Evaluation of cell death after thermal ablation in patients with bone tumors
Автор: Sitnikov P.K., Grigoryeva E.S., Anisenya I.I., Kalinchuk A.Yu., Loos D.M., Zelchan R.V., Tabakaev S.A., Pakhmurina V.V., Matyushkov S.Yu., Pakhmurin D.O., Tashireva L.A.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 3 т.24, 2025 года.
Бесплатный доступ
Introduction. Bone tumors are heterogeneous group of skeletal neoplasms characterized by frequent recurrences, aggressive clinical course and low survival rates. The development of new treatment methods continues to pose pressing challenges. Radical intraoperative thermal ablation (RIT) using high-temperature exposure is emerging as a new and promising strategy for organ-preserving treatment. This study focuses on the effect of thermal ablation (TA) on tumors. Objective of the Study to assess the impact of TA (using the RIT method) on the viability of tumor cells. Material and Methods. The study included 8 patients with bone tumors. Tumors underwent TA at a temperature of 60 °C for 30 minutes ex vivo. Apoptosis was studied in tumor tissue samples before and after TA. Apoptosis was assessed using two methods: flow cytometry and the TUNEL assay. Results. The TA procedure developed by our scientific group represents a promising organ-preserving approach for treating malignant bone tumors. A temperature regime of 60 °C for 30 minutes was effective in initiating tumor cells death. This was confirmed by two independent methods – flow cytometry and TUNEL assay – which demonstrated a significant increase in the number of apoptotic cells immediately following the procedure and a notable rise in the number of cells exhibiting signs of late apoptosis one hour post thermal ablation. Therefore, the collected data confirm a pronounced antitumor effect immediately after implementing RIT. Conclusion. The findings confirm that RIT is a viable organ-preserving method for treating bone tumors, meriting further investigation to expand its application in clinical practice.
Âone tumors, thermal ablation, radical intraoperative thermal ablation, apoptosis, necrosis, flow cytometry, TUNEL assay, high-temperature exposure, antitumor effect
Короткий адрес: https://sciup.org/140310573
IDR: 140310573 | УДК: 616.7-006.6-091.818-089-059-076.5:615.832 | DOI: 10.21294/1814-4861-2025-24-3-38-49
Текст научной статьи Evaluation of cell death after thermal ablation in patients with bone tumors
Bone tumors represent a challenging field of medicine, where, on the one hand, the rarity of malignant tumors is compensated by their significant heterogeneity, diagnostic difficulties and aggressive clinical course, and on the other hand, 70–80 % of all skeletal bone lesions are bone metastases, which significantly worsen the quality of life and prognosis of cancer patients. With the exception of a few relatively rare malignancies such as high-grade lymphoma or germ cell tumors affecting bone, metastatic bone disease is currently incurable [1]. Adopting a multidisciplinary treatment approach has improved overall survival rates for patients with bone sarcomas – from 20 % to 70 % in cases of localized disease – with a 5-year overall survival rate for osteosarcoma (OS) being approximately 65 %, and for Ewing’s sarcoma (ES), around
50–60 %. However, once metastases occur, survival remains low – up to 30 % for OS and 20 % for ES [2, 3]. In contrast, well-differentiated chondrosarcoma, while having a relatively favorable prognosis, remains resistant to systemic treatments. For low-differentiated and dedifferentiated tumors, the 5-year overall survival rate ranges between 7 % and 24 % [4, 5]. The recurrence rate after radical combination treatment remains is high and can reach 50 % for Ewing’s sarcoma, 40 % for osteosarcoma and 30 % for chondrosarcoma [6, 7]. Bone damage in soft tissue sarcomas can reach 20 % [8], but even after radical removal of the tumor, the recurrence rate is 50 % [9].
The urgency for new treatments remains as critical today as it was five decades ago [10–13]. Over the past two decades, there has been a steadily growing interest in thermal-based methods for treating malig- nant human tumors such as hyperthermia (HT) and TA [14–18].
HT, usually mild heating of the tumor to a maximum temperature of 45 °C once or twice a week, often assists in combination with radio- and/or chemotherapy. HT affects tumor via several mechanisms such as inhibiting DNA damage repair; enhancing blood flow and reoxygenation, direct damage to tumor cells by denaturing proteins and disrupting membranes at temperatures above 43 °C, activating the antitumor activity of immune cells, and reducing thermal resistance of tumor cells. In contrast, TA destroys tumor cells by exposing them to temperatures above 50 °C for a few minutes in a single session, leading to rapid cell death either through protein coagulation and denaturation or resulting in apoptosis or necrosis, depending on the exposure duration and reached temperature [19]. Various high-temperature treatment methods are actively being refined and applied either as adjuvant regime or, in some cases, as standalone treatments for malignant bone tumors [20–23].
