Exploring the link between bone biomarkers and proinflammatory cytokines in patients with osteoarthritis
Автор: Al-Mosawi R.A., Abed Shubar S.N., Aldin B.B., Al-Fahham A.A.
Журнал: Гений ортопедии @geniy-ortopedii
Рубрика: Оригинальные статьи
Статья в выпуске: 6 т.31, 2025 года.
Бесплатный доступ
Background Osteoarthritis is multifactorial joint disorder marked by the progressive breakdown of articular cartilage, alterations in the underlying subchondral bone, and chronic inflammation of the synovial membrane. Objective To measure serum levels of bone biomarkers (osteocalcin and sclerostin) in osteoarthritis patients as compared to healthy controls and also to find out the link of these biomarkers with proinflammatory cytokines including IL-6, IL-17, IL-1β and TNF-α. Materials and methods A case-control study was implemented on 65 osteoarthritis patients and 35 healthy controls participants. Blood samples were taken from participants after obtaining written informed consent. Serum levels of cytokines and bone markers were measured using ELISA. Pain disability and intensity were measured using the "Chronic Pain Grade questionnaire". Results Compared to controls, patients with osteoarthritis had significantly higher levels of IL-1β, TNF-α, IL-6, and IL-17 (P < 0.0001 for all). Osteocalcin levels were dramatically lower in the osteoarthritis group than the controls (mean ± SD: 23.50 ± 19.30 ng/mL vs. controls 48.90 ± 5.20 ng/mL), while sclerostin levels were much higher (11.70 ± 1.10 ng/mL in osteoarthritis vs. 3.80 ± 0.90 ng/mL in controls, P < 0.0001). Osteocalcin showed a moderate positive correlation with IL-17, IL-6, and TNF-α; sclerostin showed a negative correlation with these cytokines. Discussion A strong positive correlation exists between osteocalcin and proinflammatory interleukins. The downregulation of sclerostin in OA also shares common pathways with proinflammatory cytokines that drive expression of osteocalcin. Inflammation results in osteocyte apoptosis or their dedifferentiation, and this further lowers the population of sclerostin-secreting cells in the subchondral bone. This is how the reverse correlation is explained between sclerostin and proinflammatory cytokines in OA. Conclusions Results show a robust inflammatory-bone axis in the pathogenesis of osteoarthritis. High proinflammatory cytokines might bring about osteocalcin expression and also inhibit sclerostin, leading to pathological subchondral bone alteration. These biomarkers reflect disease activity and therefore could be used for early detection as well as monitoring and phenotypic stratification of osteoarthritis.
Sclerostin, Osteocalcin, Osteoarthritis, IL-1β, TNF-α, IL-6, IL-17
Короткий адрес: https://sciup.org/142246509
IDR: 142246509 | УДК: 616.72-007.248:616-072.5:612.398.12:616.153 | DOI: 10.18019/1028-4427-2025-31-6-773-779
Текст научной статьи Exploring the link between bone biomarkers and proinflammatory cytokines in patients with osteoarthritis
Osteoarthritis is a multifactorial disease of the joints involving progressive degradation of the cartilage surface and changes in the underlying bone as well as chronic inflammation of the synovial membrane. The interplay between proinflammatory cytokines and bone biomarkers exerts a critical role in the pathophysiology of OA, influencing progression of the disease and treatment outcomes. Sclerostin is a product of osteocytes and well comparably known to suppress the differentiation of osteoblasts and promote the differentiation of osteoclasts. This idea has been recently introduced and evolved [1]. In OA, the imbalance between bone formation and resorption leads to joint degeneration. The pathway of Wnt signaling performs major functions in the formation of bones [2]. Suppression of Wnt signaling by sclerostin may lead to decreased bone formation; this can be taken as an influence on OA. Results showed normal sclerostin levels in people with type 1 diabetes but elevated levels in type 2 patients; most of them have low bone turnover [3]. This dysregulation applies to OA since that means altered bone metabolism which then compromises the