Evaluation of the nanostructure durability for mineral wool fibers using the theory of che mical corrosion
Автор: Dmitry Yu. Zheldakov, Sergei A. Tursukov, Dmitry A. Sinitsin, Alexander N. Pudovkin, Anastasia A. Parfenova
Журнал: Nanotechnologies in Construction: A Scientific Internet-Journal @nanobuild-en
Рубрика: Application of nanomaterials and nanotechnologies in construction
Статья в выпуске: 1 Vol.15, 2023 года.
Бесплатный доступ
Introduction. Mineral wool is one of the widely used materials in the construction industry. A wide range of technologies and structures with integrated mineral wool is also connected with the following: ventilated facades of buildings, facades made using FICS (facade insulation composite system) technology, insulation of roofs and attics, and much more. Methods and materials. Under different conditions, the nanostructure durability for mineral wool fibers will vary significantly. Currently, there are no scientifically based methods for assessing the durability of the nanostructure. Results. The article proposes to evaluate the nanostructure durability for the of mineral wool fibers based on the developed method of chemical destruction of building ceramics. The methods of laboratory analysis of the building ceramics material and their modernization for the nanostructure of mineral wool fibers are given. Discussion. According to the results of experimental studies, it was found that the corrosion process in the mineral wool material occurs by the mechanism of reaction of alkali metal hydroxides with silicon and aluminum oxides in the mineral wool material, removing them into solution and leading to chemical destruction of the material, which is generally similar to the studied process of destruction of the wall ceramics material. The results obtained allow us to conclude that the process of chemical destruction of mineral wool has a greater dependence on temperature and less dependence on the concentration of hydroxides than the process of destruction of brick material. Conclusions. The results of the conducted studies allow us to calculate the temperature coefficient of the destruction process rate in the Van't-Hoff formula, the coefficients in the Arrhenius equation and the value of the activation energy of the destruction process. Examples of field studies are given.
Mineral wool, durability, laboratory tests, calculation
Короткий адрес: https://sciup.org/142235807
IDR: 142235807 | DOI: 10.15828/2075-8545-2023-15-1-59-71
Текст научной статьи Evaluation of the nanostructure durability for mineral wool fibers using the theory of che mical corrosion
Original article
I n the research works [1,2], the process of chemical destruction of the wall ceramics material is revealed and the possibility of calculating the durability of the material in time units based on the laws of physical chemistry is proved.
The chemical destruction of bricks and masonry is described by a multi-stage process. Humidification of the building ceramics material should be considered the first non-chemical stage of the process [1].
At the first chemical stage of the process, the formation of alkalis from oxides of alkaline and alkaline earth metals occurs in the brick material, and the formation of soluble potassium and sodium silicates is also possible. The alkali can also enter the brick from a cementsand mortar. Basically, it is calcium hydroxide formed in a cement-sand solution during the leaching process [3, 6].
At the second chemical stage of the process, the interaction of the amorphous phase of the material formed in the brick material or / and entering it from the cementsand solution, alkalis with silicon and aluminum oxides occurs. Since the amorphous component is the binding phase of the material, the material of the wall ceramics is completely destroyed to the size of crystalline particles of the order of 10–5 – 10–6 m.
The theoretical justification of the proposed scheme of chemical processes is an analysis based on the laws of
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION chemical thermodynamics. The thermodynamic method of studying reactions is an evaluation method for understanding the probability of the process as a whole and allows us to determine the preference of reactions and the stability of the compounds formed in solutions, their energetic possibility of proceeding and the direction of the processes. The Gibbs – Helmholtz equation makes it possible to calculate the estimation of the possibility of a chemical process by the value of the isobaric-isothermal potential ∆G [4, 5].
It is quite a difficult task to consider the whole complex of reactions that can occur in the mineral wool material when it is moistened. The author considers 178 possible reactions at different stages of the process. Let’s consider some stages of the chemical destruction process based on thermodynamic calculations.
As noted above, the first stage of the process of interaction of mineral wool material with water is described by reactions of alkali formation during hydrolysis of oxides of alkaline and alkaline earth metals. The hydration reactions and the resulting equations of the isobaric-isothermal potential of the analyzed reactions are given in Table 1.
The values of the isobaric potential of reactions at temperatures ranging from minus 20оС to 100оС, that is, in the operating temperature range, are shown in Figure 1. As can be seen from the graph, the process of formation of potassium and sodium alkalis will be most active.
Table 1
Hydration reactions and equations of isobaric-isothermal potential of the analyzed reactions
|
Reaction equation / equation of isobaric-isothermal reaction potential |
Reaction number |
|
Na2О + Н2О → 2NaОН |
2.7 |
|
ΔG = –121320 + 102.76 ТlnТ –89.87*10–3 Т 2–9.76*105/ Т –631.92 Т |
|
|
К2О + Н2О → 2КОН |
2.8 |
|
ΔG = –195159 + 39.86Т lnТ –32.19*10–3 Т 2+ 3.62*105/ Т –664.39 Т |
|
|
MgО + Н2О → Mg(ОН)2 |
2.9 |
|
ΔG = –28891 + 40.99 ТlnТ –5.61*10–3 Т 2+ 0.52*105/ Т –237.11 Т |
|
|
СаО + Н2О → Са(ОН)2 |
2.10 |
|
ΔG = –56663 + 21.98 ТlnТ + 3.48*10–3 Т 2+ 0.36*105/ Т –123.42 Т |
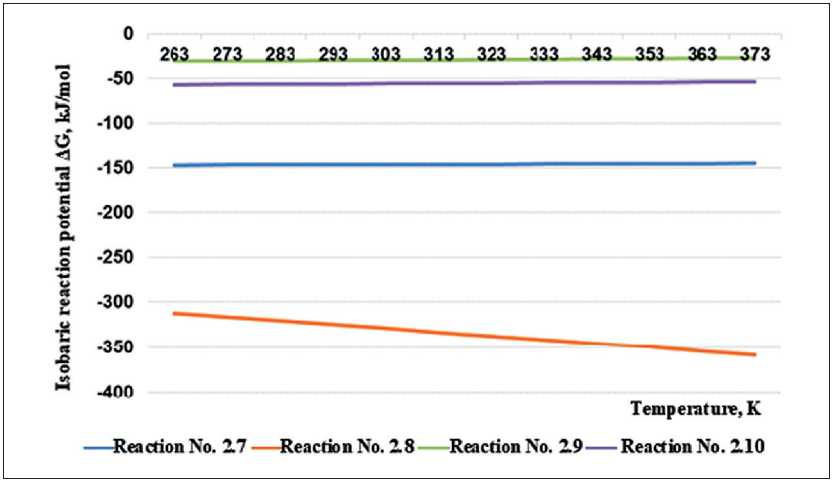
Fig. 1. Calculations results of the dependence of the isobaric potential on temperature for hydration reactions
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
At the next stage of the destruction process, chemical reactions of the interaction of alkalis with silicon and aluminum oxide of the amorphous phase of the wall ceramics material take place. It is these reactions that lead to destruction, since silicon oxide is the main component of the material [21]. For example, Table 2 shows the reactions of potassium hydroxide with silicon oxide of the brick material. Figure 2 shows a graphical analysis of the possibility of reactions and the determination of thermodynamically probable reaction products.