A new treatment method – RIT – was developed at the Cancer Research Institute of Tomsk NRMC [24]. This technique preserves the patient’s bone, potentially delaying or obviating the need for complex reconstructive surgery phases [25–28]. Its distinctive attribute is in the use of flexible plate electrodes that completely encase the diaphyseal bone segment affected by the tumor, permitting an intraoperative TA session.
The energy source for this process is the “Phoenix-2” localized hyperthermia system, which functions based on the propagation of thermal waves through the patient’s body, generated by direct current flowing through the flexible plate electrode. This setup achieves targeted temperatures ranging from 45 °C to 110 °C.
A major challenge encountered from the inception of our research was determining the antitumor effect immediately after performing RIT. Although no significant morphological changes indicating a clear antitumor effect could be observed at the light microscopy level in postoperative biopsy material, long-term follow-up revealed no clinical or radiological signs of recurrence in areas treated with TA. The absence of recurrence was confirmed histologically through biopsies and surgical specimens collected at different time points during a patient’s treatment, or across different patients.
To date, more than 20 types of cell death are known, with apoptosis and necrosis being the most prevalent [29]. Currently, there is no definitive data indicating whether RIT leads to apoptosis or necrosis. Thus, evaluating the viability of tumor cells following our TA method has been a primary objective of our study.
Objective of the Study to assess the impact of TA (using the RIT method) on the viability of tumor cells.
Material and Methods
Patients
The study included 8 patients with bone tumors: four with primary malignant bone tumors, three with soft tissue sarcomas, and one with breast cancer metastasis (Table). All patients underwent wide resection of the affected bone followed by oncological endoprosthetic replacement of the adjacent joint as indicated.
Material Collection Technique and RIT Methodology
A standard surgical approach was utilized for all patients. This involved en-bloc excision of the tumor within healthy tissue margins, ensuring at least a 3 cm margin from the tumor edge. Following the removal of the tumor, the reconstructive phase of the surgery was carried out. The excised specimen was placed in a separate sterile tray. From the tumor, two fragments of tumor tissue, each measuring 0.5 × 0.5 cm, were excised using a medical saw, scalpel and forceps from the area planned for TA. These fragments were placed in a buffer solution for suspension preparation. The affected bone segment was then wrapped with flexible heaters, which were insulated from the surrounding environment with thermal insulating material. A temperature sensor was placed at the center of the thermoablation site to monitor the temperature in real time throughout the procedure. A session of TA was conducted at a temperature of 60°C for 30 minutes, with the target temperature reached within 10 minutes. After the procedure, a second tissue sampling was performed, with the material also placed in a buffer solution for suspension preparation. The remaining specimen was sent for routine histological examination (Fig. 1).
All patients who underwent intraoperative experimental procedure on the excised specimen were informed about the study’s plan, objectives, and goals within the framework of the research project “Radical Intraoperative Thermoablation in the Combined Treatment of Bone Tumors”.
Detection of apoptosis rate by flow cytometry
Tissue samples were mechanically disrupted using BD Medimachine System (BD Biosciences, San Jose, CA, USA). Tumor fragments were placed in a sterile microblade-equipped polyethylene chamber (Medicon, BD Biosciences, San Jose, CA, USA) with 1 ml of RPMI 1640 medium (PanEco, Russia) and dissociated 3 to 4 times for 1 min at a constant speed of 100 rpm. The cell suspension was filtered through a 40-um porous Cell Strainer (Corning, NY, USA). After filtration, cells were washed twice with PBS, pH 7.0 (Gibco, Thermo Fisher Scientific, MA, USA) at 300 g. The obtained cell pellet was resuspended in PBS and stained for flow cytometry.
