Features of thermal decomposition of a new precursor for Mg-Zr mixed oxide preparation
Автор: Krivtsov I.V., Kasatkina D.D., Avdin V.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Краткие сообщения
Статья в выпуске: 1 т.7, 2015 года.
Бесплатный доступ
A new peroxo-method for Mg-Zr mixed oxide preparation has been applied. It has been established that this precursor after being heated up to 700 ºC is in the form of poorly crystalline Mg-Zr oxide. Thermal and X-ray diffractional analyses have shown that the oxide phase is formed directly from amorphous peroxocomplex, avoiding formation of intermediate Mg(OH) 2 phase. The peroxocomplex-mediated route allows achieving high degree of molecular homogeneity in the mixed oxide and high distribution of the active sites due to its low crystallinity. The prepared material is a potential catalyst for aldol condensation reaction.
Zro 2, mgo, mgo/zro 2, mixed oxides, sol-gel, peroxocomplex, aldol condensation
Короткий адрес: https://sciup.org/147160305
IDR: 147160305 | УДК: 544.77+546.05
Текст краткого сообщения Features of thermal decomposition of a new precursor for Mg-Zr mixed oxide preparation
Mixed zirconia-magnesia oxides are known for their unique properties and high activity as catalysts for aldol condensation reaction [1]. The presence of acid and basic sites, acid-base pairs and their distribution on the surface of the mixed oxides determine activity and selectivity of Mg–Zr catalysts in furfural aldol condensation and acetone self-condensation [2, 3]. Modification of the preparation technique is the tool for tuning material’s surface properties. The main focus of the researchers dealing with synthesis of Mg–Zr mixed oxides was on the modification and control of their morphology and specific surface area, applying alkoxide-based sol-gel procedure [4] or ultra-dilution method [5] to reach their goal, or dispersing a catalyst precursor on the high surface area supports [3]. However, the other approach, that could enhance molecular homogeneity of the catalyst and suppress its crystallization, thus improving the distribution of the active sites, has not been considered yet. Here we report the thermal decomposition features of a new precursor for Mg–Zr mixed oxide synthesis based on zirconium peroxocomplex.
Experimental
Zirconium oxychloride (ZrOCl 2 ) solution was supplied by MEL Chemicals, magnesium sulfate heptahydrate (MgSO 4 7H 2 O), hydrogen peroxide (H 2 O 2 ) 30 wt% water solution and citric acid monohydrate were purchased from Aldrich, sodium hydroxide (NaOH) was obtained from Prolabo.
The conventional precipitation technique described by Aramendia [6] was applied to synthesize Mg-Zr gel. Initially 50 mL of the aqueous solutions of MgSO 4 (0.1 M) and ZrOCl 2 (0.1 M) were mixed and precipitated by the addition of NaOH (2 M) until pH value was 10.7. Then the precipitate was isolated by centrifugation at 3000 r.p.m. and washed 8 times with deionized water. After the washing step 2.1 g of citric acid monohydrate, dissolved in 10 mL of H 2 O 2 , was added to the precipitate. The obtained suspension was heated to 100 ºC, while stirring. When the most part of the precipitate was dissolved the suspension was centrifuged at 3000 r.p.m. and the liquid phase was collected. Water was evaporated from the peroxocomplex and the solid phase was dried at 50 ºC for 24 h.
XRD patterns were registered using Rigaku Ultima IV diffractometer with Cu Kα source of radiation. X-ray thermodiffractional studies were carried out on the PAN analytical X`Pert Pro Philips diffractometer. The samples were dried at 400 ºC prior to thermodiffractional experiment, then they were heated at the heating rate of 5 K/min in air and the XRD patterns were collected from 500 ºC to 1000 ºC with a step of 100 ºC. Thermogravimetry (TG) and differential scanning calorimetry (DSC) data were
Краткие сообщения obtained by means of Netzsch STA 449F1 thermal analyzer in air atmosphere at the heating rate of 5 K/min.