The analysis of the graphs allows us to conclude that the reaction of potassium hydroxide with silicon oxide by the reaction mechanism (2.20) with the formation of potassium pyrosilicate K2Si4O9. The course of other analyzed reactions is also thermodynamically justified.
The resulting potassium and sodium silicates are highly soluble and represent a well-studied “liquid glass”. Alkaline silicates are typical electrolytes and almost completely dissociate in aqueous solutions [7].
Table 2
The resulting equations of isobaric-isothermal potential for reactions of interaction of potassium hydroxide with silicon oxide
|
Reaction equation / equation of isobaric-isothermal reaction potential |
Reaction number |
|
2KOH + SiО2 → K2SiO3+ H2O |
2.18 |
|
ΔG = –98136–57.23 ТlnТ + 58.05*10–3 Т 2+ 1.51*105/ Т + 340.25 Т |
|
|
4КOH + SiО2 → К4SiO4 + 2H2O |
2.19 |
|
ΔG = –111602–85.08 ТlnТ + 123.43*10–3 Т 2–12.89*105/ Т + 532.59 Т |
|
|
2КOH + 4SiО2 → К2Si4O9 + 3H2O |
2.20 |
|
ΔG = –716809–138.95 ТlnТ –5.61*10–3 Т 2–33.46*105/ Т + 1141.62 Т |
|
|
2KOH + 2SiО2 → K2Si2O5 + H2O |
2.21 |
|
ΔG = –78578 + 37.12 ТlnТ + 0.3*10–3 Т 2+ 3.96*105/ Т –278.73 Т |
|
|
2КOH + 3SiО2 → К2Si3O7 + H2O |
2.22 |
|
ΔG = –36207–48.57 ТlnТ + 104.6*10–3 Т 2–20.57*105/ Т + 230.05 Т |
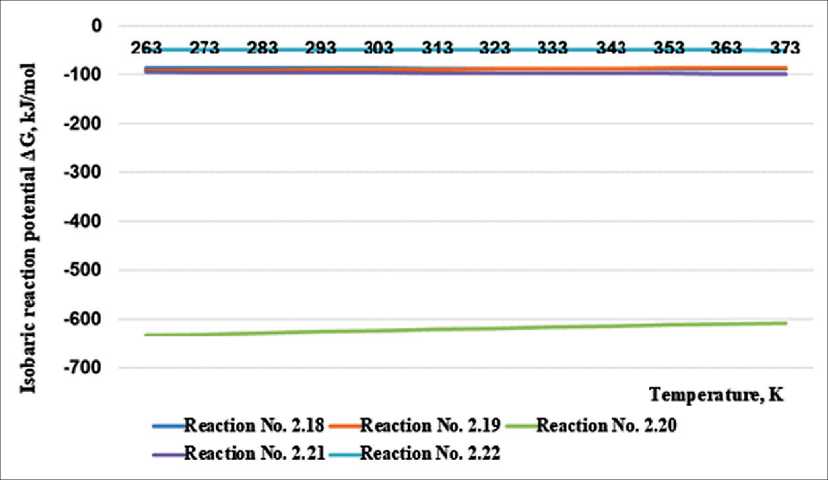
Fig. 2. Results of calculations of the dependence of the isobaric potential on temperature for chemical reactions of interaction of potassium hydroxide with silicon oxide
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
At the next stage, they easily react with calcium oxide or hydroxide, forming insoluble calcium silicates and shifting the entire process of destruction of the material to the right [8–10]. The process of interaction of liquid glass and special additives that cause the formation of insoluble reaction products has been well studied and received the term “modification” of liquid glass. The mechanism of formation of insoluble reaction products of liquid glass modified with calcium-containing and magnesium-containing additives can proceed in two directions: the formation of insoluble silicates and calcium hydrosilicates and the formation of silicic acid gel in the system. Under the conditions of the ratio of the amount of calcium and magnesium in the composition of hydroxides to the amount of potassium and sodium in the composition of “liquid glass” 0.5 and higher, the reactions will take place with the formation of silicates and hydrosilicates of calcium and magnesium. Table 3 discusses the reactions of calcium hydroxide with potassium silicates.
Figure 3 shows a graphical thermodynamic analysis of the chemical reactions of the interaction of calcium hydroxide with potassium silicates.
To assess the rate of destruction of the material, a chemical destruction coefficient is introduced – the percentage of the sample, determined as a percentage, which is destroyed during chemical exposure during the time specified in hours, Cd, [%/hour]. The coefficient of chemical destruction is equal to the ratio of the chemical resistance of the material to the time during which this value of the chemical resistance of the material is achieved. In general, the coefficient of chemical destruction of the material determines the total rate of chemical reactions of the process of destruction of the material.