Surface markers (CD45, EpCAM (CD326)), as well as Annexin-V и 7-AAD were stained by first step, intracellular staining was performed on second step. Samples were incubated at RT for 10 min with 5 μL
Table/Таблица
Clinicopathological data in patients with bone tumors Клинико-патологические данные пациентов с опухолевым поражением костей
|
№ |
Gender/ Пол |
Age, years/ Возраст, лет |
Histotype/ Гистотип |
Stage/ Стадия |
Localization of the lesion/ Локализация опухоли |
Tumor size, mm/ Размер опухоли, мм |
|
1 |
Female/ Жен |
28 |
Ewing's sarcoma G2/ Саркома Юинга G2 |
IIb (Т2N0M0) |
Right tibia/ Правая большеберцовая кость |
98 |
|
2 |
Female/ Жен |
63 |
Dedifferentiated liposarcoma/ Дедифференцированная липосар-кома |
IIIa (Т2N0M0) |
Soft tissues of the left thigh/ Мягкие ткани левого бедра |
73 |
|
3 |
Female/ Жен |
45 |
Breast cancer metastasis/ Метастаз рака молочной железы |
IV (Т4N0M1) |
Right femur/ Правая бедренная кость |
200 |
|
4 |
Female/ Жен |
75 |
Chondrosarcoma G3/ Хондросаркома G3 |
IIb (Т2N0M0) |
Right femur/ Правая бедренная кость |
169 |
|
5 |
Male/ Муж |
50 |
Secondary chondrosarcoma G1/ Вторичная хондросаркома G1 |
Ib (Т2N0M0) |
Right femur/ Правая бедренная кость |
147 |
|
6 |
Female/ Жен |
53 |
Leiomyosarcoma G3/ Лейомиосаркома G3 |
IIIb (Т3N0M0) |
Soft tissue of the left thigh/ Мягкие ткани левого бедра |
138 |
|
7 |
Male/ Муж |
35 |
Biphasic synovial sarcoma G2/ Бифазная синовиальная саркома G2 |
IV (Т2N0M1) |
Soft tissues of the left forearm/ Мягкие ткани левого предплечья |
82 |
|
8 |
Female/ Жен |
21 |
Chondrosarcoma G2/ Хондросаркома G2 |
IIb (Т2N0M0) |
Right humerus/ Правая плечевая кость |
125 |
Примечание: таблица составлена авторами.
Note: created by the authors.
of Fc Receptor Blocking Solution (Human TruStain FcX, Sony Biotechnology, USA). Next monoclonal antibodies were added and incubated at RT for 20 min: APC-Cy7-anti-CD45 (clone HI30, IgG1, Sony Biotechnology, USA), BV 605-anti-CD326 (clone 9C4, IgG2b, Sony Biotechnology, USA), FITC-conjugated Annexin V (Sony Biotechnology, USA) and 7-AAD (Sony Biotechnology). The unstained control and antibody quality control was performed. The appropriate isotype antibodies were added to the isotype control sample at the same concentration. After incubation, red blood cells were lysed by 250 μL OptiLyse C buffer (Beckman Coulter, France) at RT for 10 min in dark and washed in 1mL Cell Wash buffer (BD Biosciences, USA) at 800 x g for 6 min.
For intracellular staining cells were permeabilized by 250 μL BD Cytofix/Cytoperm (BD Biosciences, USA) at 4 °С for 30 min in the dark and washed twice in 1mL BD Perm/Wash buffer (BD Biosciences, USA) at 800 × g for 6 min. After samples were diluted in 50 μL BD Perm/Wash buffer (BD Biosciences, USA) and incubated at 4 °С for 10 min in dark with 5 μL of Fc Receptor Blocking Solution (Human TruStain FcX, Sony Biotechnology, USA). Next, monoclonal antibodies were added and incubated at 4 °С for 20 min: BV 605-anti-CD326 (clone 9C4, IgG2b, Sony Biotechnology, USA) pan-cytokeratin (clone
TUNEL assay
To assess tumor cell apoptosis in tissue samples subjected to thermal ablation, a TUNEL analysis (terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling) was conducted using the FITC TUNEL Cell Apoptosis Detection Kit (Servicebio, China). Deparaffinization and antigen retrieval of paraffin-embedded, formalin-fixed tumor tissue sections were performed using the BOND RXm Automated Immunostainer (Leica, Germany) with the Epitope Retrieval Solution 2 (Leica, Germany). Subsequent steps were carried out manually according following procedure. Tissue sections were washed in PBS buffer, further staining was carried out in a humid chamber, preventing the tissue from drying out.
