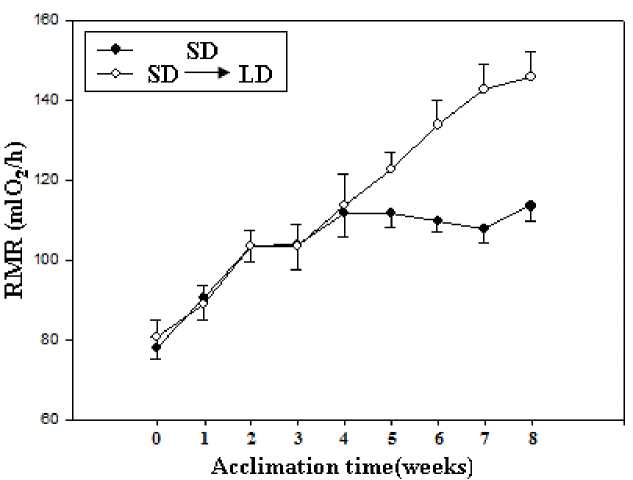
Figure∙3 Effect of RMR exposed to short and long day photoperiods during cold exposure in E miletus
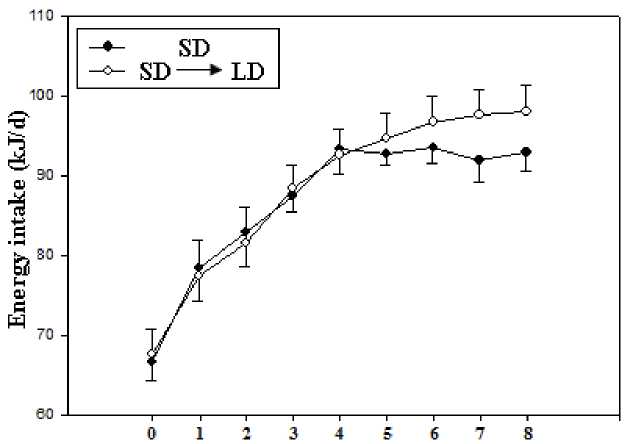
Acclimation time (weeks)
Figure∙4 Effect of energy intake exposed to short and long day photoperiods during cold exposure in E. miletus
Changes of photoperiod are considered to be an important cue affecting RMR in many small mammals (Haim et al., 1999). In the present study, LD voles after 4 weeks photoperiodic acclimation showed higher RMR than that in SD voles during the cold exposure. These data clearly indicated that RMR was affected by changes of photoperiod in E. miletus. Energy intake also showed the similar trend. The increases of RMR and energy intake were the cause of increased in body mass in E. miletus. In addition, themorphology of the organs was found to be altered to meet the increase in energy intake. Livers, kidney, and small intestine were enlarged due to photoperiod treatment during cold exposure. Liver, as an important energy-expending organ, is considered to make a large contribution to RMR. In our study, kidney and small intestine in LD voles was significantly higher than that in SD voles, which was consistent with the changes in BMR and energy intake.
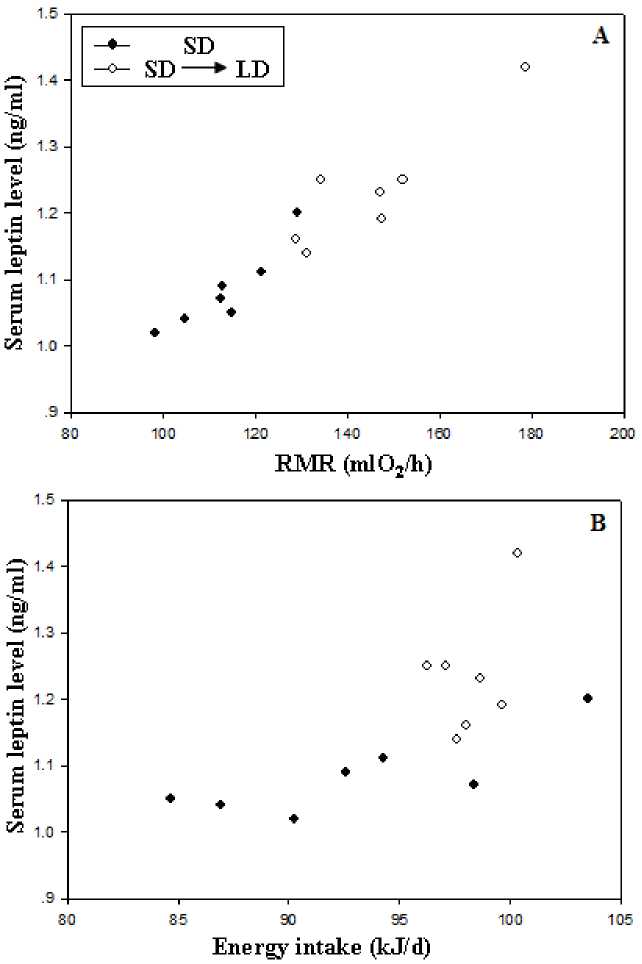
Figure 5 Correlation of serum leptin levels with RMR (A), energy intake (B) in E. miletus
Leptin level may reflect the contents of fattiness, could serve as a signal to the brain, to regulate food intake and energy expenditure and resistance to obesity (Michael et al., 2000). There was a positive correlation between leptin level and body mass (Friedman and Hallas, 1998). Subsequent parabiosis experiments in rodents supported the tenets of the lipostasis theory in that the serum “satiety signal” was at a higher concentration in obese animals than in lean animals (Concannon et al., 2001). In our result, although SD voles showed relatively lower serum leptin levels compared with LD voles. Further correlation analysis showed that serum leptin levels were positively correlated with body mass and body fat mass, indicating that leptin may act as "adiposity indicator" (Zhao and Wang, 2005). To regulate body mass, leptin influences energy balance via its effects on both food intake and energy expenditure (Concannon et al., 2001). Leptin administration is thought to increase energy expenditure, it has been reported that lower serum leptin levels were accompanied with higher energy intake in Brandt's voles, indicating that leptin acted as a starvation signal (Liu et al., 2007). Furthermore, correlation analysis showed that serum leptin levels were positively correlated with RMR and energy intake, it was opposite to our previous studies (Zhu et al., 2010a; 2011b). It may indicated that the anorectic and thermogenic effects of leptin may be dissociated in cold-acclimated (5 °C) in Eothenomys miletus by transferring them from a short (SD, 8h :16h L: D) to long day photoperiod (LD, 16h: 8h L:D). It need further study.