integrity of joints. It has been suggested that anti-sclerostin therapies serve as a therapeutic pathway for OA by restoring the balance of bone remodeling and, hence, reestablishing healthy joints [2]. Osteocalcin, like a marker mainly for bone formation, has received more attention for its systemic functions, including modulation of glucose metabolism and energy homeostasis. In other words, since its levels correlate with changes in cartilage structure over time, osteocalcin levels could be indicative of the quantity of cartilage volume loss in OA patients [4]. The interplay of sclerostin with osteocalcin is very important in the context of OA. Control of osteocalcin by other factors, for example leptin, may indirectly influence its levels and therefore bone formation and the health of cartilage [5]. Abnormal leptin production by osteoblasts in OA could also drive altered osteocalcin levels further complicating the metabolic aspects of this disease. Also, using sclerostin and osteocalcin levels as markers in osteoarthritis could give clues into how the disease works. Latest studies hint that although osteocalcin levels might not vary much between OA patients and controls, they are important for the picture of bone and cartilage metabolism [6]. Osteoarthritis has long been known as a non-inflammatory disorder of the joints, but recently it has been established that there is the involvement of low-grade inflammation which remains sustainable for the development and progression of the disease. The major pro-inflammatory cytokines participating in inducing that resultant inflammatory state are IL-6, TNF- α , IL-1 β , and IL-17 in the OA disease state which are key mediators in the molecular cascade leading to cartilage degradation, synovial hyperplasia, osteophyte formation, and pain sensitization [7]. Though the existing literature is very insightful, there are gaps in knowledge regarding the exact roles of sclerostin and osteocalcin in OA and their link to cytokines. The aim of this work is to assess serum levels of bone biomarkers (osteocalcin and sclerostin) in OA patients as compared to healthy controls and also to find out the link of these biomarkers with proinflammatory cytokines including IL-6, IL-17, IL-1 β and TNF- α .
Objective To measure serum levels of bone biomarkers (osteocalcin and sclerostin) in Osteoarthritis patients as compared to healthy controls and also to find out the link of these biomarkers with proinflammatory cytokines including IL-6, IL-17, IL-1 β and TNF- α .
MATERIALS AND METHODS
Patients and data collection
This case-control cross-sectional study was carried out at the Medical City complex in Baghdad, Iraq. It extended from August 2024 to April 2025. A sample of 65 patients diagnosed with osteoarthritis was enrolled from the rheumatology and orthopedic outpatient clinics. Controls were a group of 35 age- and sex-matched apparently healthy individuals free of joint diseases or any systemic inflammatory condition, recruited during the same period. Demographic data (age and body mass index) were collected through direct interviews and physical measurements. The classification of BMI adopted WHO criteria. Levels of IL-6, IL-17, IL-1 β , TNF- α , osteocalcin, and sclerostin in the serum were measured by performing commercially utilized sandwich ELISA kits (R&D Systems, USA) as per instructions provided by the manufacturer. For sclerostin, samples were added to Wells of a microplate already coated with capture antibody specific to sclerostin. Then detection was carried out using an enzyme-linked secondary antibody. After thoroughly washing away unbound reagents, substrate solution was added and allowed to incubate until color development occurred. Absorbance was read at 450 nm; use was made of a standard curve to determine concentration in each sample. Pain disability and intensity were measured using the "Chronic Pain Grade questionnaire", a validated 7-item self-report tool assessing pain intensity and pain-related disability. The scores are reported on a 0–100 scale, where greater severity and impact on function correspond to higher scores.