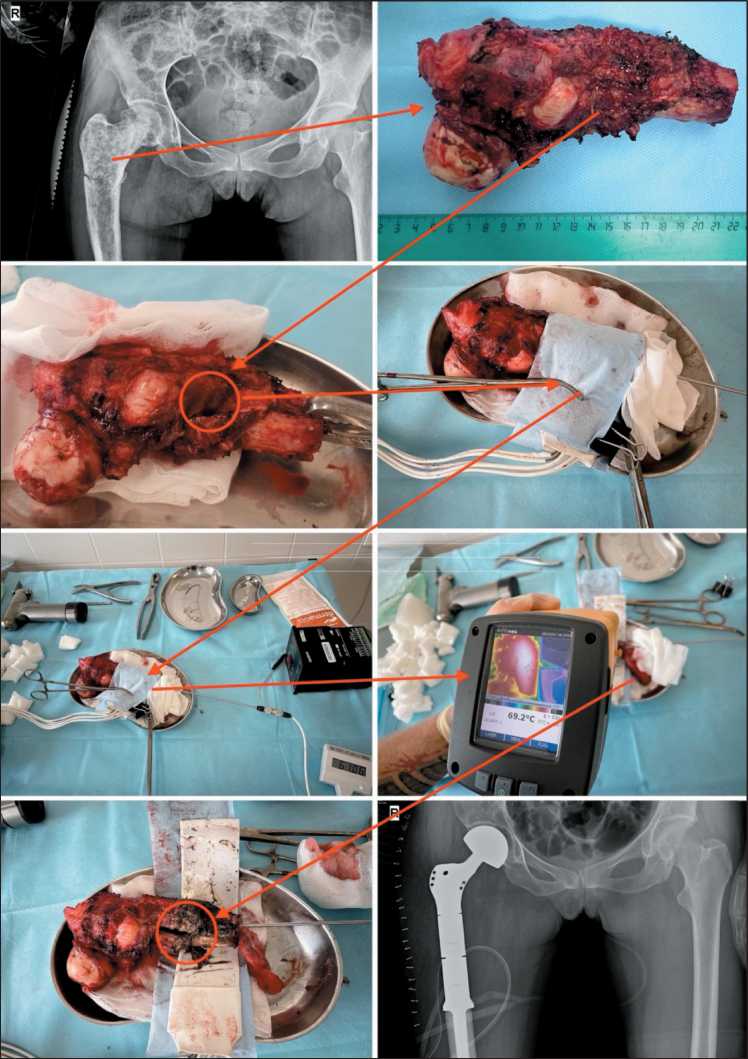
Fig. 1. Stages of a thermal ablation session ex vivo. Note: created by the authors Рис. 1. Этапы сеанса термоабляции ex vivo . Примечание: рисунок выполнен авторами
A working solution of proteinase K (100 μl, diluted 1:9) was applied to each section and then incubated at 37 ℃ for 20 minutes. The tissue sections were washed in PBS buffer three times, each time for 5 minutes. Nuclei were stained using Fluoroshield™ with DAPI (Sigma-Aldrich, USA). Visualization was performed using fluorescence microscope Axio Imager M2 (Zeiss, Germany) and the number of TUNEL-positive cells was counted (Fig. 3).
Statistical analyses
Statistical analyses of the results were performed using the Prism 10 (GraphPad, USA). Non-parametric Fridman’s tests for dependent variables were used to compare differences before and after therapy. The data are presented as median and interquartile range (Me (Q1–Q3)). All tests were two-tailed, and differences with a p-value of less than 0.05 were considered statistically significant.
Results
In our study, we employed two methods to assess apoptotic cell death. Flow cytometry enabled us to evaluate early and late stages of apoptosis separately in tumor and immune cells using population-specific markers. The TUNEL assay was utilized to confirm cell death rates induced by TA.