In conclusion, E. miletus decreased body mass during the first 4 weeks of exposure to SD. After the next 4 weeks of exposure to LD, which the average difference between body masses of LD and SD voles was 4.76 g. This increase in body mass reflected significant increases in absolute amounts of body components, including wet carcass mass, dry carcass mass and body fat mass. Livers, kidney, and small intestine were enlarged due to photoperiod treatment during cold exposure. E. miletus increased RMR and energy intake exposure to LD, but maintained a stable level to SD after 28 days. Serum leptin levels were positively correlated with body mass and body fat mass. Leptin is potentially involved in the photoperiod induced body mass regulation and thermogenesis in E. miletus during cold exposure.
ACKNOWLEDGMENTS
The project was financially supported by National Science Foundation of China (No.31071925); Project of Basic research for application in Yunnan Province (No. 2011FZ082).
Список литературы Effects of photoperiod on body mass, thermogenesis and body composition in Eothenomys miletus during cold exposure
- Aneta, K., Jan, C., Marek, K. (2009) Phenotypic flexibility of traits related to energy acquisition in mice divergently selected for basal metabolic rate(BMR). J. Exp. Biol. 212, 808-814
- Arnold, W., Ruf, T., Frey-Roos, F., Bruns, U. (2011) Diet-independent remodeling of cellular membranes precedes seasonally changing body temperature in a Hibernator. PLoS ONE 6(4), 18641.
- Barb, C.R., Kraeling, R.R. 2004. Role of leptin in the regulation of gonadotropin secretion in farm animals. Animal Reproduction Science 82-83:155-167.
- Concannon, P., Levac, K., Rawson, R., Tennant, B., Benadoun, A. (2001) Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. Am. J. Physiol. 281, 951-959.
- Demas, G.E., Bowers, R.R., Bartness, T.J., Gettys, T.W. (2002) Photoperiodic regulation of gene expression in brown and white adipose tissue of Siberian hamsters (Phodopus sungorus). Am. J. Physiol. 282, 114-121.
- Drozdz,A.(1975) Metabolic cages for small rodents. In: Grodzinski W, Klekowski RZ, Duncan A. eds: Methods for Ecological Bioenergetics. Oxford: Blackwell Scientific Press, 346-351.
- Ebling,F.J.P.(1994) Photoperiodic differences during development in thehamsters Phodopus sungorusand Phodopus campbelli. Gen. Comp. Endocrinol. 95, 475-482.
- Friedman, J.M., Hallas, J.L. (1998) Leptin and the regulation of body weight in mammals. Nature 395: 763-770.
- Geiser, F., McAllan, B.M., Kenagy, G.J., Hiebert, S.M. (2007) Photoperiod affects 10 daily torpor and tissue fatty acid composition in deer mice. Naturwissenschaften 94, 319-325.
- Haim, A., Shabtay, A., Arad, Z. (1999) The thermoregulatory and metabolic responses to photoperiod manipulations of the Macedonian mouse (Mus macedonicus), a post-fire invader. J. Therm. Biol. 24, 279-286.
- Heldmaier, G., Steinlechner,S., Rafael,J.(1982)Nonshivering thermogenesis and cold resistance during seasonal acclimation in the Djungarian hamster. J Comp Physiol B149:1-9.