Statistical Analysis
Data were analyzed using IBM SPSS Statistics version 25.0 (SPSS Inc., Chicago, IL, USA). The test normality was assessed by the Kolmogorov – Smirnov test. Data that showed normal distribution were displayed in mean ± standard deviation (SD). The OA patients and controls were compared using an independent samples t-test. Pearson’s correlation coefficient evaluated correlations between biomarkers and clinical parameters. The results of the ROC curve analysis for the discriminative ability of sclerostin and osteocalcin to distinguish OA patients from healthy individuals are provided below. A P-value < 0.05 was regarded as a significant difference.
RESULTS
Table 1 reveals the age data in the subgroups, BMI categories, and pain scores for healthy controls and osteoarthritis (OA) patients. Statistical comparisons of age P = 0.22 and BMI ( P = 0.17) between the two groups were not significant, indicating good demographic matching. However, pain intensity and pain-related disability scores were greater in OA patients than in healthy participants; 58.33 ± 16.23 versus 9.67 ± 2.5 and 53.33 ± 16.23 versus 7.89 ± 4.2, respectively; P < 0.001 for both comparisons clinically burdensome results regarding pain and disability in the OA group.
Table 1
Comparison of age, BMI and pain scores between OA patients and control
|
Items |
OA Patients N = 65 |
Control N = 35 |
T-Test ( P -value) |
||
|
Mean |
SD |
Mean |
SD |
||
|
Age |
56.87 |
13.93 |
52.67 |
20.5 |
2.79 (0.22) |
|
BMI |
29.56 |
9.72 |
23.98 |
10.3 |
3.79 (0.17) |
|
Pain Intensity |
58.33 |
16.23 |
9.67 |
2.5 |
6.54 (0.000) |
|
Pain Disability |
53.33 |
16.23 |
7.89 |
4.2 |
5.36 (0.000) |
The findings indicated that patients with osteoarthritis had significantly raised levels of serum sclerostin (11.70 ± 1.10 ng/mL) compared to healthy controls who had levels of 3.80 ± 0.90 ng/mL (Fig. 1). In turn, the levels of osteocalcin were dramatically lower in patients (23.50 ± 19.30 ng/mL) vs controls (48.90 ± 5.20 ng/mL), and both variations were significant at P < 0.0001 (Fig. 2). This indicates that bone metabolism is deranged in osteoarthritis with increased inhibition of bone formation via sclerostin and decreased osteoblastic activity shown by the lower osteocalcin level.
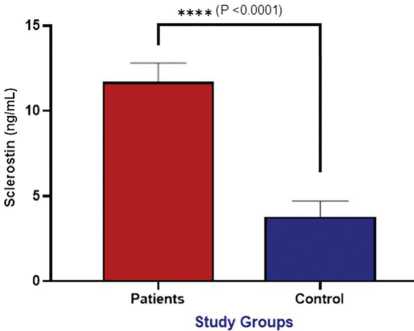
Fig. 1. Assessment of serum sclerostin (ng/mL) between patient and control groups
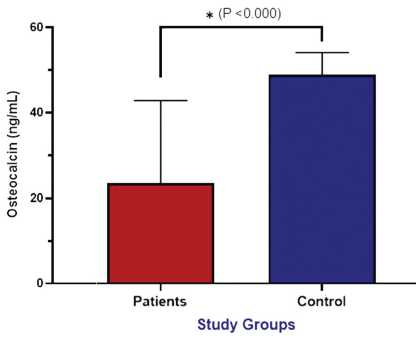
Fig. 2. Assessment of serum osteocalcin (ng/mL) between patients and control groups
The comparison of serum proinflammatory cytokine levels between the osteoarthritis patients and healthy controls showed that the concentrations of all the cytokines measured were statistically increased in the osteoarthritis group. IL-1 β , TNF, IL-6, and IL-17 were statistically greater in osteoarthritis patients (means: 11.5, 15.34, 36.8, and 45.8 pg/mL, respectively) compared to controls (means: 8.4, 11.39, 8.4 and 13 .8 pg/mL respectively). The variations were all extremely significant at a probability level of ( P < 0.0001). These findings further emphasize the central role of systemic inflammatory response in the pathophysiology of osteoarthritis and confirm these cytokines' modes of action regarding disease progression and symptom severity (Table 2).