Before TA, the proportion of tumor cells showing signs of early apoptosis was 0.33 % (0.02–1.39 %) (Fig. 4). Immediately after TA, which lasted 40 min-
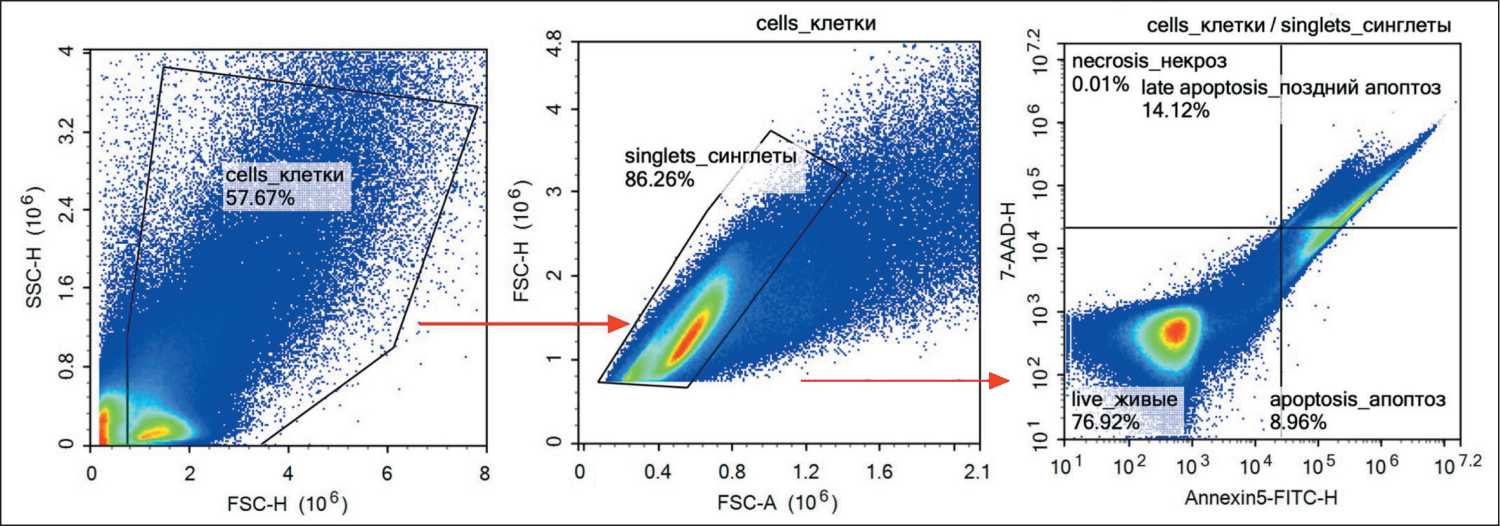
Fig. 2. Flow cytometry analysis of apoptosis in cells obtained from patients’ bone samples. Note: created by the authors Рис. 2. Оценка апоптоза опухолевых клеток методом проточной цитометрии, полученных из образцов опухоли пациентов. Примечание: рисунок выполнен авторами
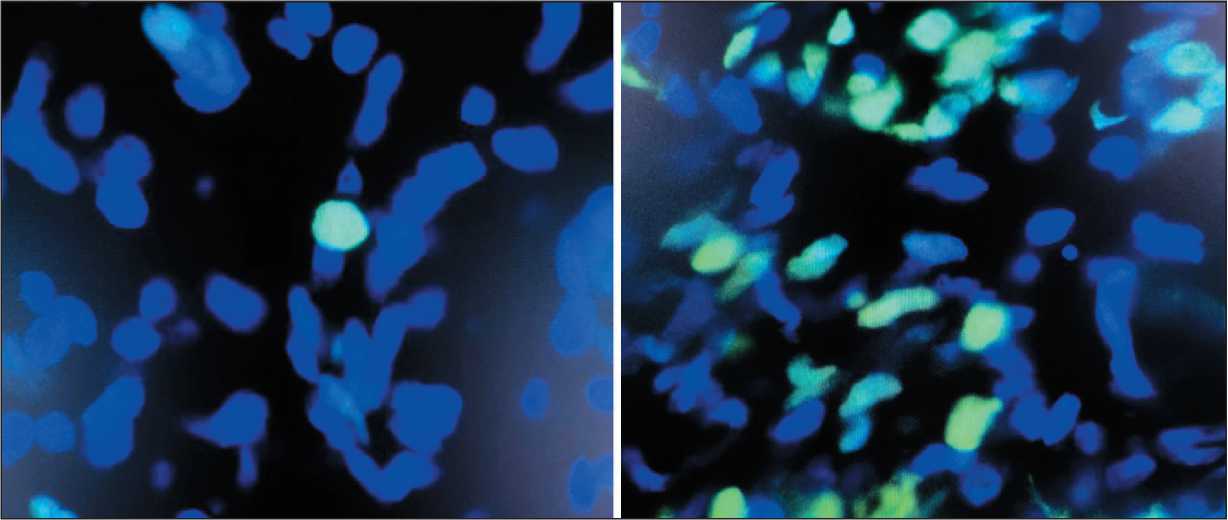
Fig. 3. TUNEL assay – before (left) and after (right) TA procedure. Note: created by the authors
Рис. 3. Метод TUNEL – до (слева) и после (справа) сеанса термоабляции. Примечание: рисунок выполнен авторами
Опухолевые клетки - ранний апоптоз/ Tumor cells - Early Apoptosis
0.0104
I1
0.0053
I1
0.0489
I1
0.8236 0.0284 0.5522
Контроль/ Контроль 1 час/ После ТА/ После ТА 1 час/
Control Control 1 hour After TA After ТА 1 hour
Точки исследования/Study points
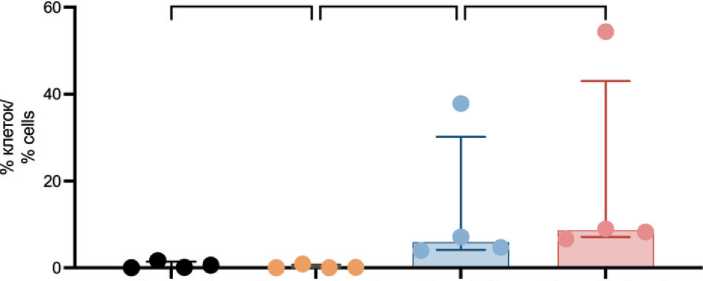
Fig. 4. Proportion of tumor cells exhibiting early apoptotic signs after TA. Note: created by the authors
Рис. 4. Опухолевые клетки с признаками раннего апоптоза после ТА. Примечание: рисунок выполнен авторами