The results of the research work carried out by the author made it possible to determine the main kinetic characteristics of the total chemical processes occurring in the brick material and on their basis to construct a phenomenological equation of the coefficient of chemical destruction of the wall ceramics material (1) [11]:
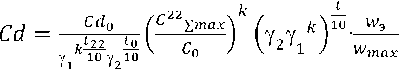
. (1)
where Сd – coefficient of chemical destruction of the material under operating conditions, %/hour;
Сd0 – the coefficient of chemical destruction of the material obtained as a result of laboratory studies according to the developed method at an alkali concentration С0 = 0,5 н and temperature t0 =100оС, %/hour;
-
γ1 – the temperature coefficient of the rate of the hydration process in the Van't-Goff formula, determined as a result of laboratory studies;
Table 3
Reactions of calcium hydroxide with potassium silicates
|
Reaction equation / equation of isobaric-isothermal reaction potential |
Reaction number according to Chapter 2 |
|
К2SiO3 + Ca(OH)2 → СaSiO3↓ + 2КOH |
2.48 |
|
ΔG = 58089 + 19.56 ТlnТ –49.64*10–3 Т 2+ 2.87*105/ Т –114.4 Т |
|
|
К4SiO4 + 2Ca(OH)2 → Сa2SiO4↓ + 4КOH |
2.49 |
|
ΔG = 93983 + 34.07 ТlnТ –127.19*10–3 Т 2+ 15.16*105/ Т –242.94 Т |
|
|
К2Si2O5 + 3Ca(OH)2 → Сa3Si2O7↓ + 2КOH + 2Н2О |
2.50 |
|
ΔG = 32780–130.45 ТlnТ + 11.42*10–3 Т 2+ 8.62*105/ Т + 827.54 Т |
|
|
К2Si2O5 + 2Ca(OH)2 → 2СaSiO3↓ + 2КOH + Н2О |
2.51 |
|
ΔG = –1517–112.45 ТlnТ + 16.51*10–3 Т 2+ 4.79*105/ Т + 730.43 Т |
|
|
К2Si3O7 + 3Ca(OH)2 → 3СaSiO3↓ + 2КOH + 2Н2О |
2.52 |
|
ΔG = –83935–64.43 ТlnТ –79.39*10–3 Т 2+ 33.7*105/ Т + 447.5 Т |
|
|
2К2SiO3 + Ca(OH)2 + 3Н2О → СaSi2O5*2H2O↓ + 4КOH |
2.53 |
|
ΔG = 154077 + 153.63 ТlnТ –74.5*10–3 Т 2+ 10.97*105/ Т –875.92 Т |
|
|
2К2SiO3 + 3Ca(OH)2 + 2Н2О → Сa3Si2O7*3H2O↓ + 4КOH |
2.54 |
|
ΔG = 172951 + 106.57 ТlnТ –108.37*10–3 Т 2+ 16.39*105/ Т –589.29 Т |
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
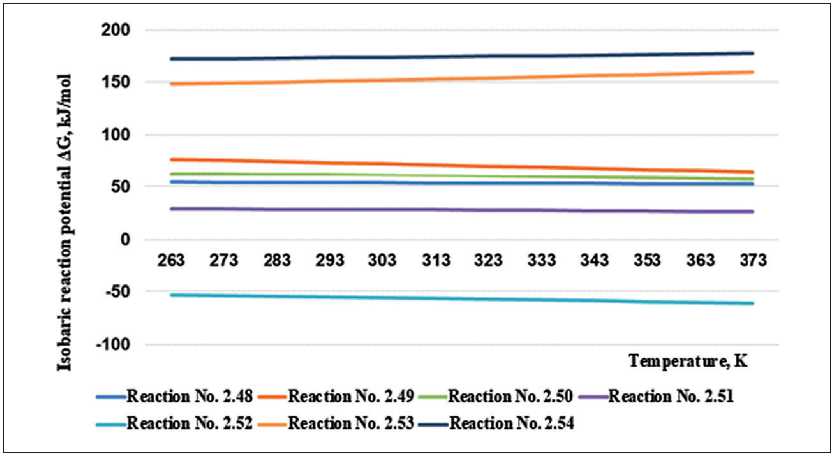
Fig. 3. Results of calculations of the isobaric potential dependence on temperature for reactions of interaction of calcium hydroxide with potassium silicates
-
γ2 – the temperature coefficient of the rate of the chemical destruction process in the Van't-Hoff formula, determined as a result of laboratory studies;
-
k – the power-law coefficient of conversion of the reaction rate of destruction from the concentration of substances;
-
t22 – laboratory experiment temperature t = 22оС;
-
t0 – laboratory experiment temperature t0 = 100оС;
-
t – operating temperature of the material;
С 22∑max – the maximum equilibrium concentration obtained as a result of laboratory studies according to the developed methodology, at a temperature of t = 22оС;
-
С0 – concentration of potassium hydroxide in the experiment, С0 = 0,5 н
-
wmax – maximum humidity of the wall ceramic material;
-
wэ – operational humidity of the wall ceramic material.
The durability of the material, in hours, will be equal to
D = 1/0,01 Cd . (2)
METHODS AND MATERIALS
The chemical composition of the materials of construction ceramics and mineral wool almost completely coincide. The composition of both materials includes oxides of silicon, aluminum, oxides of alkaline and alkaline earth metals. At the same time, the phase composition of the two materials has significant differences [12].
The phase composition of the samples was determined using an ARL X'TRA powder X-ray diffractometer. The analysis was carried out by interplanar distances in manual mode using the Hanawalt method and in semi-automatic mode using the Oxford Crystallographica Search Match software using the ICDD PDF-2 database. Quantitative X-ray phase analysis using the Rietveld method was carried out using Siroquant 3 Sietronics Pty Ltd software.
The results of the study showed that mineral wool is an amorphous aluminosilicate [13]. The proportion of amorphous matter in the brick material that participated in the study is 20%.