Results and Discussion
The formation of water-soluble peroxocomplex was confirmed by the qualitative reaction with potassium iodide, which colored the solution into orange. Elemental analysis made by EDS technique showed that the intentional equimolar composition of Mg–Zr oxide had been reached.
The thermal decomposition of the peroxocomplex goes in several stages (Fig. 1a). In the low-temperature region one can observe the mass-loss accompanied by endothermic effect, which is likely to correspond to dehydration of the complex. The second stage of the mass-loss in the range of 400–500 ºC is unambiguously the combustion of the organic part of the complex. The exothermic effects at 625– 750 ºC and 900–950 ºC are assigned to the phase transitions in the mixed oxide system. In order to corroborate the suppositions made on the basis of thermal analysis the XRD and thermodiffractional studies have been carried out.
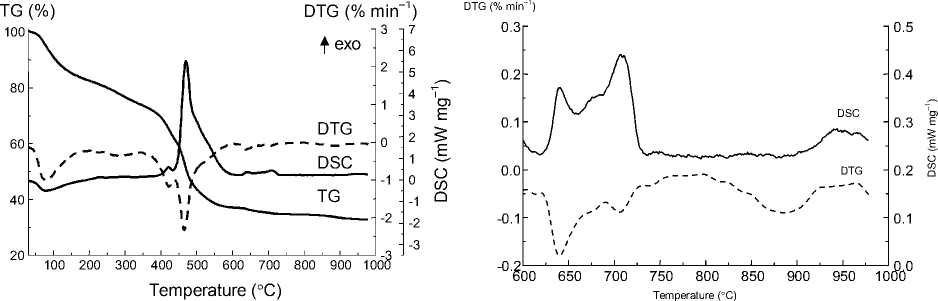
а)
b)
Fig. 1. Thermoanalytical curves of the peroxocomplex precursor (a), DSC and DTG curves in the range of 600–1000 °C (b)
The as-prepared complex is amorphous (Fig. 2a) and shows no presence of magnesium hydroxide or oxide phases. The broad reflection at 9 2Θ probably corresponds to the zirconium peroxocomplex, similar observation was reported by Ichinose et. al [7] for peroxotitanate hydrate.
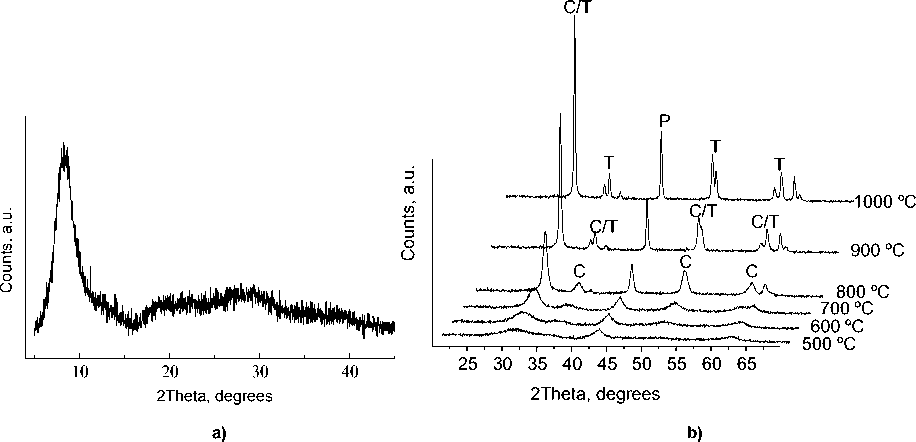
Fig. 2 XRD pattern of the amorphous complex (a) and thermodiffractional patterns of Zr-Mg mixed oxide (b)
Кривцов И.В., Касаткина Д.Д., Авдин В.В.