Опухолевые клетки - поздний апоптоз/некроз/ Tumor cells - Late Apoptosis/Necrosis
Контроль 1 час/ Control 1 hour
После ТА 1 час/ After ТА 1 hour
После та/ After ТА
Точки исследования/ Study points
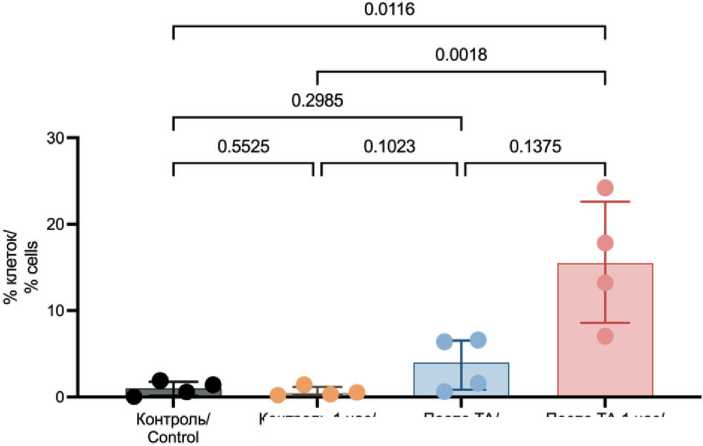
Fig. 5. Proportion of tumor cells exhibiting late apoptosis/necrosis signs after TA. Note: created by the authors
Рис. 5. Опухолевые клетки с признаками позднего апоптоза/ некроза после ТА. Примечание: рисунок выполнен авторами
Лейкоциты - ранний апоптоз/ Leukocytes - Early apoptosis
Точки исследования/ Study points
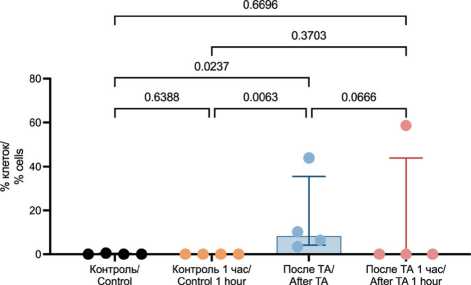
Fig. 6. Assessment of Early Apoptosis in Leukocytes Using Flow Cytometry. Note: created by the authors
Рис. 6. Оценка раннего апоптоза в лейкоцитах с помощью проточной цитометрии. Примечание: рисунок выполнен авторами utes, the proportion significantly increased to 5.88 % (4.14–30.15 %) and reached 8.63 % (7.07–43.05 %) after one hour (p=0.0489 and p=0.0104, respectively). However, no significant difference in the proportion of tumor cells exhibiting early apoptosis was observed between the samples analyzed immediately after TA and one hour later (p=0.5522).