Table 2
Comparison of proinflammatory cytokines between patients and control
|
Proinflammatory Cytokines |
OA Patients N = 65 |
Control N = 35 |
T-Test ( P -value) |
||
|
Mean |
SD |
Mean |
SD |
||
|
IL-1 β (pg/mL) |
11.5 |
2.01 |
8.4 |
2.5 |
4.61 (0.000) |
|
TNF (pg/mL) |
15.34 |
5.71 |
11.39 |
4.3 |
3.57 (0.000) |
|
IL-6 (pg/mL) |
36.8 |
7.60 |
8.4 |
1.9 |
6.2 (0.000) |
|
IL-17 (pg/mL) |
45.8 |
9.47 |
13.8 |
1.5 |
7.5 (0.000) |
The analysis of diagnostic performance has revealed sclerostin to have a strong potential as a biomarker for osteoarthritis with an area under the curve of 0.82 which is fairly good. At a cut-off value of 5.8 sclerostin reported a sensitivity of 78% and specificity of 82 % which means it can fairly well identify those individuals who have osteoarthritis from those who do not. The p -value obtained 0.029 also adds to the evidence in favor of the reliability of sclerostin in this case. These findings strengthen the potential clinical application of sclerostin as a non-invasive biomarker in the future for detecting and assessing the risk of osteoarthritis at an early stage (Table 3, Fig. 2). The correlation matrix exhibited that sclerostin showed highly significant positive correlations with TNF ( r = 0.423, p = 0.000), IL-6 ( r = 0.342, p = 0.003), and IL-17 ( r = 0.298, p = 0.005). Thus, a direct involvement of inflammatory activity in increased expression of sclerostin is suggested. The other way round, osteocalcin revealed the most significant negative correlations with the above-mentioned cytokines: TNF ( r = –0.385, p = 0.001), IL-6 ( r = –0.345, p = 0.003), and IL-17 ( r = –0.312, p = 0.004), hence an inverse relationship with inflammation status on bone formation activity. The above proinflammatory cytokine did not correlate with IL-1 β expression. The different degrees of expression of the two major genes associated with bone remodeling confirm the report by the authors that inflammatory mediators have a differential regulatory effect on the expression of these genes (Fig. 3).
Table 3
Pearson correlation coefficient between bone biomarkers and interleukins
|
Proinflammatory Cytokines |
Sclerostin |
Osteocalcin |
|
IL-1 β |
r = 0.121 (0.351) |
r = –0.106 (0.420) |
|
TNF |
r = 0.423 (0.000) |
r = –385 (0.001) |
|
IL-6 |
r = 0.342 (0.003) |
r = –0.345 (0.003) |
|
IL-17 |
r = 0.298 (0.005) |
r = –0.312 (0.004) |
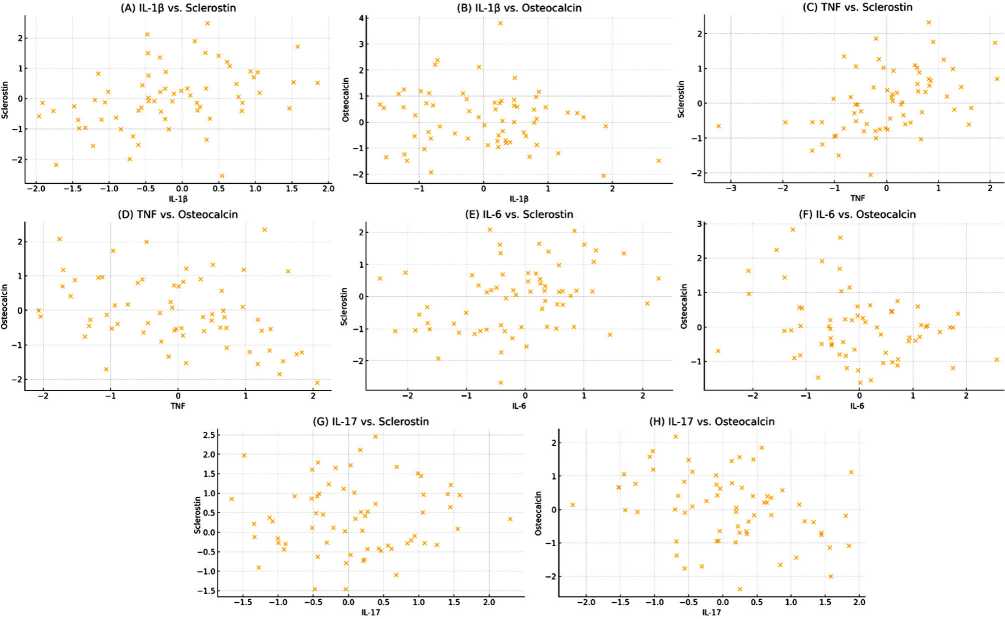
Fig. 3. Scatter plots showing the correlation and regression line between proinflammatory cytokines and bone markers in osteoarthritis patients (A) IL-1 β and sclerostin. (B) IL-1 β and osteocalcin. (C) TNF and sclerostin. (D) TNF and osteocalcin. (E) IL-6 and sclerostin. (F) IL-6 and osteocalcin (G) IL-17 sclerostin (H) IL-17 and osteocalcin
DISCUSSION