It can be assumed that the processes occurring during the hydration of mineral wool are identical to those studied in this work, and therefore, the developed research methods are applicable to them. It was hypothesized that it is possible to apply the developed theory of chemical corrosion of the building ceramics material and the calculation method to determine the durability of mineral wool.
RESULTS
Based on scientific studies of the kinetics of chemical processes occurring in the brick material, two research methods have been developed, currently undergoing the procedure of approving them as normative documents: GOST R “Brickwork enclosing structures. Method for determining the corrosion activity of moisture” and GOST R “Enclosing structures made of brickwork. Method for determining chemical resistance”. These techniques were used for laboratory studies of the process of destruction of mineral wool. In addition, some requirements were used in the development of the methodology for preparing a sample of the material under study [14–15].
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
For testing, a sample of mineral wool material is taken in accordance with the task. The pieces of material are combined into a combined sample, pre-ground in a mortar and dried in a drying cabinet at a temperature of 105–115оС to a constant mass. Immediately after drying, a weight of 2 g is prepared, weighted with an error of no more than 0.001 g.
The technique is based on the process of interaction of alkaline and alkaline earth metals present in the mineral wool material in the form of oxides with water.
The tests are carried out at a temperature of 22оС and 100оС as follows: the suspension is placed in a conical flask with a capacity of 500 ml and 250 ml of distilled water is poured. When conducting an experiment at a temperature of 22оС, the flask is tightly closed with a apped or rubber stopper and kept at a temperature of 20–22оС for 1 hour, 5 hours and 25 hours. During the experiment, the flask is periodically shaken at intervals of 5–10 minutes.
When conducting an experiment at a temperature of 100оС, 250 ml of distilled water is preheated in a flask connected to a reverse refrigerator. After the start of boiling, a weight of 2 g is placed in the flask, weighted with an error of no more than 0.001 g.
After the end of the holding time, the flask with the sample is shaken, the sample of the material is allowed to settle, the liquid is transferred to a container for subsequent analysis of the concentration of elements on an atomic emission spectrometer with inductively coupled plasma and into a container for pH measurement.
Determination of the concentration of the elements sodium, potassium, calcium and magnesium by an atomic emission spectrometer with inductively coupled plasma is carried out in accordance with GOST R 57165-2016 (ISO 11885:2007) “Water. Determination of the content of elements by atomic emission spectrometry with inductively coupled plasma” [16]. The results of the analysis are presented in units of mg/l (mg/dm3). The pH is determined using a pH meter with an accuracy of 0.1.
The developed method for determining the chemical resistance of mineral wool material is based on the process of interaction of alkali with mineral wool material with repeated exposure to alkali on the sample and determining the change in the mass of the sample before and after exposure and the time for which this change occurred. Thus, in the course of a laboratory experiment, the value of the coefficient of chemical destruction of the mineral wool material is obtained.
During the research, experiments are carried out at a process temperature of 22оС and 100оС. The tests are carried out in stages. At each stage, the following actions are carried out: the suspension is placed in a conical flask with a capacity of 500 ml and 250 ml of potassium hydroxide solution is poured. The concentration of potassium hydroxide solution in experiments is assumed to be 0.5 n and 5.0 n [17].
When conducting an experiment at a process temperature of 100оС, the flask is placed on a preheated electric stove, connected to a reverse refrigerator and boiled. The boiling time is one hour. After the boiling process, the solution is filtered. When conducting an experiment at a temperature of 22оС, the sample is placed in a flask, closed with a rubber or lapped lid and kept under traction for 10–15 days, periodically shaking the flask.
After the end of the experiment time, the solution from the flask is filtered. The filter with the sample remaining on it is placed in a drying cabinet and dried at a temperature of 105–115оС to a constant mass. The dried sample is weighed on a scale immediately after drying with a measurement error of no more than 0.001 g. The sample is returned to the flask after weighing. The tests are repeated until the mass of the sample in the two subsequent tests differs by no more than 0.01 g, that is, 0.5% of the initial mass of the sample.
DISCUSSION
The study of the hydration process was carried out for samples of mineral wool and brick material according to the method described above, developed at a temperature of 100оС for one hour. Samples of mineral wool and bricks from various manufacturers were taken for the study. The results of the study are shown in Table 1.
The obtained results on the composition of ions and elements in the aliquot, as well as on the value of the hydrogen index, confirm the course of hydration reactions of mineral wool when it is moistened. At the same time, it is obvious that, first of all, hydroxides of alkaline and alkaline earth metals, acids and salts of fluorine, chlorine and sulfur are formed.
When comparing the concentration of elements in the solution for the two materials, it is necessary to note a significant excess of the content for all elements for the mineral wool material compared with the wall ceramics material.
The corrosion process takes place inside the mineral wool material in the same way as in the studied process of destruction of the wall ceramics material: alkali metal hydroxides can actively react with silicon oxides and, to a lesser extent, with aluminum oxides in the mineral wool material, removing them into solution and leading to chemical destruction of the material. A significant amount of silicon and aluminum in the solution (Table 4) indicate the course of this process. Thus, the possibility of the chemical destruction process proceeding according to a theoretically justified mechanism is confirmed.
Laboratory experiments to determine the coefficient of chemical destruction of mineral wool material were carried out according to the method described above for rock wool and glass wool (Figure 4, 5).