The proportion of tumor cells exhibiting signs of late apoptosis/necrosis was 1.01 % (0.17–1.78 %). After TA, there was a significant increase in the number of tumor cells exhibiting signs of late apoptosis/necrosis, reaching 3.98 % (0.86–6.53 %). While, one hour after TA, a fifteen-fold increase was observed (15.52 % (8.57–22.61 %), p=0.0116) (Fig. 5).
Leukocytes and their precursors are resident cells of bone tissue, making it crucial to assess the impact of TA on these cells. It was shown that TA significantly increases the number of leukocytes exhibiting signs of early apoptosis (0.00 % (0.00–0.39 %) vs 8.31 % (4.15–35.56 %), p=0.0237), with no further increase over time (0.00 % (0.00–43.94 %), p=0.0666 and p=0.6696, respectively) (Fig. 6).
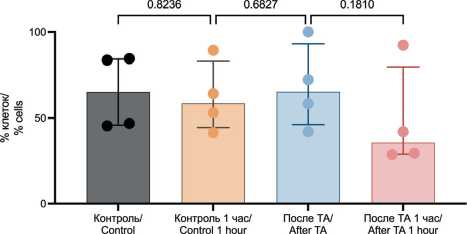
Before TA, the proportion of immune cells with signs of late apoptosis/necrosis was 65.24 % (45.79–84.29 %) (Fig. 7). No significant changes were observed in the proportion of immune cells exhibiting late apoptosis/necrosis either immediately after TA or one hour later (65.36 % (46.09–93.09 %) and 35.70 % (28.84–79.72 %), p=0.8526 and p=0.2494, respectively).
The TUNEL assay allowed us to perform morphological identification of tumor cells with precise assessment of the proportion of apoptotic cells. The obtained data confirmed that TA (using the RIT method) results in significant increase in the proportion of apoptotic cells immediately after the TA and during one hour later (Fig. 8).
Discussion
In clinical practice, surgery continues to be the primary treatment method for bone tumors, underscoring the importance of exploring new and effective tools and therapeutic approaches. The literature regarding the use of TA for bone sarcomas is limited and often merges data with other types of tumors due to their relative rarity and heterogeneity [30]. Specific data are frequently unavailable, and precise molecular mechanisms remain unclear. Evidence suggests that high temperatures can cause both direct and indirect damage to cells, with the effects varying based on
Лейкоциты - поздний апоптоз/некроз/ Leukocytes - Late apoptosis/Necrosis
0.2494 I---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------1
0.3529 I-----------------------------------------------------------------------------------------------------------------------------1
0.8526
I-----------------------------------------------------------------------------------------------------------------------------1
Точки исследования/ Study points
Fig. 7. Assessment of Late Apoptosis/Necrosis in Leukocytes Using Flow Cytometry. Note: created by the authors
Рис. 7. Оценка позднего апоптоза/некроза в лейкоцитах с помощью проточной цитометрии. Примечание: рисунок выполнен авторами
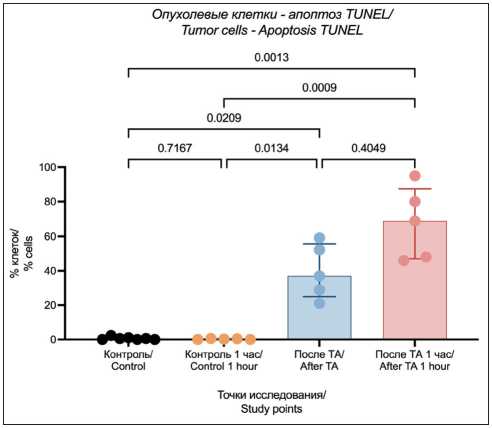
Fig. 8. Apoptosis in tumor cells evaluated using TUNEL assay. Note: created by the authors
Рис. 8. Оценка апоптоза в опухолевых клетках методом TUNEL. Примечание: рисунок выполнен авторами
temperature and duration of exposure [31]. Proteins undergo irreversible damage resulting in coagulative necrosis at temperatures above 60 °C [32]. Such high temperatures compromise membrane integrity, lipid fluidity, and microtubule functionality, impairing substance transport and resulting in cell death [33]. Proton leakage through mitochondrial membranes causes organelle swelling and destruction [34]. Thermal damage also inhibits DNA polymerases, disrupts DNA replication and repair, and affects chromatin condensation [35]. Delayed cell damage is triggered by apoptosis, ischemia, reperfusion injury, lysosomal enzyme release, and immune response [36]. Immunomodulation is enhanced by the release of cytokines such as IL-6 and TNFα, as well as antigens entering lymph nodes [37]. Several studies have demonstrated that hyperthermia induces apoptosis in numerous tumor cell lines [38, 39], including sarcomas. Exposure at 43 °C for 60 minutes increased apoptosis in the human sarcoma U-2 cell line, but not in normal osteoblast cell lines. Additionally, hyperthermia caused an increase in intracellular reactive oxygen species and activation of caspase-3 in U-2 cell line [40].