Osteoarthritis is the most prevalent degenerative joint disorder. In osteoarthritis (OA), the subchondral bone gets remodeled and the inflammatory microenvironment greatly affects both the expression and systemic levels of related proteins such as osteocalcin and sclerostin. Osteocalcin is a non-collagenous protein secreted by osteoblasts that has key importance in marking bone formation. It has been found elevated in patients with OA; thus, its synthesis reflects increased turnover of bone characteristic of the disease, along with the formation of osteophytes [8]. Sclerostin from osteocytes, a known inhibitor in the pathway of Wnt/β-catenin signaling, is mostly inhibited in OA particularly where there is active subchondral bone formation, i.e., in osteophyte regions [9]. On the other hand, recent data have shifted this view since chronic low-grade inflammation mainly mediated by proinflammatory cytokines appears to be central to the pathogenesis and evolution of OA. The proinflammatory cytokines included in the current study are strong mediators in the molecular cascade that leads to degradation of the cartilage; hyperplasia of synovium, osteophyte formation, and pain sensitization [10]. The concentration levels of IL-1β in OA synovial fluid are typically within 10–150 pg/mL. Healthy individuals normally have significantly lower concentrations; their levels are often undetectable or < 10 pg/mL [11]. Higher levels of IL-1β have been accompanied by a positive correlation with disease severity, joint space narrowing, and radiographic grading in OA patients [12]. It underscores the importance not just as a mechanistic contributor but a potential biomarker for disease activity and progression. Another essential inflammatory cytokine in OA is TNF-α. Its producing cells are macrophages, synovial fibroblasts, and chondrocytes; that is, practically any cell residing within the joint. Levels of TNF-α in serum and synovial fluid in OA patients (normally run between 15 and 100 pg/mL control values) are below 10–15 pg/mL [13]. Higher levels of TNF-α have also been correlated with pain, stiffness, and limitation of function in joints among OA population [12]. Similarly, in OA joints, IL-6 is secreted by chondrocytes, synoviocytes, and osteoblasts; it is more commonly in response to the stimulation by IL-1β and TNF-α [14]. Raised levels of IL-6 have been determined in the blood and joint fluid of OA patients, usually between 10–300 pg/mL, much higher than in healthy people (< 10 pg/mL) [15]. Greater amounts of IL-6 are linked to more pain in the joints, severity seen on X-rays, and loss of cartilage measured by MRI images [16]. IL-6 helps in central pain sensitization and could explain why some OA patients have worse symptoms than would be expected from the amount of damage. It is mostly secreted by T helper cells 17 (Th17) and this proinflammatory cytokine is ever more identified in the pathology of OA. It has been found that IL-17 levels are greatly elevated in the serum and synovial fluid of OA patients as compared to the norm, and its concentrations most times range between 20–150 pg/mL [17]. These cytokines hardly ever act alone. They typify the pro-inflammatory milieu seen within the OA-affected joint. For instance, IL-17 can signal chondrocytes to secrete IL-1β and TNF-α which further improve IL-6 secretion, creating a positive feedback loop [18]. Such crosstalk perpetuates the high degree of local and systemic inflammation seen in concert with ongoing joint destruction. Raised levels of TNF-α, IL-1β, IL-17, and IL-6 together have been linked with poorer clinical outcomes; in other words, the higher pain scores and the greater functional limitations are the faster radiographic