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
Table 4
The content of ions and elements in an aqueous solution
|
Element |
The content of ions and elements in an aqueous solution, mg/dm3 |
|||||||
|
Sample, № p/p |
||||||||
|
Mineral wool |
Brick |
|||||||
|
1 |
2 |
3 |
4 |
1 |
2 |
3 |
4 |
|
|
F– |
0.55 |
0.50 |
0 |
2.35 |
– |
– |
– |
– |
|
Cl– |
8.58 |
3.60 |
4.42 |
2.42 |
0.22 |
0.23 |
– |
– |
|
S |
0 |
10.90 |
2.53 |
1.74 |
1.05 |
1.26 |
0.38 |
0.78 |
|
K |
2.30 |
0.98 |
0.52 |
4.79 |
1.45 |
1.17 |
0.64 |
0.95 |
|
Na |
7.64 |
10.44 |
4.06 |
2.80 |
1.72 |
0.99 |
0.40 |
0.21 |
|
Mg |
3.45 |
5.24 |
1.64 |
0.54 |
1.03 |
1.51 |
0.46 |
0.10 |
|
Ca |
11.79 |
9.89 |
11.78 |
17.08 |
4.2 |
6.22 |
3.30 |
1.83 |
|
Si |
17.66 |
13.86 |
12.40 |
15.85 |
1.67 |
3.71 |
5.21 |
4.09 |
|
Al |
3.91 |
2.33 |
1.66 |
1.91 |
0.34 |
0.30 |
1.34 |
0.50 |
|
pH |
9.37 |
8.69 |
10.5 |
10.70 |
8.50 |
9.18 |
8.15 |
8.20 |

Fig. 4, 5. The course of the experiment with mineral wool

The results of an experiment to study the process of destruction of mineral wool material are shown in Figure 6. For comparison, Figure 6 shows a graph of the coefficient of chemical destruction of brick material. It can be seen that the coefficient of destruction of the stone wool material is less than for glass wool and brick material. This determines that, under equal conditions, stone wool will collapse more slowly [22]. However, it should be borne in mind that the concentration of alkali and alkaline earth metal hydroxides in the mineral wool material, as shown in Table 4, is significantly higher than in the brick material. According to equations (1) and (2), this will lead to a decrease in the durability of the material.
Figure 7 shows the results of the experiment when exposed to the material of stone wool and brick with potassium hydroxide with a concentration of 5.0 n.