The available literature lacks data on apoptosis and the effects of high temperatures on tumor cells in sarcoma patients. In this study, we investigated the antitumor effect of TA on human bone tumors. Our data demonstrates that the effect of RIT is mediated through the initiation of programmed cell death. We found that the number of tumor cells exhibiting signs of early apoptosis significantly increased immediately after the TA session (60 °C for 30 minutes) conducted during RIT. It is well known that the effector phase of apoptosis can last from several minutes to several hours, depending on the stimulus [41]. Indeed, our study observed an increase over time in the proportion of tumor cells in the state of early apoptosis. Early stages of apoptosis, such as caspase activation, changes in mitochondrial membrane potential, or external exposure of phosphatidylserine (determined in our study using Annexin V), are potentially reversible if the cell receives recovery signals [42]. In this context, we also assessed the proportion of tumor cells in the state of late apoptosis/necrosis, which significantly increased after RIT. To confirm this observation, the TUNEL method was used to detect DNA fragmentation, indicative of the irreversible phase of apoptosis [43].
The lack of a significant increase in the number of leukocytes showing early apoptosis one hour after RIT suggests that the applied temperature regimen (60° C for 30 minutes) selectively affects tumor cells and their microenvironment. In the control tumor tissue sample, the proportion of leukocytes with signs of late apoptosis/necrosis was relatively high – 65 %. There is scant literature on the proportion of tumorinfiltrating leukocytes that are in the terminal stages of apoptosis or in a necrotic state. For instance, in a study by T.L. Whiteside, it was shown that in 13 out of 27 patients with oral cavity carcinoma, tumor-infiltrating lymphocytes exhibited pronounced apoptosis. More- over, in the circulation of patients with head and neck tumors, 74 ± 15 % of CD3+ T cells expressed Fas, and 29 ± 16 % of Fas+CD3+ T cells bound Annexin V [44]. These high percentages are similar to our findings and might indicate an immunological imbalance in patients with solid tumors.
The TA method we developed and our subsequent studies are focused on enhancing the understanding of the mechanisms underlying the antitumor response to high-temperature exposure. However, it is necessary to expand the patient cohort and conduct additional studies to investigate cell death processes over more extended periods following RIT, as well as to determine the optimal temperature regimen that achieves the ideal balance between affecting malignant and normal cells.
Standard treatments for tumors, such as chemotherapy and radiation therapy, have prescribed doses outlined in treatment guidelines. The lack of precise temporal and temperature parameters forces clinicians to use thermal ablation with caution. Additionally, variations in device designs for the same TA method complicate the implementation of multicenter studies. There is an urgent need to explore the sensitivity of bone tumors to TA, and the development of additional criteria for evaluating pathomorphosis after RIT, which is a crucial task.
Our developed method of RIT expands the arsenal of organ-preserving approaches in the treatment of diaphyseal bone tumors. Its application allows for the preservation of the bone segment affected by the tumor process, which is particularly relevant in cases with an uncertain disease prognosis. This method can serve as either a temporary or a definitive alternative to radical reconstructive interventions (such as wide resection with oncologic endoprosthesis replacement) and may also be used as an adjunct to them. This opens up additional therapeutic options for patients in whom traditional reconstructive surgeries are technically or economically unfeasible.
Conclusion
Despite the aforementioned findings, further investigation is necessary to assess the long-term effects of RIT on bone tumors and their microenvironment. These results will enhance our understanding of the effects of TA, helping to minimize potential complications, improve treatment outcomes, and integrate new methods for treating bone tumors into routine clinical practice. The high variability observed in some data points underscores the need for additional research to more accurately evaluate the efficacy of RIT at later stages of cell death. Employing a broader range of apoptosis markers would be beneficial and could also help determine whether tumor cells of different origins (mesenchymal and epithelial) exhibit varying sensitivities to RIT. Extending the observation period for cellular changes would answer a critical question: how much time is required for RIT to induce complete tumor cell death.