- Heldmaier,G., Steinlecher,T., Ruf,T., Wiesinger,H., Kliongenspor,M.(1989)Photoperiod and thermoregulation in vertebrate: Body temperature rhyme and thermgenic acclimation. J. Biol. Rthym4:251-265.
- Hill,R.W.(1972) Determination of oxygen consumption by use of the paramagnetic oxygen analyzer. J Appl Physiol33(2):261-263.
- Hunter,H.,Nagy,T.R.(2002) Body composition in a seasonal modelobesity: longitudinal measures and validation of DXA. Obes. Res.10,1180-1187.
- Klingenspor,M., Niggemann,H., Heldmaier,G.(2000). Modulation ofsensitivity by short photoperiod acclimation in the Djungarian, Phodopus sungorus. J. Comp. Physiol.B 170, 37-43.
- Kristan,D.M.,Hammond,K.A.(2006)Effects of three simultaneous demands glucose transport, resting metabolism and morphology of laboratory mice. J.Comp. Physiol. B176, 139-151.
- Krol,E., Redman,P., Thomoson,P.J., Williams,R., Mayer,C., Mercer,J.G., Speakman,J.R.(2005) Effect of photoperiod on body mass, food intake and body composition in thevole, Microtus agrestis.The Journal of Experimental Biology208, 571-584.
- Li, X.S., Wang, D.H. (2005) Regulation of body weight and thermogenesis in seasonally acclimatized Brandt's voles (Microtus brandti). Horm Behav 48: 321-328.
- Liu, J.S., Sun, R.Y., Wang, D.H. (2007) Tract Morphology in Three Rodent Species. Chin J Zool 42(1): 8-13.
- Mercer, J.G., Speakman, J.R. (2001) Hypothalamic neuropeptide for regulating energy balance: from rodent models to human. Neurosci. Biobehav. Rev. 25, 101-116.
- Michael,W.S., Stephen, C.W., Danlel, P.Jr., Randy, J.S., Denls, G.B. (2000) Central nervous system control of food intake. Nature 404: 661-621.
- Naya,D.E., Ebensperger,L.A., Sabat,P., Bozinovic,F.(2008) Digestive and Metabolic Flexibility Allows Female Degus to Cope with Lactation Costs. Physiol Biochem Zool 81(2), 186 -194.
- Rosenmann,M., Morrison,P.(1974) Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2. Am. J. Physiol. 226: 490-495.
- Sabat, P., Bozinovic, F. (2000) Digestive plasticity and the cost of acclimation to chemistry in the omnivorous leaf-eared mouse Phyllotis darwini. J. Comp. Physiol. B 170, 411-417.
- Silva, J.E. (2006). Thermogenic mechanisms and their hormonal regulation. Physiological Reviews 86:435-464.
- Steinlechner, S., Heldmaier, G., Becker, H. (1983) The seasonal cycle of body weight in the Djungarian hamster: photoperiod control and the influence of starvation and melatonin. Oecologia 60: 401-405.
- Villarin, J.J., Schaeffer, P.J., Markle, R.A., Lindstedt, S.L. (2003) Chronicexposure increases liver oxidative capacity in the marsupial Monodelphis. Comp. Biochem. Physiol. 136, 621-630.
- Zhang, X.Y., Wang, D.H. (2007) Thermogenesis, food intake and serum leptin in cold-exposed lactating Brandt's voles Lasiopodomys brandtii. The Journal of Experimental Biology 210:512-521.
- Zhao, Z.J., Wang, D.H. (2005) Short photoperiod enhances thermogenic capacity in Brandt's voles. Physiology and Behavior 85, 143-149.
- Zhu, W.L., Jia, T., Lian, X., Wang, Z.K. (2008) Evaporative water loss and energy metabolism in two small mammals, voles(Eothenomys miletus) and mice(Apodemus chevrieri) in Hengduan mountains region. J of Ther Bio 33: 324-331
- Zhu, W.L., Jia, T., Lian, X., Wang, Z.K. (2010a) Effects of cold acclimation on body mass, serum leptin level, energy metabolism and thermognesis in Eothenomys miletus in Hengduan Mountains region. J. Therm. Biol. 35(1), 41-46.
- Zhu, W.L., Cai, J.H., Lian, X., Wang, Z.K. (2010b) Adaptive character of metabolism in Eothenomys miletus in Hengduan Mountains region during cold acclimation. J. Therm. Biol. 35(8), 417-421.
- Zhu, W.L., Wang, B., Cai, J.H., Lian, X., Wang, Z.K. (2011a)Thermogenesis, energy intake and serum leptin in Apodemus chevrieri in Hengduan Mountains region during cold acclimation. Journal of Thermal Biology 36(3):181-186.
- Zhu, W.L., Cai, J.H., Lian, X., Wang, Z.K. (2011b) Effects of photoperiod on energy intake, thermogenesis and body mass in Eothenomys miletus in Hengduan Mountain region. J. Therm. Biol. 36(8), 380-385.