The author also conducted studies of the coefficient of chemical destruction of brick and mineral wool material at a process temperature of 22оС. At the same time, the sample holding time for each experiment was 10–15 days. The results of the experiment at concentrations of potassium hydroxide 0.5 n and 5.0 n are shown in Figures 8 and 9.
The analysis of the process of chemical destruction of mineral (stone) wool material is presented in Figures 10 and 11. The graphs compare the rate of chemical de-
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
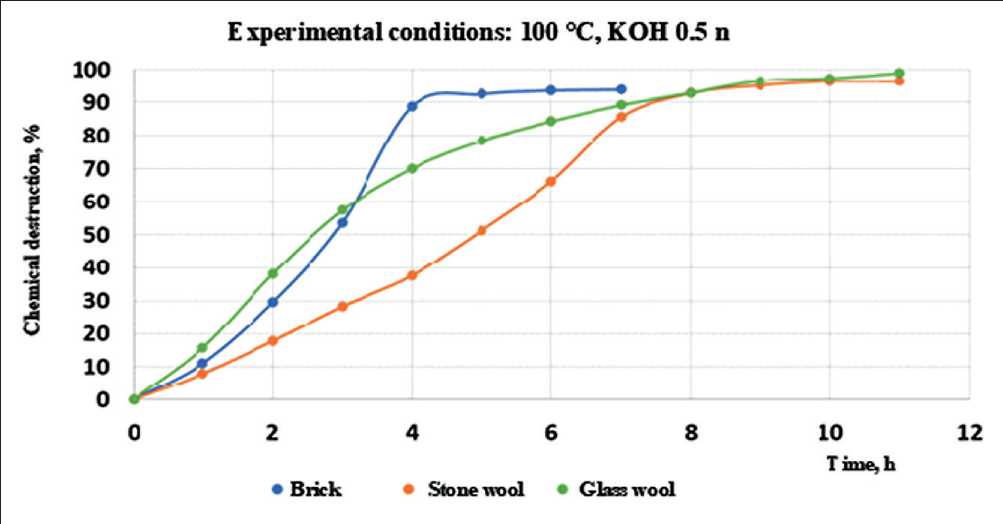
Fig. 6. Chemical degradation coefficient values for stone and glass wool in contact with 0.5 n potassium hydroxide at 100оС
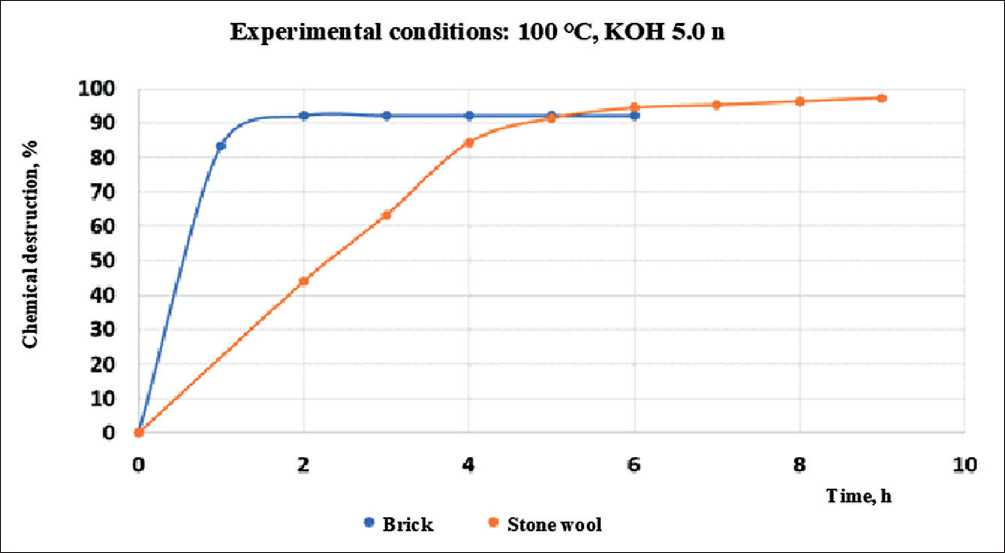
Fig. 7. The values of the chemical destruction coefficient for stone and glass wool in contact with 5.0 n potassium hydroxide at 100оС
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
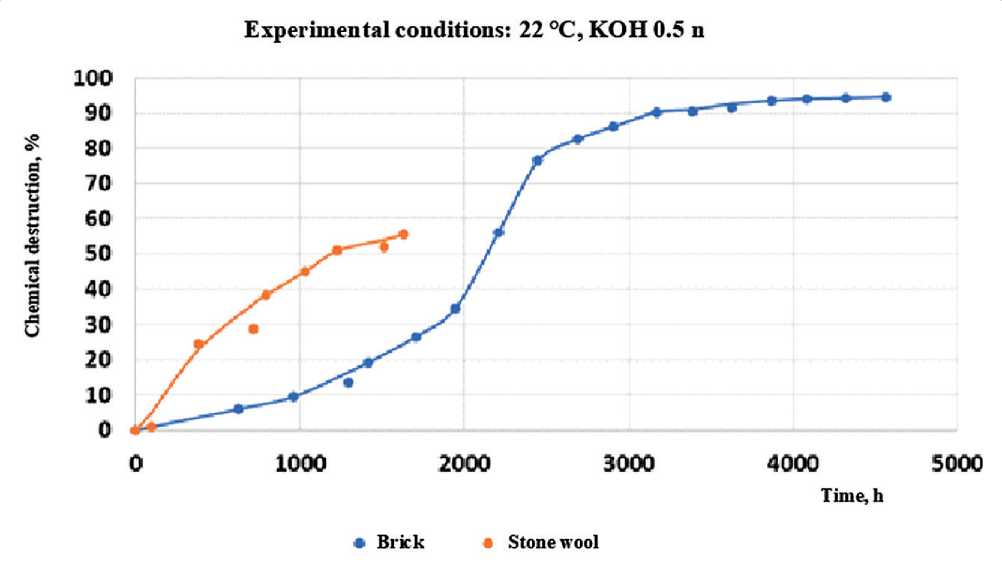
Fig. 8. The values of the chemical destruction coefficient for stone and glass wool in contact with 5.0 n potassium hydroxide at 22оС
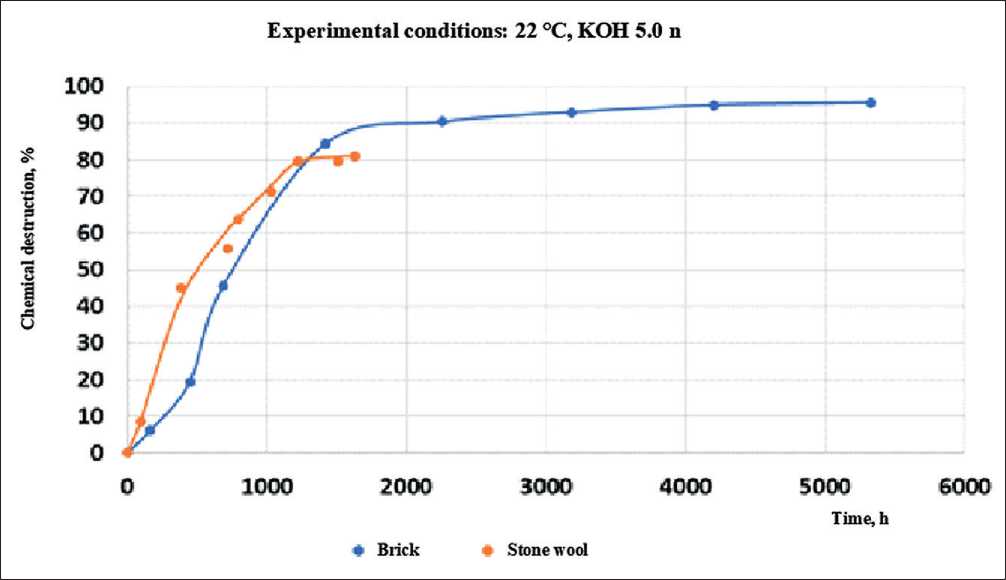
Fig. 9. The values of the chemical destruction coefficient for stone and glass wool in contact with 5.0 n potassium hydroxide at 22оС
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
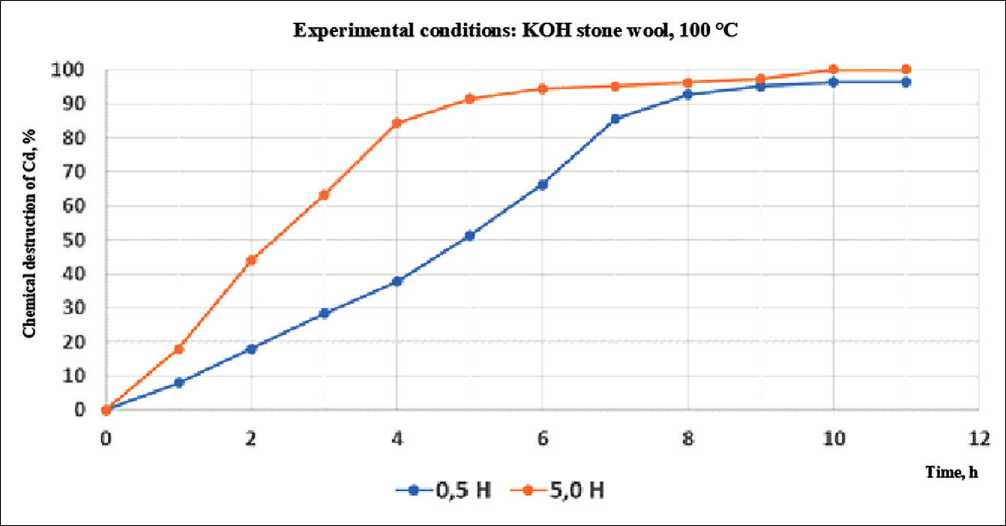
Fig. 10. Chemical degradation coefficient values for rock wool in contact with 0.5 n and 5.0 n potassium hydroxide at 100оС
Experimental conditions: KOH stone wool 22 °C
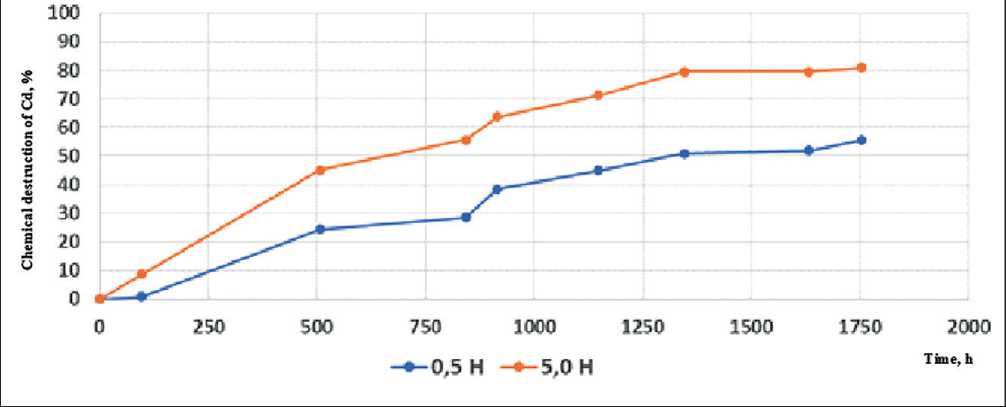
Fig. 11. Chemical degradation coefficient values for rock wool in contact with 0.5 N and 5.0 n potassium hydroxide at 22оС
struction of rock wool at a concentration of potassium hydroxide of 0.5 and 5.0 N and process temperatures of 100оС and 22оС.