Особенности термического разложения нового прекурсора смешанных оксидов Mg–Zr
From Fig. 2b it is seen that the material up to 700 ºC is the mixture of poorly crystalline phases of MgO (ICDD PDF2 99-200-4113) and cubic magnesia-stabilized zirconia Mg x Zr 1-x O 2-x (ICDD PDF2 00080-0967 and 00-080-0964). Thus, the exothermic effect on the DSC curve (Fig. 1a) in the range of 400–600 ºC could not be assigned to the phase transition in the mixed oxide, but only to the combustion of the organic residue. The doubled exothermic effect at 625–750 ºC (Fig. 1b) is attributed to the coalescence of the small crystallites of magnesia-stabilized zirconia and magnesia into larger crystallites, that is obvious from the improved crystallinity (Fig. 2b). The last exothermic effect near 950 ºC (Fig. 1b), according to thermodiffractinal study, reflects the process of phase transition of magnesia-stabilized zirconia Mg x Zr 1-x O 2-x phase into tetragonal zirconia (ICDD PDF2 00-081-1544) accompanied by separation of periclase.
Conclusion
The process of thermal decomposition of the new precursor for Zr–Mg mixed oxide synthesis shows that the formation of oxide phases goes directly from amorphous precursor. Up to 700 ºC the sample contains the mixture of poorly crystalline phases of cubic magnesia-stabilized zirconia and periclase phases. The coalescence of the small crystallite near 700 ºC accompanied by two exothermic effects leads to the formation of highly crystalline material. The cubic magnesia-stabilized zirconia phase starts decomposing at 900 ºC, forming tetragonal ZrO 2 and cubic MgO. The hindered crystallization behavior of the mixed oxide prepared via proposed technique could be the feature of high importance for its application as a catalyst for aldol condensation reactions.
We are grateful for financial support of The Ministry of Education and Science of the Russian Federation (grant No 16.2674.2014/K).
Список литературы Features of thermal decomposition of a new precursor for Mg-Zr mixed oxide preparation
- Faba L., Diaz E., Ordonez S. Improvement of the Stability of Basic Mixed Oxides Used as Catalysts for Aldol Condensation of Bio-derived Compounds by Palladium Addition. Biomass and Bioenergy, 2013, vol. 56, pp. 592-599.
- Faba L., Diaz E., Ordonez S. Aqueous-phase Furfural-acetone Aldol Condensation Over Basic Mixed Oxides. Applied Catalysis B: Environmental, 2012, vol. 113, pp. 201-211.
- Faba L., Diaz E., Ordonez S. Gas Phase Acetone Self-condensation Over Unsupported and Supported Mg-Zr Mixed-oxides Catalysts. Applied Catalysis B: Environmental, 2013, vol. 142-143, pp. 387-395.
- Sadaba I., Ojeda M., Mariscal R., Richards R., Lopez Frandos M. Preparation and Characterization of Mg-Zr Mixed Oxide Aerogels and Their Application as Aldol Condensation Catalysts. A European Journal of Chemical Physics and Physical Chemistry, 2012, vol. 13, pp. 3282-3292.
- Gawande M.B. Rathi A.K., Branco P.S., Potewar T.M., Velhinho A., Nogueira I.D., Tolstogouzov A., Amjad A.G, Orlando M. N. D. Teodoro. Nano-MgO-ZrO2 Mixed Metal Oxides: Characterization by SIMS and Application in the Reduction of Carbonyl Compounds and in Multicomponent Reactions. RSC Advances, 2013, vol. 3, pp. 3611-3617.
- Aramendia M.A., Boráu V., Jiménez C, Marinas A., Marinas J.M., Navı́o J.A., Ruiz J.R., Urbano F.J. Synthesis and Textural-structural Characterization of Magnesia, Magnesia -titania and Magnesia -zirconia Catalysts. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, vol. 234, pp. 17-25.
- Ichinose H., Terasaki M., Katsuki H. Synthesis of Peroxo-modified Anatase Sol from Peroxo Titanic Acid Solution. Journal of Ceramic Society of Japan, 1996, vol. 104, pp. 715-718.