progression is [19]. In osteoarthritis, the cross-talk bone remodeling and inflammation hold a central position in the pathogenesis of the disease. Important molecular mediators of bone metabolism are osteocalcin and sclerostin. These two proteins quite opposed in function and regulation. In OA, osteocalcin is upregulated and sclerostin suppressed simultaneously, reflecting a shift toward a bone-anabolic phenotype from a chronic low-grade inflammatory state. A negative correlation was reported between sclerostin and studied interleukins [20]. The increase in osteocalcin seems to be very much related to proinflammatory cytokines. These cytokines, IL-6, IL-1β, and TNF-α have been proven to activate osteoblast progenitor cells through intracellular signaling cascades delivering messages that eventually result in gene expression of osteocalcin [21–22]. Moreover, under conditions of chronic inflammation these same cytokines can stimulate Wnt/β-catenin signaling that paradoxically increases osteoblast differentiation and matrix protein synthesis such as osteocalcin [23]. In OA, at the bone-cartilage interface, the inflammatory microenvironment together with mechanical stress allows for osteoblastic hyperactivity and angiogenesis to come about. This further raises osteocalcin levels and pathological bone formation [9]. Therefore, a strong positive correlation exists between osteocalcin and proinflammatory interleukins. The more these latter cytokines are involved in inflammation, the more they accelerate bone turnover in OA. The downregulation of sclerostin in OA also shares common pathways with proinflammatory cytokines that drive expression of osteocalcin. The inflammatory signaling pathways under which IL-1β, IL-17, and TNF-α suppress SOST gene expression involved that allowed for unrestrained osteogenesis. Inflammation results in osteocyte apoptosis or their dedifferentiation, and this further lowers the population of sclerostin-secreting cells in the subchondral bone. Thus, it furthers the abnormal bone formation environment to prevail [20]. This is how the reverse correlation is explained between sclerostin and proinflammatory cytokines in OA.
CONCLUSION
The studied proinflammatory cytokines (IL-17, IL-1 β , TNF- α , IL-6) mediate osteoarthritis; they act by enhancing inflammation and damage to cartilage as well as changing the subchondral bone. In this regard, they increase osteocalcin levels by promoting the activity of osteoblasts and inhibit sclerostin allowing pathological bone formation. The positive correlation of osteocalcin with cytokines and inverse correlation with sclerostin reflects an inflammatory-driven imbalance in bone remodeling. Collectively, these molecules provide a clue to the pathophysiology of osteoarthritis and could perhaps be biomarkers indicative of disease activity and progression.
Conflict of interest Not declared.
Funding The authors rely only on their own financial support.
Ethics approval The proposal in this research was recommended by the bioethical board of the College of in the University of Baghdad (No. 245 in 2025).
Consent to participate Before data collection and blood sampling, all patients included in the study were asked to provide written informed consent.