Based on these studies, the coefficient of chemical destruction was calculated for various conditions of the chemical corrosion process of the stone wool material. The calculation results are shown in Table 5.
These results made it possible to calculate the temperature coefficient of the reaction rate in the Van’t-Hoff equation γ = 2,08 and find the power factor k in the equation of the dependence of the rate of the destruction process on the concentration k = 0.202.
For the brick material, these values are equal γ = 1.25 and k = 0.374. These results allow us to conclude that the process of chemical destruction of mineral wool material depends more on temperature than the process of destruction of brick material and depends less on the concentration of alkali in the material.
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
Table 5
Values of the chemical destruction coefficient for the rock wool material under different experimental conditions
|
Concentration of KOH in the experiment |
Temperature, оС |
|
|
22 |
100 |
|
|
0.5 н |
0.037 |
11.5 |
|
5.0 н |
0.059 |
18.0 |

Fig. 12. A sample of mineral wool exposed to moisture
The results of the experiment are shown in the figure 1 3
Based on the data obtained, the coefficients in the Arrhenius equation for the rate of destruction of the studied mineral wool material were calculated. The Arrhenius equation will be written:
ln Cd = –8198.8/ T + 24.5. (3)
The activation energy of the destruction process will be Е* = 68132.0 J/mol. For the brick material, the previously calculated activation energy of the destruction process Е* = 86450 J/mol. That is, “starting” the process of destruction of mineral wool material requires significantly less energy.
The results of the research were tested on a full-scale experiment. During the dismantling of building structures, samples of mineral wool were taken. One sample was inside the structure, the other on the edge and was actively exposed to moisture (Figure 12).
In the graphs shown in Figure 13, the first points are of the greatest interest. The active destruction of a sample that has already undergone the process of chemical destruction under field conditions is recorded experimentally. Presumably, by the difference in the values of the chemical destruction coefficient, it is possible to determine a decrease in the durability of mineral wool.
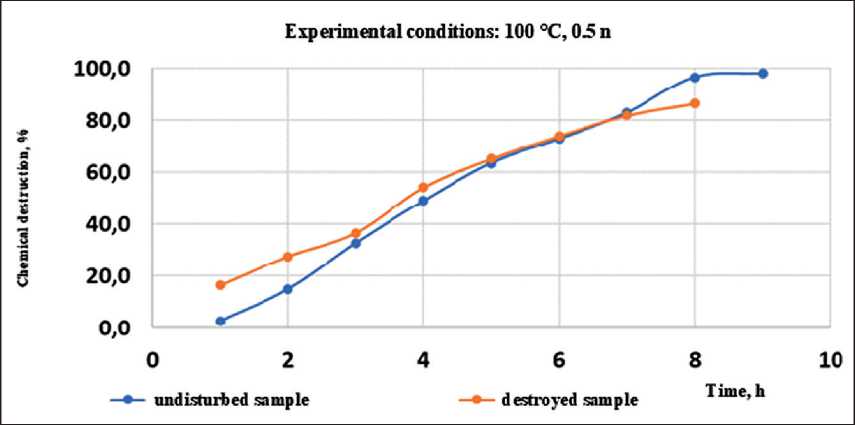
Fig. 13. Results of comparison of chemical destruction of natural samples of mineral wool
APPLICATION OF NANOTECHNOLOGIES AND NANOMATERIALS IN CONSTRUCTION
CONCLUSION
As a result of the conducted research, it has been proved that the nature of the chemical reactions of mineral wool hydration processes and mineral wool degradation processes under the action of hydroxides is identical with similar processes occurring in the material of wall ceramics. This suggests that the theory of durability calculation developed for the wall ceramics material is broader in nature and can be used for a significant range of building materials [23, 24].
Based on the performed studies and calculations of the temperature coefficient of the destruction process rate in the Van’t-Hoff equation and the power coefficient of the process rate on the concentration of reacting substances, it can be concluded that the process of chemical destruction of mineral wool has a greater dependence on temperature
and a lesser dependence on the concentration of hydroxides than the process of destruction of brick material.
The activation energy of the process of destruction of mineral wool is calculated. The activation energy of the process of destruction of mineral wool is 21% less than the activation energy of the process of destruction of the material of wall ceramics, which gives reason to assert that the process of destruction of the material of mineral wool in the system proceeds much easier.
The mineral wool material has a different rate of destruction, and therefore different durability [25]. The glass wool material undergoes destruction much faster than the stone wool material.
To calculate the durability of the mineral wool material according to the phenomenological equation (1), it is necessary to conduct additional studies of the effect of temperature on the rate of the hydration process.
Список литературы Evaluation of the nanostructure durability for mineral wool fibers using the theory of che mical corrosion
- Zheldakov D.Yu. Methods of investigation of the kinetics of the process of chemical corrosion of brick masonry materials. Izvestiya higher educational institutions. Construction. 2019; 11(731): 74-86.
- Zheldakov D.Yu. Features of chemical corrosion of brickwork. International science and technology conference EarthScience IOP Conf. Series: Earth and Environmental Science. 459 (2020) 062089. https://doi.org/10.1088/1755-1315/459/6/062089
- Zheldakov D.Y. Chemical corrosion of brickwork. The course of the process. Building materials. 2019; 4:36-43. https://doi.org/10.31659/0585-430X-2019-769-4-36-43
- Maradudina E.S. Khabarova E.E., Abramenko A.A. Mineral wool as an effective insulating material in the structures of residential buildings. Innovative methods of designing building structures of buildings and structures: collection of scientific papers of the All-Russian Scientific and Practical Conference. Kursk. November 21. 2019; 147-149.
- Yartsev V.P. Mamontov A.A., Mamontov S.A. The influence of external influences on the thermophysical and long-term mechanical properties of mineral wool plates. Issues of modern science and practice. V.I. Vernadsky University. 2014;1(50):125-134.
- Gazizov A.M., Zairov A.A., Yangirova R.R., Timerov M.R. Environmental impact assessment of various thermal insulation materials. Electronic scientific journal Oil and Gas business. 2021; 1:40-59. https://doi.org/10.17122/ogbus-2021-1-40-59
- Abdrakhimov V.Z. The use of waste from the production of mineral wool for the production of wall materials. Ecology of industrial production. 2019; 2(106): 9-12.
- Yartsev, V.P. Mamontov A.A., Mamontov S.A. Operational properties and durability of thermal insulation materials (mineral wool and expanded polystyrene) . Roofing and insulation materials. 2013; 1:8-11.
- Rakhmankulov D.L., Bugai D.E., Gabitov A.I., Gonik A.A., Akhiyarov R.Zh., Kalimullin A.A. Corrosion inhibitors. Vol. 4 Theory and practice of anticorrosive protection of oilfield equipment and pipelines. Moscow; 2007.
- Burlakov D.V. Medvedeva N.L., Ishchuk N.V. Analysis of thermal insulation materials. Research in construction, heat and gas supply and energy supply: Materials of the international scientific and practical conference. Saratov. Ed. F.K. Abdrazakov. November 17-18. 2016; 43-46.
- Methodology for assessing the properties of mineral fibers / E.Y. Bobrova, A.A. Medvedev, A.D. Zhukov, A.I. Poserenin. Innovations in life. 2018; 4(27): 97-102.
- Garkushin I.K., Lavrentieva O.V., Istomova M.A., Kalmykova O.Y. Structural materials: composition, properties, application. Samara: Samara State Technical University; 2015.
- Abramyan S.G., Mikhailova N.A., Kotlyarevsky A.A., Semochkin V.O. Thermal insulation materials ensuring energy efficiency of facade systems. Engineering Bulletin of the Don. 2018; 4(51): 221.
- Kupriyanov V.N., Kupriyanov V.N., Ivantsov A.I. Thermal aging of polymer-containing thermal insulation materials in external walls. Expert: theory and practice. 2020; 3(6):31-36. https://doi.org/10.24411/2686-7818-2020-10022
- Rumyantsev B.M., Zhukov A.D., Bobrova E.Y., Smirnova T.V. Technological aspects of the operational resistance of mineral fibers. Industrial and civil construction. 2015; 1: 32-36.
- Derevyakina V.Yu., Muravyev E.A., Erofeev A.V. The influence of freezing–thawing cycles on the change of heat-protective qualities of insulation. Sustainable development of the region: architecture, construction, transport: Materials of the 4th International Scientific and Practical Conference of the Institute of Architecture, Construction and Transport of Tambov State Technical University. Tambov. June 15-16. 2017; 241-244.
- Yartsev V.P., Strulev S.A., Mamontov A.A. Evaluation of the economic efficiency of the use of insulation in the enclosing structures of frame-panel buildings. VolgGASU Internet Bulletin. 2015; 1(37):12.
- Yartsev V.P., Strulev S.A., Mamontov A.A. Analysis of the economic feasibility of using various enclosing structures of buildings. Building materials, equipment, technologies of the XXI century. 2018; 7-8 (234-235):24-27.
- Kravchenko K.S. Segaev I.N. Analysis of insulation materials for thermal insulation of buildings and structures. Alley of Science. 2018; 1. 4(20): 242-246.
- Enyushin V.N. Nurmukhametova A.D., Khaeretdinova A.D. Energy efficiency of modern enclosing structures. Proceedings of the Kazan State University of Architecture and Civil Engineering. 2016;4(38): 217-221.
- Gnip I.Ya., Vaitkus S.I. Analytical description of creep deformations of mineral wool (MW) plates under prolonged compression. Building Materials. 2013; 11: 57-62.
- Zheldakov D.Yu. Chemical destruction of mineral wool. Industrial and civil construction. 2021; 5: 26-33.
- Yartsev V.P., Mamontov A.A. Comparative analysis of the effectiveness of insulation in frame housing construction. Roofing and insulation materials. 2016; 6: 32-35.
- Chernoivan V.N., Chernoivan N.V., Chernoivan A.V. Assessment of the influence of atmospheric influences on the strength and elastic characteristics of mineral wool slabs in wall insulation systems. Industrial and civil construction. 2017; 1: 101-104.
- Ivanova T.A., Kolesnikova L.G. Thermal insulation materials of enclosing structures of external insulation systems. Young scientist. 2022; 19(414): 91-94.


